Published online Dec 6, 2019. doi: 10.12998/wjcc.v7.i23.3915
Peer-review started: October 8, 2019
First decision: November 10, 2019
Revised: November 20, 2019
Accepted: November 27, 2019
Article in press: November 27, 2019
Published online: December 6, 2019
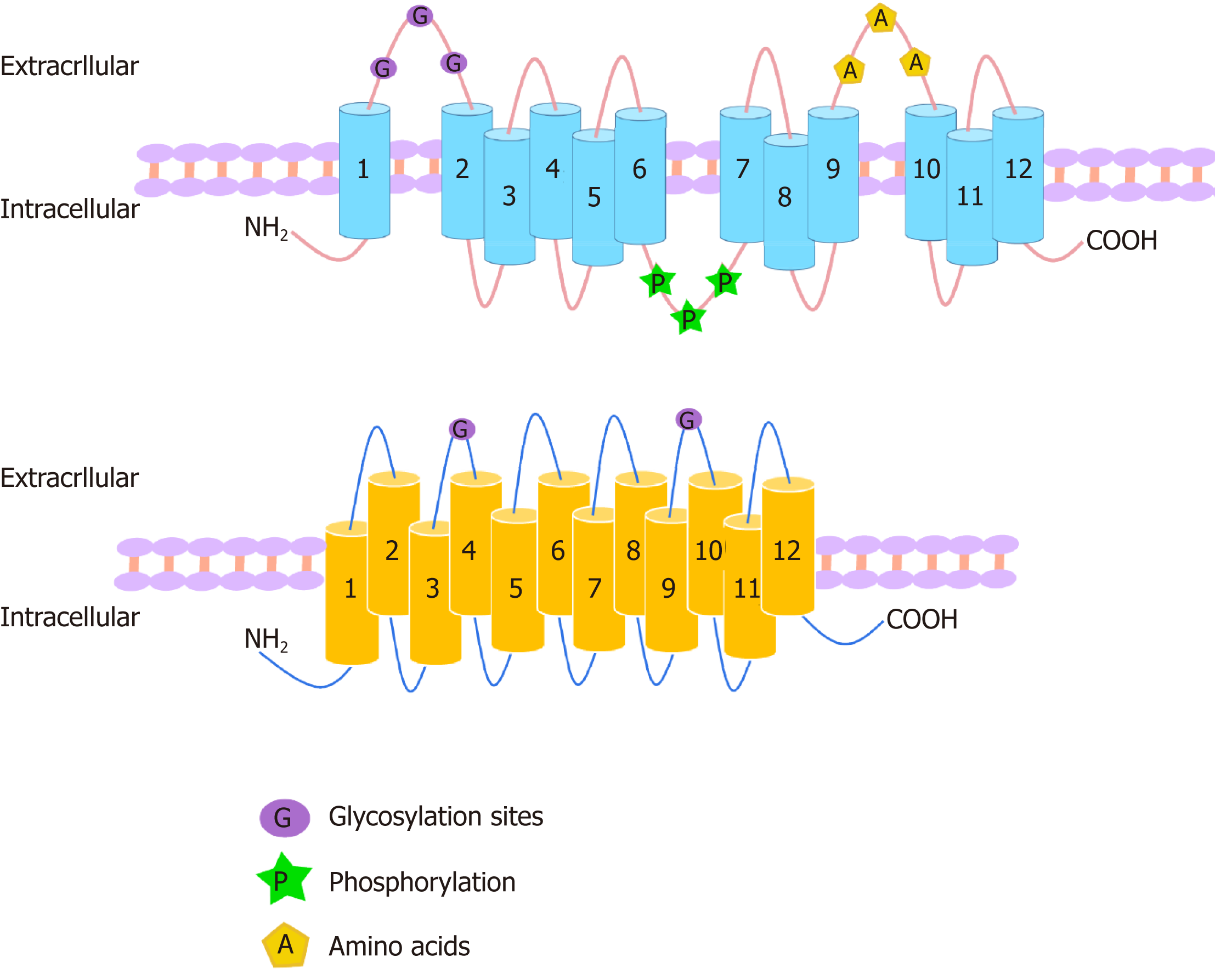
Organic anion transporters (OATs) and organic anion transporter polypeptides (OATPs) are classified within two SLC superfamilies, namely, the SLC22A superfamily and the SLCO superfamily (formerly the SLC21A family), respectively. They are expressed in many tissues, such as the liver and kidney, and mediate the absorption and excretion of many endogenous and exogenous substances, including various drugs. Most are composed of 12 transmembrane polypeptide chains with the C-terminus and the N-terminus located in the cell cytoplasm. OATs and OATPs are abundantly expressed in the liver, where they mainly promote the uptake of various endogenous substrates such as bile acids and various exogenous drugs such as antifibrotic and anticancer drugs. However, differences in the locations of glycosylation sites, phosphorylation sites, and amino acids in the OAT and OATP structures lead to different substrates being transported to the liver, which ultimately results in their different roles in the liver. To date, few articles have addressed these aspects of OAT and OATP structures, and we study further the similarities and differences in their structures, tissue distribution, substrates, and roles in liver diseases.
Core tip: As important anion transporters, organic anion transporters (OATs) and organic anion transporter polypeptides (OATPs) have similar structures and transport substrates. So far, the role of some members of OATs and OATPs in the liver has been reported, but studies on both families are still rare. In this paper, we study their structure, distribution, substrate of action, and regulatory mechanisms in various diseases of the liver. With the further study of the relationship between OATs/OATPs and various liver diseases, targeted therapy with OATs/OATPs is expected to improve the adverse reactions of drugs in the liver and improve the survival rate of patients with liver diseases.
- Citation: Li TT, An JX, Xu JY, Tuo BG. Overview of organic anion transporters and organic anion transporter polypeptides and their roles in the liver. World J Clin Cases 2019; 7(23): 3915-3933
- URL: https://www.wjgnet.com/2307-8960/full/v7/i23/3915.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i23.3915
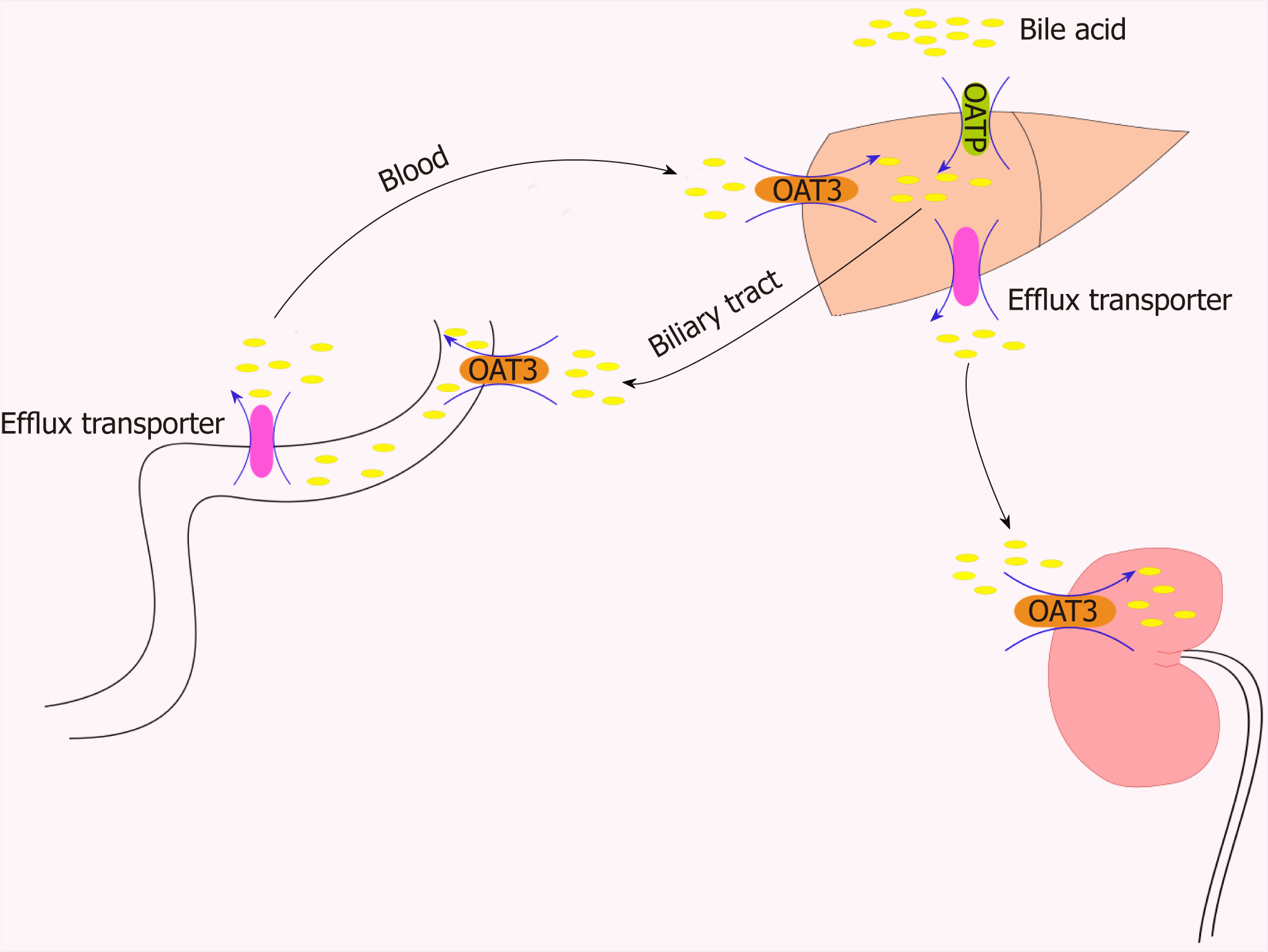
Organic anions are a general term for a class of materials containing a carbon skeleton with a net negative charge under physiological pH conditions. Both organic anion transporters (OATs) and organic anion transporter polypeptides (OATPs) belong to the SLC family. OATs are classified in the SLC22A superfamily. SLC22 transporters have at least six subfamilies: OAT, OAT-like, OAT-related, organic cation transporter (OCT), organic cation/carnitine transporter (OCTN), and OCT/OCTN-related[1]. OATPs are encoded by genes in the SLCO superfamily. This superfamily was originally named SLC21A. In 2004, it was updated and standardized according to phylogenetic relationships. This superfamily was renamed SLCO, the solute carrier family of OATPs[2]. OATs and OATPs are important transmembrane transporters that are usually composed of 12 transmembrane polypeptide chains with extracellular glycosylation regions (Figure 1). OATs and OATPs are abundantly expressed in the liver and are mainly distributed on the basolateral membrane. They mediate the transport of a variety of endogenous and exogenous substrates through the cellular membrane[3]. The substrates for OATs are small and hydrophilic organic anions such as dicarboxylates and cyclic nucleotides[4], while OATPs transport large hydrophobic organic anions such as bile acids, thyroid hormones, prostaglandins, testosterone, and steroid hormone conjugates. However, they are all involved in the intestinal-hepatic circulation of bile. To maintain this important physiological process, hepatocytes recover bile acids from portal vein blood through some members of the OATP family, such as OATP1B1, OATP1B3, and OATP2B1[5]. OAT3, a member of the OAT family, plays a central role in the movement of bile acids through the “gut-liver-kidney” axis and participates in the absorption, metabolism, and excretion of bile acids[6] (Figure 2). OATs and OATPs, together with other SLC transporters, play a key role in inter- and intra-tissue molecule communication, in neuroendocrine, growth factor-cytokine, and other homeostatic systems and in the regulation of local and systemic homeostasis[1]. In liver disease, such as liver fibrosis, cirrhosis, and liver cancer, the expression of these uptake transporters changes, eventually affecting the rate of endogenous and exogenous drug transport, causing intracellular and extracellular signal transduction dysfunction and increasing the accumulation of metabolites in the plasma. This brief review will summarize our current understanding of the similarities and differences between these two transporter family members, with an emphasis on tissue composition and substrates, regulation of their expression, and their roles in liver diseases.
The OAT family members have similar membrane topologies[7] and consist of 12 α-helical transmembrane domains (TMD) with three highly conserved regions in their structure that are important for their function: A large extracellular loop with many glycosylation sites between TMD1 and TMD2; a large intracellular loop in the central region between TMD6 and TMD7 with conserved residue phosphorylation; and some motifs in TMD9 and TMD10 with amino acids critical for protein transport activity[8]. No specific residues or domains were found to determine the specificity of the substrates, indicating that the three domains are compatible[9]. OATs contain 526 to 568 amino acid residues, and the C-terminus and N-terminus of OATs are located in the cell cytoplasm[10,11] (Figure 1). There are more than 20 identified human OAT subtypes, which can be divided into three subclades: (1) OAT: OAT1 (SLC22A6), OAT2 (SLC22A7, originally known as novel liver-specific transporters [NLTs])[12], OAT3 (SLC22A8), OAT7 (SLC22A9), OAT5 (SLC22A10), OAT4 (SLC22A11), urate transporter 1 (URAT1; SLC22A12), OAT6 (SLC22A20), SLC22A orphan (S22AO), SLC22A24, OAT8 (SLC22A25, also known as unknown substrate transporter 6 [UST6]), SLC22A26, OAT9 (SLC22A27), SLC22A28, SLC22A29, and SLC22A30, which is formed by the best functionally characterized OATs; (2) OAT-like: OAT10 (SLC22A1) and organic cation transporter-like 2 (OCTL2; SLC22A14); and (3) OAT-related: BOIT (SLC22A17), ORCTL2 (SLC22A18), Boct2 (SLC22A23), and SLC22A31, the members of which seem to be able to transport organic cations[8,13]. Most OATs are highly expressed in the human kidneys and/or liver and are expressed at lower levels in the brain, placenta, prostate, and testis[11]. OAT1, OAT2, and OAT3 are located on the basolateral membrane of renal proximal tubule cells, where they are involved in the secretion of drugs and toxins that are subsequently eliminated in urine[14]. In contrast, OAT4, OAT10, and URAT1 are expressed on the apical membrane of proximal tubular cells and participate in the reabsorption of substances from tubular fluids[14]. OAT2, OAT5, and OAT7 are located on the sinus membrane of hepatocytes and participate in the liver detoxification process[15]. Recently, there has been controversy over the main distribution of OAT2. Ohtsuki et al[16] compared the protein expression levels with corresponding mRNA expression levels and activities in 17 human liver samples and found that OAT2 protein expression did not correlate with the corresponding mRNA expression. The difference between the mRNA and protein expression levels may be due to posttranscriptional modification, intracellular trafficking, and/or membrane sorting. Thus, mRNA expression may not be a surrogate marker of transporter function, and as such, the use of it can cause misleading results because some of the transmembrane proteins localize to the membranes of intracellular organelles. Vildhede et al[17] found that OAT2 protein expression was 30-100-fold lower than that of other liver uptake transporters, including OATP1B1 and OATP1B3. Low OAT2 expression may result in its low activity in the human liver. However, recently, Nakamura et al[18] adopted a larger-scale proteomics approach and determined that the liver expression of OAT2 is comparable to that of OATP. Surprisingly, although it is hypothesized that human OAT2 is located in the sinus membrane of hepatocytes, the immunohistochemical staining for OAT2 protein has not been confirmed in human liver, which is the main organ where it is expressed. In addition, studies have shown that OAT2 is expressed in the embryonic liver, kidney, and other tissues, suggesting a role in the formation and maintenance of these tissues. OAT2 can play an important developmental role independent of its transport function[19]. OAT8 (UST6) is a slc22 transporter homolog in flies, worms, and humans, which contributes to the definition of a subfamily within the OATs, the USTs. The expression of UST6 is restricted to the liver in adults and fetuses and may play a role in the development and differentiation of the liver[20]. Other OATs include OAT6, which has less pharmacological relevance and are primarily expressed in the olfactory mucosa but not in the kidney or liver, and S22AO and OCTL2, which are poorly understood[21] (Table 1).
| Protein name | Alias | Chromosome localization | Distribution location | Substrates (Km value) |
| OAT1 | SLC22A6 | 11q13.1 | Kidney, brain, liver, stomach, pancreas | P-aminohippurate (PAH; 5 μmol), prostaglandin E2 (PGE2; 0.97 μmol), α-ketoglutaric acid, captopril, methotrexate |
| OAT2 | SLC22A7 | 6p21.1 | Liver, kidney, embryonic liver and kidney | PAH, PGE2 (0.71 μmol), estrone-3-sulfate (E3S), α-ketoglutaric acid (17.8 μmol), cGMP, succinic acid, paclitaxel (0.14 μmol), dehydroepiandrosterone (DHEA) sulfate, methotrexate, diclofenac, acyclovir, penciclovir, theophylline (12.6 μmol), erythromycin (18.5 μmol), salicylate (88.8 μmol) |
| OAT3 | SLC22A8 | 11q11.7 | Kidney, brain, liver, skeletal muscle, adrenal gland | PAH (87 μmol), PGE2 (0.34 μmol), E3S (3 μmol), zidovudine, cimetidine, bumetanide, ciprofloxacin, benzylpenicillin, pravastatin, methotrexate, bile acids, amino acids, MTX (11 μmol) |
| OAT4 | SLC22A11 | 11q12.3 | Kidney, placenta, adrenal gland | Urate, PGE2 (0.15 μmol), E3S (1 μmol), PAH, olmesartan, methotrexate, bumetanide |
| OAT5 | SLC22A10 | 11q12.3 | Liver | Unknown |
| OAT6 | SLC22A20 | 11q13.1 | Olfactory mucosa | E3S, PAH, PGE2, ibuprofen, ochratoxin A (OTA) |
| OAT7 | SLC22A9 | 11q12.3 | Liver | E3S, DHEA, sulfate, butyrate |
| OAT8 | UST6 | 11q12.3 | Liver | Unknown |
| OAT9 | SLC22A27 | Unknown | ||
| OAT10 | SLC22A13 | 3p22.2 | Kidney, brain, heart, colon | Lactate, nicotinate, succinate, glutathione urate |
| URAT1 | SLC22A12 | 11q13.1 | Kidney, vascular smooth muscle cells, liver, lung | Urea, chloride |
| S22AO | SLC22A24 | 11q12.3 | Kidney | Unknown |
| OCTL2 | SLC22A14 | 3p22.2 | Testis, kidney | Unknown |
OATPs consist of 643-722 amino acids and have 12 transmembrane segments, which can form six extracellular loops and five intracellular loops, and both the C-terminus and N-terminus are located in the cytoplasm[13]. OATP1B1 has been shown to be glycosylated in the second and fifth extracellular loops, and the unglycosylated protein remains in the endoplasmic reticulum (Figure 1). Therefore, all mammalian OATPs may be glycosylated in these two extracellular loops. Disulfide bonds can also have an effect on the proper folding and function of these proteins[22]. Based on site-directed mutagenesis of ten conserved cysteine residues in the large extracellular loop 5 of OATP2B1, all ten cysteine residues were determined to be usually disulfide-linked, and these disulfide bonds are the targets important for transporting OATP2B1 to the plasma membrane[23]. The OATP family contains 11 members, which share 40% of their amino acid sequence identity. These members show similar structures in their 12 putative transmembrane regions[24]. In the human genome, OATPs encoded by the solute carrier OAT (former SLC21) genes constitute an important transporter subfamily that consists of 11 members: OATP1A2 (SLCO1A2), OATP1B1 (SLCO1B1, formerly known as LST-1), OATP1B3 (SLCO1B3, formerly known as LST-2), OATP1C1 (SLCO1C1), OATP2A1 (SLCO2A1, also known as prostaglandin transporter PGT), OATP2B1 (SLCO2B1), OATP3A1 (SLCO3A1), OATP4A1 (SLCO4A1), OATP4C1 (SLCO4C1), OATP5A1 (SLCO5A1), and OATP6A1 (SLCO6A1)[25]. OATPs are expressed in many human organs such as the gastrointestinal tract, liver, kidney, heart, lung, and brain[25] and commonly expressed in various tumors. The OATP1A2 protein is expressed in the cells of the brush border membrane of the duodenal midgut[26], the biliary cells of the liver, the distal nephron of the kidney, and the luminal membrane of the capillary endothelial cells of the brain[27]. OATP1C1 is present in the brain and testis[28]. OATP2A1 is expressed in a variety of tissues[4]. Its mRNA is found in several tissues, including the brain, colon, heart, kidney, liver, lung, and small intestine[29]. Recently, OATP2A1 protein expression has been shown in the gastrointestinal tract, localized to the gastric antrum and the parietal cells of the stomach wall[30]. OATP3A1 mRNA expression is high in the brain, heart, and testis, followed by the lung, spleen, peripheral blood leukocytes, and thyroid[31]. OATP4A1 mRNA expression is high in the heart and placenta, followed by the lung, liver, skeletal muscle, kidney, and pancreas[32]. OATP4C1 is expressed in the kidney, liver, and human colon[33]. OATP5A1 is expressed in the fetal brain, prostate, skeletal muscle, and thymus. OATP6A1 is highly expressed in the testis, followed by the spleen, brain, fetal brain, and placenta[34,35]. OATP1B1, OATP1B3, and OATP2B1 are mainly expressed in the liver[15]. OATP1B1 is expressed in hepatocytes throughout the lobules, and OATP1B3 is mainly expressed around the central vein[36]. The mRNA expression level of OATP1B1 in the liver is generally higher than that of OATP1B3[10]. The level of OATP2B1 mRNA is highest in the liver, where the protein is located on the basolateral membrane of hepatocytes[37] (Table 2).
| Protein name | Coding gene | Chromosome localization | Distribution location | Substrates (Km value) |
| OATP1A2 | SLCO1A2 | 12p12.1 | Brain, liver, kidney | Bile salts, bilirubin, ciprofloxacin, estrone-3-sulfate (59 μmol), methotrexate, N-methylquinidine (26 μmol), norfloxacin, PGE2, taurochenodeoxycholate (19 μmol) |
| OATP1B1 | SLCO1B1 | 12p12.1 | Liver | Benzylpenicillin, bile acid, bilirubin (8 μmol), estradiol-17b-glucuronide (8 μmol), estrone-3-sulfate (0.2 μmol), methotrexate, olmesartan, pravastatin (35 μmol), taurocholate (10-34 μmol), steroid hormone |
| OATP1B3 | SLCO1B3 | 12p12.2 | Liver | Benzylpenicillin (penicillin G), bilirubin (39 μmol), bile acid, cholecystokinin octapeptide (CCK-8; 11 μmol), docetaxel, eicosanoid, estradiol-17β-glucuronide (5.4 μmol), methotrexate (25 μmol), paclitaxel (7 μmol), rosuvastatin, saquinavir, steroid hormone, taurocholate (6 μmol) |
| OATP1C1 | SLCO1C1 | 12p12.2 | Brain, testis | Bromosulfophthalein, estrone-3-sulphate, thyroid hormones (0.1 μmol) |
| OATP2A1 | SLCO2A1 | 3q22.1-q22.2 | Brain, colon, heart, kidney, liver, small intestine, stomach | PGH2, PGE1 (82 μmol), PGE2 (100 μmol), PGF2a (92 μmol), thromboxane B2 (182 μmol) |
| OATP2B1 | SLCO2B1 | 11q13.4 | Liver, intestinal epithelium, placenta, epidermal keratin, breast, heart, skeletal muscle, brain | Benzylpenicillin, bilirubin, dehydroepiandrosterone-3-sulphate, estrone-3-sulphate (5 μmol), fexofenadine, montelukast, pravastatin, pitavastatin, PGE2, talinolol, statins, steroids, taurocholate |
| OATP3A1 | SLCO3A1 | 15q26.1 | Testis, brain, heart, lung, spleen | Benzylpenicillin, estrone-3-sulphate, PGE1, PGE2, PGF2a, steroid hormone |
| OATP4A1 | SLCO4A1 | 20q13.33 | Heart, placenta, lung, liver, skeletal muscle, kidney, pancreas | Bile salts, estrone-3-sulphate, PGE2, taurocholate (15 μmol), steroid hormone |
| OATP4C1 | SLCO4C1 | 5q21.1 | Liver | Bile salts, cAMP, digoxin (8 μmol), estrone-3-sulphate, methotrexate, steroid hormone |
| OATP5A1 | SLCO5A1 | 8q13.3 | Fetal brain, prostate, skeletal muscle | Satraplatin |
| OATP6A1 | SLCO6A1 | 5q21.1 | Testis, spleen, brain, fetal brain, placenta | Unknown |
OATs and OATPs belong to the SLC superfamily and are anion transporters with 12 transmembrane structures (Figure 1). However, differences in glycosylation sites, amino acid sites, and phosphorylation sites in the transmembrane structure of the OATs and OATPs and the tissue distribution of them lead to differences in substrate apparent affinity (Km) (Tables 1 and 2) and functions.
OATs can transport a variety of anionic endogenous metabolites and xenobiotic molecules, including many drugs[21]. In 1999, OAT1 was used for the initial functional characterization of a multispecific organic anion-dicarboxylic acid exchanger[38,39]. OAT1 plays an important role in the elimination of various toxins in the kidney. In addition to transporting p-aminohippurate (PAH)[40], OAT1 has been shown to transport prostaglandins, a-ketoglutaric acid, NSAIDs, antiviral drugs, and anticancer drugs[39,40]. OAT2 was the first mammalian OAT to be cloned. OAT2 was renamed OAT2 due to its close homology to OAT1 and its interaction with organic anions[41]. OAT2 has three variants, OAT2-546aa, OAT2-548aa, and OAT2-539aa[42,43]. The OAT2-546aa protein is localized on the plasma membrane, and the OAT2-548aa protein is localized to the intracellular compartment. The OAT2-539aa differs greatly from OAT2-546aa and OAT2-548aa because the C-termini are significantly different between species[44]. Different variants have different substrate specificities. Many previously identified OAT2-548aa substrates, such as PAH, estrone-3-sulfate (E3S), alpha-ketoglutarate, succinic acid, paclitaxel, and dehydroepiandrosterone (DHEA) sulfate, are not transported by OAT2-546aa[45]. OAT2-546aa was found to be capable of transporting guanine nucleotide-related compounds and cGMP[44], as well as other endogenous substrates, suggesting that OAT2 may play a regulatory role in intracellular signaling[45]. OAT2 is an important determinant of drug elimination due to the expression of OAT2 in the liver and its ability to transport and hence affect the deposition of multiple pharmacologically active agents[21]. Therefore, some antitumor drugs interfere with OAT2-mediated transport, while others, such as methotrexate[46] or irinotecan[47], are substrates for this transporter. In addition, the drug substrates of OAT2 also include many antibiotics[48,49], antimetabolites[46,50], H-2 receptor antagonists[51], diuretic agents[49], nonsteroidal anti-inflammatory drugs[52], and antiviral drugs[53]. Many of the substrates of OAT2 are also substrates of OAT1 and/or OAT3. Notably, the three OAT2 splice variants OAT2-546aa, OAT-548aa, and OAT2-539aa with different transport specificities have been used in different laboratories and have resulted in conflicting findings[45]. OAT1 and OAT3 have similar specific drug substrates. However, it is not clear whether they have similar specific endogenous substrates[6]. Typically, the substrates of OAT3 are bulkier and more lipophilic than those of OAT1. OAT3 can transport E3S[54], zidovudine[55], cimetidine[51], diuretics[56], antibiotics[57], and statins such as methotrexate[58]. In addition, Bush et al[6] demonstrated that OAT3 is involved in the bile acid (Figure 2) and lipid metabolism pathways. OAT4 is unique in that it can absorb and transport certain substrates, such as urate and steroid sulfates (such as E3S)[59,60], but is an efflux transporter for other substances, such as PAH and olmesartan[61]. Little is known about human OAT5, although Northern blot analysis has shown mRNA expression in the liver[46]. Mouse and rat oat5 (Slc22a19) proteins have been shown to be expressed only in the kidney[62] and are improbable homologs of the human OAT5 protein[7]. OAT6 is capable of interacting with a variety of small organic anions of physiological, pharmacological, and toxicological significance, such as estrone sulfate, PAH, and prostaglandin E2 (PG-E2). The preferred ligand for this transporter is an odor molecule[63]. OAT7 is highly expressed in the liver. It transports butyric acid and other short-chain fatty acids into hepatocytes and affects the pathway of short-chain fatty acid metabolism but does not promote hepatocyte uptake of bile acids[11,64]. In addition, OAT7 may be critical for the release of steroid hormones such as estrogen-3-sulfate into the bloodstream. Impaired function of OAT7 may result in slower metabolism of short-chain fatty acids and impaired steroid responses[11]. OAT10 acts as an antiporter and exchanges extracellular nicotinate with intracellular lactate, nicotinate, succinate, or glutathione[65]. URAT1 was originally cloned as a “renal specific transporter (Rst)”, and the human homolog transported urate; therefore, it was named “urate transporter 1 (URAT1)”[66]. URAT1 is similar to other family members and operates as an anion exchanger. URAT1 can transport urea and chloride[66,67] (Table 1).
Most OATPs transport a wide range of compounds. Although most substrates are anionic, some OATPs can also transport neutral and ionic compounds[68]. Typically, the substrate is an amphiphilic molecule with a molecular weight greater than 350 Daltons, including bile acids, conjugated steroids, thyroid hormones, linear and cyclic peptides and mushroom toxins, and many drugs, including statins, sartans, antibiotics, and antitumor drugs[4]. Many of these compounds are substrates for a variety of OATPs. For example, both OATP1B1 and OATP1B3 regulate the transport of bile acids, eicosanoids, peptides, and some drugs, including the antitumor drug methotrexate[69]. It has been reported that OATP-mediated transport may be affected by pH. Some studies have shown that OATP2B1 transport activity increases in acidic pH conditions[70-72]. Under normal physiological pH, OATP2B1 transports estrone and DHEA though the membranes, while under acidic conditions, it can transport many other compounds such as taurocholate, bilirubin, fexofenadine, statins, and it is also a transporter of steroids that increases the amount of estradiol in tumor cells[73,74] (Table 2).
Although the substrates of OATs are mainly small hydrophilic organic anions, OATPs mainly transport large hydrophobic organic anions. However, many members of OAT and OATP families are capable of transporting the same endogenous substrates such as PG-E2 (transported by OAT1, OAT6, and OATP1A2) and E3S (transported by OAT2, OAT3, OAT4, OAT6, OAT7, OATP1A2, OATP1B1, OATP1C1, OATP2B1, OATP3A1, OATP4A1, and OATP4C1), and exogenous drugs such as benzylpenicillin (transported by OAT3, OATP1B1, and OATP2B1), ciprofloxacin (transported by OAT3 and OATP1A2), diclofenac (transported by OAT2 and OATP1B3), methotrexate (transported by OAT1, OAT2, OAT3, OAT4, OATP1A2, OATP1B1, OATP1B3, and OATP4C1), olmesartan (transported by OAT1, OAT4, and OATP1B1), paclitaxel (transported by OAT2 and OATP1B3), and pravastatin (transported by OAT3, OATP1B1, OATP1B1, and OATP2B1)[4,14,21,45,63,69,73].
In liver fibrosis and cirrhosis, scar tissue spreads throughout the liver, which may result in a decrease in membrane protein. Changes in the liver uptake transporter OAT2 can have a profound impact on the pharmacokinetics (PKs) of drugs administered to patients with liver cirrhosis, which may result in unexpected adverse effects and/or potential changes in drug efficacy[75,76]. For example, downregulation of OAT2 may affect liver uptake of entecavir. Entecavir, a guanosine cyclopentanoate analog, is a first-line drug used for hepatitis B and has a significant effect against hepatitis B virus (HBV) by inhibiting HBV polymerase[77]. Entecavir needs to be organized in the liver to act as an anti-HBV agent. Previous studies have shown that entecavir is a substrate for OAT 1/3[78]. Xu et al[78] first reported that liver fibrosis causes changes in the distribution of entecavir in the liver, and after liver fibrosis, the ability of the intestine to transport entecavir to the liver is significantly reduced due to the downregulation of OAT2[79], ultimately reducing the distribution of entecavir in the liver. In addition, studies have found that there are increased plasma hepatocyte growth factor (HGF) levels in patients with hepatic failure or liver cirrhosis[80]. HGF is a ligand of the c-Met membrane receptor tyrosine kinase and a potent stimulator of DNA synthesis in hepatocytes, contributing to liver regeneration[81,82]. Le et al[83] found that HGF (20 ng/mL) treatment for 48 h downregulated OAT2 mRNA levels. OAT2 downregulation may affect the liver’s transport of endogenous substances or drugs, thereby further promoting the development of cirrhosis. Besides, a non-alcoholic steatohepatitis (NASH) rat model induced by methionine-choline deficiency showed that two different stages of non-alcoholic fatty liver disease (NAFLD; simple fatty liver and more serious NASH) would lead to decreased liver uptake of transporter, such as OAT2, OAT3, OATP1a1, and OATP1b2. Furthermore, NAFLD may alter the plasma retention time of clinically relevant drugs that are dependent on these transporters and may increase potential drug toxicity. The impact of NAFLD on human hepatic uptake transporters is the focus of ongoing research[84].
The hepatitis-fibrosis-cirrhosis progression eventually leads to liver cancer. Hepatocellular carcinoma (HCC) is the most common primary liver cancer, and HCC patients have a poor prognosis. Enhanced surveillance of hepatitis/fibrosis/cirrhosis patients and additional risk analysis are important to prevent the development of HCC. OAT2 is not only an important independent risk factor for HCC but also the best predictor in the HCC recurrence index MO. Yasui et al[85] examined the association between de novo HCC development and OAT2 expression at baseline in 38 patients with hepatitis C without HCC who subsequently developed HCC, whose age, gender, and fibrosis stage data were matched with those of 76 hepatitis C patients who did not develop HCC. It was found that a decrease in the expression of OAT2 in the liver indicates a high risk of HCC for patients with chronic hepatitis C regardless of other risk factors[85]. Based on current data, assessment of the transporter function from liver biopsy samples provides additional valuable predictors. In addition, serum albumin levels differ in patients with and without HCC, with serum albumin level of 4.0 g/dL being a critical predictor of HCC development. Low serum albumin levels constituted an independent risk factor for HCC development in patients matched by age, gender, and liver fibrosis stage[84]. Nonetheless, in patients with higher serum albumin levels (≥4.0 g/dL), decreased expression of OAT2 remained an important independent risk factor for HCC development[85]. A study showed that OAT2 is responsible for the uptake of orotic acid[86], which is reported to promote liver carcinogenesis[87,88]. In a clinical setting, orotic aciduria was also detected in HCC patients without cirrhosis[89]. Furthermore, gene set enrichment analysis showed that OAT2 expression was significantly associated with mitochondrial oxidoreductase activity and fatty acid metabolism. Mitochondrial dysfunction and oxidative stress are considered to be key mechanisms for the development of HCC[85]. Taken together, the results from these studies suggest that reduced OAT2 expression may contribute to liver cancer by increasing the concentration of orotate around hepatocytes and promoting oxidative stress and mitochondrial dysfunction. It has been hypothesized that these microenvironmental changes may occur in patients with early chronic HCV infection[85]. In fact, the precise mechanism of the association between OAT2 expression and HCC development requires further investigation. Clinically, OAT2 may be a predictive tool for HCC, and patients with reduced expression of OAT2 and reduced serum albumin levels are candidates for enhanced HCC surveillance, even if they do not exhibit risk factors for HCC. In addition, OAT2 and UST6 expressed in the embryonic liver may indicate involvement in liver differentiation and development. They may play a distinct role in the formation and maintenance of liver tissues. Although their most likely role seems to be in the transport of organic molecules, it is also conceivable that they have a role in an independent transport function[20]. These speculations lead to the prediction that the high expression of embryonic OAT2 and UST6 is likely to be interesting in the context of cancer occurrence and regeneration. However, these effects have not been analyzed in detail, and their roles as embryonic transporters require further study.
HCC is an aggressive malignancy primarily due to tumor metastasis or recurrence, even after potentially curative treatment. Intrahepatic recurrence after hepatectomy for HCC includes intrahepatic metastasis (IM) and multicenter occurrence (MO)[89]. The following MO criteria are defined as HCC characteristics: (1) Recurrent tumors consist of well-differentiated HCC cells that are found in different liver segments and were moderately or poorly differentiated in the previous HCC case; (2) Primary and recurrent tumors have well differentiated HCC cells; (3) Recurrent tumors include areas of dysplastic nodules in the peripheral zone; and (4) Multiple HCCs have a nodule of well-differentiated HCC cells and contain some nodules consisting of moderately or poorly differentiated HCC cells. MO is a type of intrahepatic HCC recurrence, in which the new HCC lesions are formed due to chronic liver disease, and the extant noncancerous liver tissue with oncogenic potential may explain the risk of MO after hepatectomy[90]. It is unclear how liver dysfunction involving OAT failure leads to the development of HCC. Studies have focused on elucidating the relationship between liver dysfunction and MO after radical hepatectomy. According to the Gene Ontology database (GO: 0015711) of the OAT genes for hepatocyte function, the best predictor of HCC MO is OAT2[91]. Kudo et al[91] first elucidated the relationship between HCC and OAT2 expression in noncancerous liver tissues. They examined 49 noncancerous liver tissues from Milanese patients with standard HCC and found that high OAT2 expression prevented HCC after hepatectomy [odds ratio (OR) = 0.2; P = 0.004]. In contrast, a new HCC may occur 1 year after hepatectomy in patients with low OAT2 expression[91].
There are many types of cirrhosis, including primary biliary cirrhosis (PBC), alcoholic cirrhosis, and hepatitis C virus (HCV)-related cirrhosis. The expression of OATP in different types of cirrhosis differs. In alcoholic cirrhosis, although the expression of OATP1B1 and OATP1B3 is decreased, the expression of OATP2B1 is increased[92]. In PBC, uptake transporter expression is similar to that observed in the alcoholic cirrhotic liver[93,94]. Ogasawara et al[95] found a significant decrease in mRNA expression of OATP1B1, OATP1B3, and OATP2B1 in HCV-associated cirrhosis. However, Wang et al[92] found that the expression of OATP1B1 and 2B1 increased in HCV-associated cirrhotic liver. The reasons for these differences are not clear, but they may reflect ethnic differences (liver tissues from Japanese patients compared with those from Caucasian patients), endpoints (expression of mRNA and protein), disease severity, or sample size. This difference could also be due to different mechanisms of alcohol transporter regulation in the livers affected by the HCV vs that from livers affected by PBC. In addition, studies have shown that OATP1B1-mediated transport determines the rate of repaglinide uptake in the liver[96]. Hatorp et al[97] found that repaglinide plasma absorption increased four-fold in patients with cirrhosis, as determined by the area under the curve. This increase may be due to a decrease in the expression of OATP1B1[98]. The PKs of drugs for hepatobiliary transporter clearance vary between patients with different types of cirrhosis and liver fibrosis, and patients should be differentiated in clinical PK studies.
OATPs, especially OATP1B3, play an increasingly important role in detecting liver diseases. Gd-EOB-DTPA-enhanced MRI (EOB-MRI) is increasingly used to detect and assess liver lesions[99]. It was found that OATP1B3 expression was associated with an increase in EOB-MRI, indicating that it transports Gd-EOB-DTPA into HCC cells. It is generally believed that 85% of HCCs emit a low signal in the hepatobiliary phase of Gd-EOB-MRI compared to the noncancerous liver background, and the expression of OATP1B3 is reduced in tumors[100,101]. Gd-EOB-DTPA can be used to assess the vascular distribution of liver lesions and the activity of the OAT OATP1B3 to further understand the prognosis of the disease. Yamashita et al[102] found that EOB-MRI combined with serum alpha-fetoprotein (AFP) status reflects the stem/maturation status of HCC determined with different biological and prognostic information. Moreover, upregulation of OATP1B3 increased the uptake of Gd-EOB DTPA in the hepatobiliary phase and downregulated the low levels of serum AFP, an outcome that was associated with the maintenance of hepatocyte function and good prognosis. In contrast, HCC cells showed reduced Gd-EOB-DTPA uptake that was associated with high serum AFP levels, poor prognosis, and activation of the oncogene FOXM1[102]. In addition, the identification of specific subclasses to which the tumor belonged prior to treatment informs the molecular targeted drug therapy. Activation of the Wnt/β-catenin signaling pathway has been identified as an important molecular marker in HCC and has been used to define specific HCC subclasses[103]. Studies have found that HCC with OATP1B3 upregulation is a good candidate for inclusion into a specific subclass of Wnt/β-catenin-activated HCC. Defining an OATP1B3-upregulated HCC subclass would be useful in the current era in which molecular targeted therapies are rapidly developed, particularly with the advantage of being able to classify tumors noninvasively using EOB-MRI[103]. In addition to being the most useful imaging modality for diagnosing HCC, Gd-EOB DTPA may be a very useful tool for detecting and describing pathological sinus syndrome, also known as hepatic venous occlusive disease, at a relatively early stage[104]. It has been reported that Gd-EOB-DTPA can also be used to distinguish some liver diseases that are otherwise difficult to distinguish, such as focal nodular hyperplasia of alcoholic cirrhosis and HCC[105]. Recently, studies have classified OATP1B3 into two types: (Lt)-OATP1B3 (hepatic type specifically expressed in the human liver) and (Ct) OATP1B3 (cancer type identified in colon cancer, lung cancer, and pancreatic cancer tissues and cell lines). Lt-OATP1B3 and Ct-OATP1B3 have different transport functions and membrane localization characteristics. Lt-OATP1B3 is mainly expressed on the plasma membrane, while Ct-OATP1B3 is mainly retained in the cytosol[106]. Lt-OATP1B3 has significantly higher transport activity than Ct-OATP1B3 does. The expression and role of Lt-OATP1B3 in drug treatments are beneficial for drug deposition in the liver[107]. The characterization of extrahepatic expression of Lt-OATP1B3 in cancer expands our understanding of the potential role of OATP1B3 in the influx of OATP1B3 substrates serving as anticancer drugs in cancer cells. Lt-OATP1B3 mediates the uptake of many clinically important anticancer drugs[108-111]. Therefore, understanding the specific Lt-OATP1B3 expression in cancer has potential clinical relevance for cancer treatment. Future studies will need to compare the relationship between the appropriate levels of Lt-OATP1B3 and Ct-OATP1B3 expression in liver tissue.
In addition, studies have shown that decreased expression of OATP is significantly associated with HCC-related death after relapse. Vasuri et al[112] correlated the expression of OATP1B1 and OATP1B3 with HCC morphological features and the expression of bile keratin K7 and K19 [associated with a poor prognosis after orthotopic liver transplantation (OLT)] by observing the liver of 69 patients with HCC liver transplantation (OLT). OATP1B1 and OATP1B3 were hepatocyte-specific, while bile cells and biliary malignancies were always negative[113]. The phenotypic expression of K19 implied a major risk of repaid recurrence and poor overall prognosis[114]. They found a significant negative correlation between OATP and K7 and K19 expression (P < 0.001). In HCC patients with K7 and/or K19 positive expression, the OATP detection was always negative. Thus, there is an inverse correlation between OATP expression on the basolateral hepatocyte membrane and a biliary “phenotype” determined for the same hepatocytes. Although the meaning of this correlation is unclear, since OATP and keratin are molecules that have different functions and intracellular localizations, these findings seem to support the existence of morphological profiles for hepatic malignancies[112].
OATs and OATPs play important roles in a series of diseases, such as hepatitis, liver fibrosis, cirrhosis, and liver cancer. When liver diseases occur, changes in the expression of OATs and OATPs reduce the uptake of many drugs in the liver, such as entecavir and repaglinide, which ultimately has a profound impact on the PKs of drugs, including an increase in side effects. OATs and OATPs are closely related to the occurrence, recurrence, and prognosis of HCC. They can be used as important indicators to predict, detect, and distinguish different liver diseases.
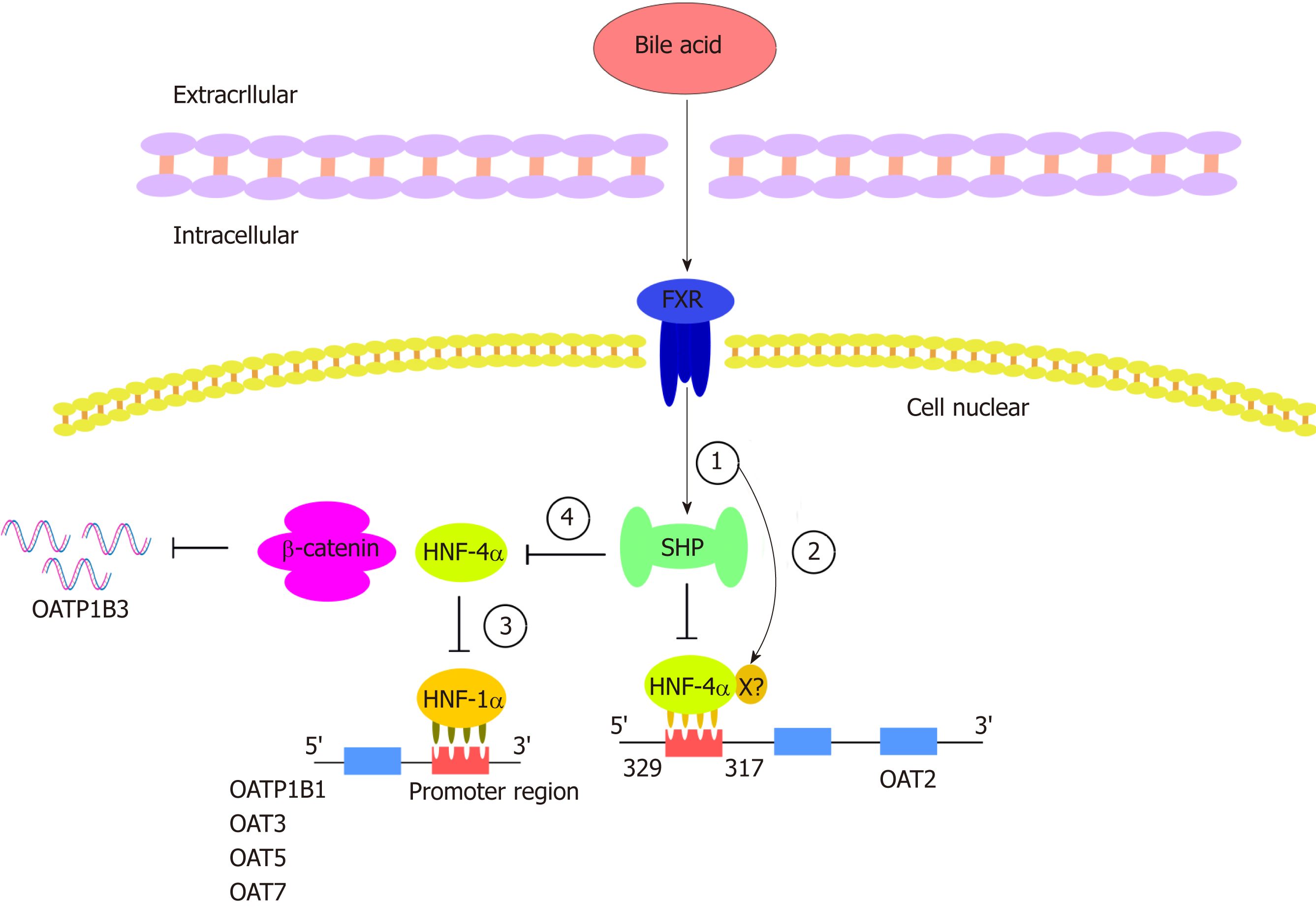
The expression of OATs is regulated by a variety of transcription factors. Hepatocyte nuclear factors (HNFs) constitute a class of transcription factors that regulate gene-specific expression in the liver. These transcription factors and their interactions constitute a complex regulatory network that precisely controls liver development and hepatocyte function. HNF-1a plays an important role in regulating a variety of hepatocyte-specific genes, although it is also expressed in other tissues[115]. HNF-1α and/or HNF-1β were previously reported to increase the promoter activity of OAT3[116] and affect the expression of URAT1 in the kidney[117]. Klein et al[115] showed that HNF-1a is able to increase human hepatocyte OAT5 and OAT7 promoter activities, and HNF-1a binds to two functional binding elements in the proximal OAT5 promoter and binds to one element in the OAT7 promoter; this binding is critical for the HNF-1α-mediated increase in promoter activities in liver-derived cells. They found a decrease in OAT5 and OAT7 mRNA expression when endogenous HNF-1a was knocked down[115]. In contrast, human OAT1 and OAT2 mRNA expression was not affected by the regulation of HNF-1a expression levels in hepatic cell lines. Human OAT1[118] and OAT2[119] promoter activities are increased by another liver-enriched transcription factor: HNF-4a, which is in the nuclear receptor family and is known to have an indispensable role in hepatocyte differentiation and the maintenance of liver gene expression. Target genes encode proteins involved in a variety of physiological processes, particularly cholesterol and glucose metabolism[120-122]. HNF-4a is also a gene encoding a transcriptional regulator of HNF-1a[123]. Through a computer analysis of the 5’ flanking regions of the OAT2 gene, Popowski et al[119] identified a common binding site for the liver-enriched HNF-4a between 329 and 317 nt upstream of the transcription initiation site. It was found that HNF-4α is involved in the transactivation of the OAT2 promoter, whereas chenodeoxycholic acid (CDCA) reduces the transactivation potential of HNF-4a and thereby inhibits the expression of endogenous OAT2 mRNA in Huh7 cells[119]. It is well known that HNF plays an important role in bile acid metabolism and transport through the transcriptional control of specific genes[124]. A primary bile acid, CDCA, and secondary bile acids, deoxycholic acid (DCA) and lithocholic acid (LCA), have been identified as endogenous ligands and effective activators of farnesoid X receptor (FXR)[125]. FXR is considered to play a key role in bile acid feedback regulation systems[126]. Bile acids enhance the transactivation ability of FXR, which results in the induction of FXR target genes, such as the transcriptional repressor SHP[127]. SHP is an orphaned member of the nuclear receptor family that inhibits other DNA-binding transcription factors, such as HNF-4a[128], through protein-protein interactions[129]. Overexpression of the FXR-induced transcriptional repressor SHP can counteract HNF-4α-mediated endogenous activity. In addition to acting through a SHP-dependent mechanism, FXR may directly bind to a negative response element located in the HNF-4a promoter[130,131], thereby counteracting HNF-4α-mediated OAT2 promoter activation (Figure 3).
In addition to certain members of the OAT family, promoter regions of other OATP genes, such as OATP1B1 and OATP1B3, have also been shown to be regulated by HNF[132]. Studies have shown that the OATP1B1 proximal promoter contains a functional HNF1a response element that is responsible for the expression of liver OATP1B1[132,133]. In human hepatoma cell lines, HepG2 and Huh7, bile acids, including CDCA, DCA, LCA, cholic acid, and ursodeoxycholic acid (UDCA), were shown to inhibit HNF1a-mediated OATP1B1 gene activation through a cascade reaction. In this cascade, the HNF-1a promoter is regulated by HNF-4a, and SHP negatively targets HNF-4a (HNF-4a is a common target for SHP), thereby downregulating HNF-1a activity and ultimately inhibiting OATP1B3[132]. Studies have found that UDCA increased the exposure of rosuvastatin and serum bilirubin in 12 healthy volunteers, probably because UDCA inhibited the transcription factor HNF1a and decreased the expression of the OATP1B1 transporter, which affected the liver uptake of sulvastatin and serum bilirubin[133]. Previous studies have reported that transcription of the OATP1B3 gene is regulated by three transcription factors: FXR, HNF1α, and HNF3β. Liver-specific expression of OATP1B3 is highly dependent on the liver-rich transcription factors HNF1α and HNF3β, and its expression is upregulated by CDCA and DCA via FXR[126]. In addition, some studies have reported that HNF4α interacts with β-catenin to promote the expression of hepatocyte target genes[134], such as OATP1B3[135]. This interaction is attenuated when the activity of HNF4α is inhibited (Figure 3).
The clinical relevance of drug transporters depends on their distribution in human tissues, their vector orientation, and the therapeutic indices and individual differences in the PK and pharmacodynamic properties of the substrate drugs. It has been shown that gene polymorphisms in OATs affect the PKs of substrate drugs. For example, some single nucleotide polymorphisms of the gene encoding OATP1B1 on sinus membranes are associated with changes in the uptake and excretion of organic anionic compounds. This discovery indicates that these transporters have important clinical significance[136-140]. OATs play a very important role in drug absorption, distribution, metabolism, and excretion, and changes in these transporters in the liver and/or kidney may affect the rate of drug metabolism, excretion, and drug residence time and half-life[141]. Studies have shown that pravastatin is a highly effective lipid-lowering drug that targets the liver, where it inhibits hydroxy methylglutaryl coenzyme A and cholesterol synthesis. Pravastatin is weakly soluble and can be transported into cells by OATP1B1, where it exerts its pharmacological effects before being discharged into the bile by multidrug resistance-associated protein (MRP2) and bile salt export pump on the bile duct-side membrane and then to the duodenum. Then, it is reabsorbed in the intestine where it is integrated into the intestinal-hepatic circulation, improving its bioavailability and pharmacological effects[142,143]. In addition, OAT3 is critical to the elimination of liver-derived phase II metabolites, particularly those that undergo glucuronidation. Bush et al[6] analyzed the pathways of OAT3-KO using the robust metabolomics data from mice, which indicated that OAT3 plays a central role in the movement of metabolites through the “gut-liver-kidney” axis, participating in the absorption, metabolism, and excretion of endogenous metabolites, particularly the gut microbial metabolites, bile acids, and nutrients that have undergone modification by phase 2 liver drug metabolizing enzymes involved in sulfation and glucuronidation reactions. A large number of these metabolites may be involved in “metabolite signaling” and signaling via G protein-coupled receptors throughout the body[6] (Figure 2).
Liver cancer is one of the most common causes of cancer death worldwide. Although surgery is a common treatment, the removal of these tumors is not always feasible and it is necessary to use alternative therapies for this disease. Systemic chemotherapy is the most common option for the treatment of advanced disease[50]. Unfortunately, pharmacological approaches are very ineffective because of resistance to antitumor drugs and/or development of chemical resistance during treatment, despite the number of drugs available to treat these tumors. The reduced therapeutic efficacy of anticancer drugs may be caused by changes in the expression and/or activity of the transporters involved in drug uptake, which have been identified as mechanisms of chemoresistance 1a (MOC-1a)[144]. OATP1B1 is considered an OAT belonging to the MOC-1a group that plays an important role in the uptake of anionic antitumor drugs in the liver, such as irinotecan[145], paclitaxel[146], and cytostatic cisplatin-conjugated bile acid derivatives[147]. Due to the abundant expression of OATs in liver cancer and their high activity in transporting many anticancer drugs, they can be considered important therapeutic targets in the design of anticancer drugs. In the liver tissues of patients with nonalcoholic fatty liver, HCC, inflammatory cholestasis, PBC, or chronic hepatitis, the expression of OATP1B is usually reduced[148]. Inhibition of OATP1B function may also result in elevated levels of bilirubin and affect liver function. However, the combined use of substrates of OATP1B used as drugs may result in unexpected toxicity and fatal consequences[149,150]. These findings suggest that OATPs can be important targets for anticancer therapy in three ways: (1) OATP-mediated hormone expression, formation of hormone conjugates, or uptake of growth-promoting chemicals can be prevented with OATP inhibitors; (2) New anticancer drugs can be designed as substrates of OATP to increase their uptake by OATP-expressing cancer cells; and (3) Allosteric stimulators can enhance the uptake of anticancer drugs. Since OATs may determine the extent of distribution of some drugs to target sites and nontarget sites, the transport capacity of liver transporters can be used to enhance the distribution of drugs to target sites and facilitate the intracellular accumulation of various compounds in the liver. OATs might be important with respect to the discovery of novel cancer agents.
As our understanding of organic anions increases, the mechanisms of OAT and OATP regulation in the liver will be further elucidated. How to effectively target transporters to improve the distribution of drugs in the liver and reduce the adverse reactions of the delivered drugs are urgent problems that need to be resolved. It has been confirmed that many drugs are substrates of OATs and OATPs, and hepatic-specific expression of these transporters can mediate the uptake of specific substrates. Then, these drugs are discharged into bile and finally enter the intestinal tract, facilitating the targeted therapy with clinical drugs, improving their efficacy, and reducing their adverse reactions. Although the application of transporters in clinical practice is not imminent, these studies may help establish the target therapy of cancer or increase drug bioavailability. It is believed that with further research on these transporters and liver cancer and other liver diseases, targeted therapy with OATs and OATPs is expected to be an ideal and effective new way to treat and improve the survival rate of patients with liver disease.
Manuscript source: Invited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chen GX, Huang HC, Ocker M S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Xing YX
| 1. | Nigam SK. The SLC22 Transporter Family: A Paradigm for the Impact of Drug Transporters on Metabolic Pathways, Signaling, and Disease. Annu Rev Pharmacol Toxicol. 2018;58:663-687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 137] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 2. | Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/ SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 2004;447:653-665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 741] [Cited by in F6Publishing: 706] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 3. | Shitara Y, Horie T, Sugiyama Y. Transporters as a determinant of drug clearance and tissue distribution. Eur J Pharm Sci. 2006;27:425-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 397] [Cited by in F6Publishing: 311] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 4. | Roth M, Obaidat A, Hagenbuch B. OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol. 2012;165:1260-1287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 502] [Cited by in F6Publishing: 532] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 5. | Meier PJ. Molecular mechanisms of hepatic bile salt transport from sinusoidal blood into bile. Am J Physiol. 1995;269:G801-G812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Bush KT, Wu W, Lun C, Nigam SK. The drug transporter OAT3 (SLC22A8) and endogenous metabolite communication via the gut-liver-kidney axis. J Biol Chem. 2017;292:15789-15803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 7. | Koepsell H, Endou H. The SLC22 drug transporter family. Pflugers Arch. 2004;447:666-676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 392] [Cited by in F6Publishing: 366] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 8. | Zhu C, Nigam KB, Date RC, Bush KT, Springer SA, Saier MH, Wu W, Nigam SK. Evolutionary Analysis and Classification of OATs, OCTs, OCTNs, and Other SLC22 Transporters: Structure-Function Implications and Analysis of Sequence Motifs. PLoS One. 2015;10:e0140569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Kaler G, Truong DM, Khandelwal A, Nagle M, Eraly SA, Swaan PW, Nigam SK. Structural variation governs substrate specificity for organic anion transporter (OAT) homologs. Potential remote sensing by OAT family members. J Biol Chem. 2007;282:23841-23853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Michalski C, Cui Y, Nies AT, Nuessler AK, Neuhaus P, Zanger UM, Klein K, Eichelbaum M, Keppler D, Konig J. A naturally occurring mutation in the SLC21A6 gene causing impaired membrane localization of the hepatocyte uptake transporter. J Biol Chem. 2002;277:43058-43063. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 101] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Koepsell H. The SLC22 family with transporters of organic cations, anions and zwitterions. Mol Aspects Med. 2013;34:413-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 275] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 12. | Simonson GD, Vincent AC, Roberg KJ, Huang Y, Iwanij V. Molecular cloning and characterization of a novel liver-specific transport protein. J Cell Sci. 1994;107:1065-1072. [PubMed] [Cited in This Article: ] |
| 13. | VanWert AL, Gionfriddo MR, Sweet DH. Organic anion transporters: discovery, pharmacology, regulation and roles in pathophysiology. Biopharm Drug Dispos. 2010;31:1-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 14. | Burckhardt G, Burckhardt BC. In vitro and in vivo evidence of the importance of organic anion transporters (OATs) in drug therapy. Handb Exp Pharmacol. 2011;29-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 15. | Hilgendorf C, Ahlin G, Seithel A, Artursson P, Ungell AL, Karlsson J. Expression of thirty-six drug transporter genes in human intestine, liver, kidney, and organotypic cell lines. Drug Metab Dispos. 2007;35:1333-1340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 431] [Cited by in F6Publishing: 423] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 16. | Ohtsuki S, Schaefer O, Kawakami H, Inoue T, Liehner S, Saito A, Ishiguro N, Kishimoto W, Ludwig-Schwellinger E, Ebner T, Terasaki T. Simultaneous absolute protein quantification of transporters, cytochromes P450, and UDP-glucuronosyltransferases as a novel approach for the characterization of individual human liver: comparison with mRNA levels and activities. Drug Metab Dispos. 2012;40:83-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 337] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 17. | Vildhede A, Wiśniewski JR, Norén A, Karlgren M, Artursson P. Comparative Proteomic Analysis of Human Liver Tissue and Isolated Hepatocytes with a Focus on Proteins Determining Drug Exposure. J Proteome Res. 2015;14:3305-3314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 18. | Nakamura K, Hirayama-Kurogi M, Ito S, Kuno T, Yoneyama T, Obuchi W, Terasaki T, Ohtsuki S. Large-scale multiplex absolute protein quantification of drug-metabolizing enzymes and transporters in human intestine, liver, and kidney microsomes by SWATH-MS: Comparison with MRM/SRM and HR-MRM/PRM. Proteomics. 2016;16:2106-2117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 19. | Pavlova A, Sakurai H, Leclercq B, Beier DR, Yu AS, Nigam SK. Developmentally regulated expression of organic ion transporters NKT (OAT1), OCT1, NLT (OAT2), and Roct. Am J Physiol Renal Physiol. 2000;278:F635-F643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 93] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Eraly SA, Monte JC, Nigam SK. Novel slc22 transporter homologs in fly, worm, and human clarify the phylogeny of organic anion and cation transporters. Physiol Genomics. 2004;18:12-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Lozano E, Briz O, Macias RIR, Serrano MA, Marin JJG, Herraez E. Genetic Heterogeneity of SLC22 Family of Transporters in Drug Disposition. J Pers Med. 2018;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Stieger B, Hagenbuch B. Organic anion-transporting polypeptides. Curr Top Membr. 2014;73:205-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 23. | Hänggi E, Grundschober AF, Leuthold S, Meier PJ, St-Pierre MV. Functional analysis of the extracellular cysteine residues in the human organic anion transporting polypeptide, OATP2B1. Mol Pharmacol. 2006;70:806-817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Liu T, Li Q. Organic anion-transporting polypeptides: a novel approach for cancer therapy. J Drug Target. 2014;22:14-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | König J, Seithel A, Gradhand U, Fromm MF. Pharmacogenomics of human OATP transporters. Naunyn Schmiedebergs Arch Pharmacol. 2006;372:432-443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 266] [Cited by in F6Publishing: 253] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 26. | Lee W, Glaeser H, Smith LH, Roberts RL, Moeckel GW, Gervasini G, Leake BF, Kim RB. Polymorphisms in human organic anion-transporting polypeptide 1A2 (OATP1A2): implications for altered drug disposition and central nervous system drug entry. J Biol Chem. 2005;280:9610-9617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 258] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 27. | Schulte RR, Ho RH. Organic Anion Transporting Polypeptides: Emerging Roles in Cancer Pharmacology. Mol Pharmacol. 2019;95:490-506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 28. | Nomura T, Lu R, Pucci ML, Schuster VL. The two-step model of prostaglandin signal termination: in vitro reconstitution with the prostaglandin transporter and prostaglandin 15 dehydrogenase. Mol Pharmacol. 2004;65:973-978. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 111] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 29. | Schuster VL. Prostaglandin transport. Prostaglandins Other Lipid Mediat. 2002;68-69:633-647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 152] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 30. | Mandery K, Bujok K, Schmidt I, Wex T, Treiber G, Malfertheiner P, Rau TT, Amann KU, Brune K, Fromm MF, Glaeser H. Influence of cyclooxygenase inhibitors on the function of the prostaglandin transporter organic anion-transporting polypeptide 2A1 expressed in human gastroduodenal mucosa. J Pharmacol Exp Ther. 2010;332:345-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Huber RD, Gao B, Sidler Pfändler MA, Zhang-Fu W, Leuthold S, Hagenbuch B, Folkers G, Meier PJ, Stieger B. Characterization of two splice variants of human organic anion transporting polypeptide 3A1 isolated from human brain. Am J Physiol Cell Physiol. 2007;292:C795-C806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 32. | Fujiwara K, Adachi H, Nishio T, Unno M, Tokui T, Okabe M, Onogawa T, Suzuki T, Asano N, Tanemoto M, Seki M, Shiiba K, Suzuki M, Kondo Y, Nunoki K, Shimosegawa T, Iinuma K, Ito S, Matsuno S, Abe T. Identification of thyroid hormone transporters in humans: different molecules are involved in a tissue-specific manner. Endocrinology. 2001;142:2005-2012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 116] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Kleberg K, Jensen GM, Christensen DP, Lundh M, Grunnet LG, Knuhtsen S, Poulsen SS, Hansen MB, Bindslev N. Transporter function and cyclic AMP turnover in normal colonic mucosa from patients with and without colorectal neoplasia. BMC Gastroenterol. 2012;12:78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Sebastian K, Detro-Dassen S, Rinis N, Fahrenkamp D, Müller-Newen G, Merk HF, Schmalzing G, Zwadlo-Klarwasser G, Baron JM. Characterization of SLCO5A1/OATP5A1, a solute carrier transport protein with non-classical function. PLoS One. 2013;8:e83257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Svoboda M, Riha J, Wlcek K, Jaeger W, Thalhammer T. Organic anion transporting polypeptides (OATPs): regulation of expression and function. Curr Drug Metab. 2011;12:139-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 36. | König J, Cui Y, Nies AT, Keppler D. Localization and genomic organization of a new hepatocellular organic anion transporting polypeptide. J Biol Chem. 2000;275:23161-23168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 402] [Cited by in F6Publishing: 372] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 37. | Kullak-Ublick GA, Ismair MG, Stieger B, Landmann L, Huber R, Pizzagalli F, Fattinger K, Meier PJ, Hagenbuch B. Organic anion-transporting polypeptide B (OATP-B) and its functional comparison with three other OATPs of human liver. Gastroenterology. 2001;120:525-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 556] [Cited by in F6Publishing: 572] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 38. | Cihlar T, Lin DC, Pritchard JB, Fuller MD, Mendel DB, Sweet DH. The antiviral nucleotide analogs cidofovir and adefovir are novel substrates for human and rat renal organic anion transporter 1. Mol Pharmacol. 1999;56:570-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 273] [Cited by in F6Publishing: 278] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 39. | Hosoyamada M, Sekine T, Kanai Y, Endou H. Molecular cloning and functional expression of a multispecific organic anion transporter from human kidney. Am J Physiol. 1999;276:F122-F128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 104] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 40. | Lu R, Chan BS, Schuster VL. Cloning of the human kidney PAH transporter: narrow substrate specificity and regulation by protein kinase C. Am J Physiol. 1999;276:F295-F303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Sekine T, Cha SH, Tsuda M, Apiwattanakul N, Nakajima N, Kanai Y, Endou H. Identification of multispecific organic anion transporter 2 expressed predominantly in the liver. FEBS Lett. 1998;429:179-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 234] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 42. | Cropp CD, Komori T, Shima JE, Urban TJ, Yee SW, More SS, Giacomini KM. Organic anion transporter 2 (SLC22A7) is a facilitative transporter of cGMP. Mol Pharmacol. 2008;73:1151-1158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 43. | Enomoto A, Takeda M, Shimoda M, Narikawa S, Kobayashi Y, Kobayashi Y, Yamamoto T, Sekine T, Cha SH, Niwa T, Endou H. Interaction of human organic anion transporters 2 and 4 with organic anion transport inhibitors. J Pharmacol Exp Ther. 2002;301:797-802. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 152] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 44. | Hotchkiss AG, Gao T, Khan U, Berrigan L, Li M, Ingraham L, Pelis RM. Organic Anion Transporter 1 Is Inhibited by Multiple Mechanisms and Shows a Transport Mode Independent of Exchange. Drug Metab Dispos. 2015;43:1847-1854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Shen H, Lai Y, Rodrigues AD. Organic Anion Transporter 2: An Enigmatic Human Solute Carrier. Drug Metab Dispos. 2017;45:228-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 46. | Sun W, Wu RR, van Poelje PD, Erion MD. Isolation of a family of organic anion transporters from human liver and kidney. Biochem Biophys Res Commun. 2001;283:417-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 161] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 47. | Marada VV, Flörl S, Kühne A, Müller J, Burckhardt G, Hagos Y. Interaction of human organic anion transporter 2 (OAT2) and sodium taurocholate cotransporting polypeptide (NTCP) with antineoplastic drugs. Pharmacol Res. 2015;91:78-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Babu E, Takeda M, Narikawa S, Kobayashi Y, Yamamoto T, Cha SH, Sekine T, Sakthisekaran D, Endou H. Human organic anion transporters mediate the transport of tetracycline. Jpn J Pharmacol. 2002;88:69-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 49. | Yee SW, Nguyen AN, Brown C, Savic RM, Zhang Y, Castro RA, Cropp CD, Choi JH, Singh D, Tahara H, Stocker SL, Huang Y, Brett CM, Giacomini KM. Reduced renal clearance of cefotaxime in asians with a low-frequency polymorphism of OAT3 (SLC22A8). J Pharm Sci. 2013;102:3451-3457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 50. | Kobayashi Y, Ohshiro N, Sakai R, Ohbayashi M, Kohyama N, Yamamoto T. Transport mechanism and substrate specificity of human organic anion transporter 2 (hOat2 [SLC22A7]). J Pharm Pharmacol. 2005;57:573-578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 51. | Tahara H, Kusuhara H, Endou H, Koepsell H, Imaoka T, Fuse E, Sugiyama Y. A species difference in the transport activities of H2 receptor antagonists by rat and human renal organic anion and cation transporters. J Pharmacol Exp Ther. 2005;315:337-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 144] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 52. | Zhang Y, Han YH, Putluru SP, Matta MK, Kole P, Mandlekar S, Furlong MT, Liu T, Iyer RA, Marathe P, Yang Z, Lai Y, Rodrigues AD. Diclofenac and Its Acyl Glucuronide: Determination of In Vivo Exposure in Human Subjects and Characterization as Human Drug Transporter Substrates In Vitro. Drug Metab Dispos. 2016;44:320-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 53. | Cheng Y, Vapurcuyan A, Shahidullah M, Aleksunes LM, Pelis RM. Expression of organic anion transporter 2 in the human kidney and its potential role in the tubular secretion of guanine-containing antiviral drugs. Drug Metab Dispos. 2012;40:617-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 54. | Soodvilai S, Chatsudthipong V, Evans KK, Wright SH, Dantzler WH. Acute regulation of OAT3-mediated estrone sulfate transport in isolated rabbit renal proximal tubules. Am J Physiol Renal Physiol. 2004;287:F1021-F1029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 55. | Nagle MA, Truong DM, Dnyanmote AV, Ahn SY, Eraly SA, Wu W, Nigam SK. Analysis of three-dimensional systems for developing and mature kidneys clarifies the role of OAT1 and OAT3 in antiviral handling. J Biol Chem. 2011;286:243-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 56. | Hasannejad H, Takeda M, Taki K, Shin HJ, Babu E, Jutabha P, Khamdang S, Aleboyeh M, Onozato ML, Tojo A, Enomoto A, Anzai N, Narikawa S, Huang XL, Niwa T, Endou H. Interactions of human organic anion transporters with diuretics. J Pharmacol Exp Ther. 2004;308:1021-1029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 143] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 57. | Maeda K, Tian Y, Fujita T, Ikeda Y, Kumagai Y, Kondo T, Tanabe K, Nakayama H, Horita S, Kusuhara H, Sugiyama Y. Inhibitory effects of p-aminohippurate and probenecid on the renal clearance of adefovir and benzylpenicillin as probe drugs for organic anion transporter (OAT) 1 and OAT3 in humans. Eur J Pharm Sci. 2014;59:94-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 58. | Takeda M, Khamdang S, Narikawa S, Kimura H, Hosoyamada M, Cha SH, Sekine T, Endou H. Characterization of methotrexate transport and its drug interactions with human organic anion transporters. J Pharmacol Exp Ther. 2002;302:666-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 203] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 59. | Hagos Y, Stein D, Ugele B, Burckhardt G, Bahn A. Human renal organic anion transporter 4 operates as an asymmetric urate transporter. J Am Soc Nephrol. 2007;18:430-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 181] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 60. | Tomi M, Eguchi H, Ozaki M, Tawara T, Nishimura S, Higuchi K, Maruyama T, Nishimura T, Nakashima E. Role of OAT4 in Uptake of Estriol Precursor 16α-Hydroxydehydroepiandrosterone Sulfate Into Human Placental Syncytiotrophoblasts From Fetus. Endocrinology. 2015;156:2704-2712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 61. | Noguchi S, Nishimura T, Fujibayashi A, Maruyama T, Tomi M, Nakashima E. Organic Anion Transporter 4-Mediated Transport of Olmesartan at Basal Plasma Membrane of Human Placental Barrier. J Pharm Sci. 2015;104:3128-3135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 62. | Youngblood GL, Sweet DH. Identification and functional assessment of the novel murine organic anion transporter Oat5 (Slc22a19) expressed in kidney. Am J Physiol Renal Physiol. 2004;287:F236-F244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 63. | Wu W, Bush KT, Liu HC, Zhu C, Abagyan R, Nigam SK. Shared Ligands Between Organic Anion Transporters (OAT1 and OAT6) and Odorant Receptors. Drug Metab Dispos. 2015;43:1855-1863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 64. | Shin HJ, Anzai N, Enomoto A, He X, Kim DK, Endou H, Kanai Y. Novel liver-specific organic anion transporter OAT7 that operates the exchange of sulfate conjugates for short chain fatty acid butyrate. Hepatology. 2007;45:1046-1055. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 65. | Burckhardt G. Drug transport by Organic Anion Transporters (OATs). Pharmacol Ther. 2012;136:106-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 243] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 66. | Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, Hosoyamada M, Takeda M, Sekine T, Igarashi T, Matsuo H, Kikuchi Y, Oda T, Ichida K, Hosoya T, Shimokata K, Niwa T, Kanai Y, Endou H. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002;417:447-452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1027] [Cited by in F6Publishing: 994] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 67. | Hosoyamada M, Ichida K, Enomoto A, Hosoya T, Endou H. Function and localization of urate transporter 1 in mouse kidney. J Am Soc Nephrol. 2004;15:261-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 68. | Bossuyt X, Müller M, Meier PJ. Multispecific amphipathic substrate transport by an organic anion transporter of human liver. J Hepatol. 1996;25:733-738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 101] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 69. | Shitara Y, Maeda K, Ikejiri K, Yoshida K, Horie T, Sugiyama Y. Clinical significance of organic anion transporting polypeptides (OATPs) in drug disposition: their roles in hepatic clearance and intestinal absorption. Biopharm Drug Dispos. 2013;34:45-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 305] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 70. | Kobayashi D, Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. Involvement of human organic anion transporting polypeptide OATP-B (SLC21A9) in pH-dependent transport across intestinal apical membrane. J Pharmacol Exp Ther. 2003;306:703-708. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 337] [Cited by in F6Publishing: 338] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 71. | Varma MV, Rotter CJ, Chupka J, Whalen KM, Duignan DB, Feng B, Litchfield J, Goosen TC, El-Kattan AF. pH-sensitive interaction of HMG-CoA reductase inhibitors (statins) with organic anion transporting polypeptide 2B1. Mol Pharm. 2011;8:1303-1313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 72. | Sai Y, Kaneko Y, Ito S, Mitsuoka K, Kato Y, Tamai I, Artursson P, Tsuji A. Predominant contribution of organic anion transporting polypeptide OATP-B (OATP2B1) to apical uptake of estrone-3-sulfate by human intestinal Caco-2 cells. Drug Metab Dispos. 2006;34:1423-1431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 139] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 73. | Mikkaichi T, Suzuki T, Tanemoto M, Ito S, Abe T. The organic anion transporter (OATP) family. Drug Metab Pharmacokinet. 2004;19:171-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 154] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 74. | Hagenbuch B, Meier PJ. The superfamily of organic anion transporting polypeptides. Biochim Biophys Acta. 2003;1609:1-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 652] [Cited by in F6Publishing: 587] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 75. | Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838-851. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1446] [Cited by in F6Publishing: 1428] [Article Influence: 89.3] [Reference Citation Analysis (0)] |
| 76. | Dincer D, Besisk F, Demirkol O, Demir K, Kaymakoglu S, Cakaloglu Y, Okten A. Relationships between hemodynamic alterations and Child-Pugh Score in patients with cirrhosis. Hepatogastroenterology. 2005;52:1521-1525. [PubMed] [Cited in This Article: ] |
| 77. | Wang C, Fan R, Sun J, Hou J. Prevention and management of drug resistant hepatitis B virus infections. J Gastroenterol Hepatol. 2012;27:1432-1440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 78. | Xu Q, Wang C, Meng Q, Liu Q, Sun H, Peng J, Ma X, Kaku T, Liu K. OAT1 and OAT3: targets of drug-drug interaction between entecavir and JBP485. Eur J Pharm Sci. 2013;48:650-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 79. | Ricaurte GA, Langston JW, Delanney LE, Irwin I, Peroutka SJ, Forno LS. Fate of nigrostriatal neurons in young mature mice given 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine: a neurochemical and morphological reassessment. Brain Res. 1986;376:117-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 159] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 80. | Nakamura T, Nishizawa T, Hagiya M, Seki T, Shimonishi M, Sugimura A, Tashiro K, Shimizu S. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989;342:440-443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1628] [Cited by in F6Publishing: 1606] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 81. | Rubin JS, Bottaro DP, Aaronson SA. Hepatocyte growth factor/scatter factor and its receptor, the c-met proto-oncogene product. Biochim Biophys Acta. 1993;1155:357-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 96] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 82. | Tsubouchi H, Niitani Y, Hirono S, Nakayama H, Gohda E, Arakaki N, Sakiyama O, Takahashi K, Kimoto M, Kawakami S. Levels of the human hepatocyte growth factor in serum of patients with various liver diseases determined by an enzyme-linked immunosorbent assay. Hepatology. 1991;13:1-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 272] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 83. | Le Vee M, Lecureur V, Moreau A, Stieger B, Fardel O. Differential regulation of drug transporter expression by hepatocyte growth factor in primary human hepatocytes. Drug Metab Dispos. 2009;37:2228-2235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 84. | Fisher CD, Lickteig AJ, Augustine LM, Oude Elferink RP, Besselsen DG, Erickson RP, Cherrington NJ. Experimental non-alcoholic fatty liver disease results in decreased hepatic uptake transporter expression and function in rats. Eur J Pharmacol. 2009;613:119-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 85. | Yasui Y, Kudo A, Kurosaki M, Matsuda S, Muraoka M, Tamaki N, Suzuki S, Hosokawa T, Ueda K, Matsunaga K, Nakanishi H, Tsuchiya K, Itakura J, Takahashi Y, Tanaka S, Asahina Y, Enomoto N, Arii S, Izumi N. Reduced organic anion transporter expression is a risk factor for hepatocellular carcinoma in chronic hepatitis C patients: a propensity score matching study. Oncology. 2014;86:53-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 86. | Fork C, Bauer T, Golz S, Geerts A, Weiland J, Del Turco D, Schömig E, Gründemann D. OAT2 catalyses efflux of glutamate and uptake of orotic acid. Biochem J. 2011;436:305-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 87. | Laurier C, Tatematsu M, Rao PM, Rajalakshmi S, Sarma DS. Promotion by orotic acid of liver carcinogenesis in rats initiated by 1,2-dimethylhydrazine. Cancer Res. 1984;44:2186-2191. [PubMed] [Cited in This Article: ] |
| 88. | Jeffers LJ, Dubow RA, Zieve L, Reddy KR, Livingstone AS, Neimark S, Viamonte M, Schiff ER. Hepatic encephalopathy and orotic aciduria associated with hepatocellular carcinoma in a noncirrhotic liver. Hepatology. 1988;8:78-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 89. | Hao S, Fan P, Chen S, Tu C, Wan C. Distinct Recurrence Risk Factors for Intrahepatic Metastasis and Multicenter Occurrence After Surgery in Patients with Hepatocellular Carcinoma. J Gastrointest Surg. 2017;21:312-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 90. | Utsunomiya T, Shimada M, Imura S, Morine Y, Ikemoto T, Mori M. Molecular signatures of noncancerous liver tissue can predict the risk for late recurrence of hepatocellular carcinoma. J Gastroenterol. 2010;45:146-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 91. | Kudo A, Mogushi K, Takayama T, Matsumura S, Ban D, Irie T, Ochiai T, Nakamura N, Tanaka H, Anzai N, Sakamoto M, Tanaka S, Arii S. Mitochondrial metabolism in the noncancerous liver determine the occurrence of hepatocellular carcinoma: a prospective study. J Gastroenterol. 2014;49:502-510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 92. | Wang L, Collins C, Kelly EJ, Chu X, Ray AS, Salphati L, Xiao G, Lee C, Lai Y, Liao M, Mathias A, Evers R, Humphreys W, Hop CE, Kumer SC, Unadkat JD. Transporter Expression in Liver Tissue from Subjects with Alcoholic or Hepatitis C Cirrhosis Quantified by Targeted Quantitative Proteomics. Drug Metab Dispos. 2016;44:1752-1758. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 93. | Zollner G, Fickert P, Silbert D, Fuchsbichler A, Marschall HU, Zatloukal K, Denk H, Trauner M. Adaptive changes in hepatobiliary transporter expression in primary biliary cirrhosis. J Hepatol. 2003;38:717-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 216] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 94. | More VR, Cheng Q, Donepudi AC, Buckley DB, Lu ZJ, Cherrington NJ, Slitt AL. Alcohol cirrhosis alters nuclear receptor and drug transporter expression in human liver. Drug Metab Dispos. 2013;41:1148-1155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 95. | Ogasawara K, Terada T, Katsura T, Hatano E, Ikai I, Yamaoka Y, Inui K. Hepatitis C virus-related cirrhosis is a major determinant of the expression levels of hepatic drug transporters. Drug Metab Pharmacokinet. 2010;25:190-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 96. | Niemi M, Backman JT, Kajosaari LI, Leathart JB, Neuvonen M, Daly AK, Eichelbaum M, Kivistö KT, Neuvonen PJ. Polymorphic organic anion transporting polypeptide 1B1 is a major determinant of repaglinide pharmacokinetics. Clin Pharmacol Ther. 2005;77:468-478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 290] [Cited by in F6Publishing: 240] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 97. | Hatorp V, Walther KH, Christensen MS, Haug-Pihale G. Single-dose pharmacokinetics of repaglinide in subjects with chronic liver disease. J Clin Pharmacol. 2000;40:142-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 98. | Johnson TN, Boussery K, Rowland-Yeo K, Tucker GT, Rostami-Hodjegan A. A semi-mechanistic model to predict the effects of liver cirrhosis on drug clearance. Clin Pharmacokinet. 2010;49:189-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 99. | Huppertz A, Balzer T, Blakeborough A, Breuer J, Giovagnoni A, Heinz-Peer G, Laniado M, Manfredi RM, Mathieu DG, Mueller D, Reimer P, Robinson PJ, Strotzer M, Taupitz M, Vogl TJ; European EOB Study Group. Improved detection of focal liver lesions at MR imaging: multicenter comparison of gadoxetic acid-enhanced MR images with intraoperative findings. Radiology. 2004;230:266-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 337] [Cited by in F6Publishing: 319] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 100. | Kitao A, Matsui O, Yoneda N, Kozaka K, Shinmura R, Koda W, Kobayashi S, Gabata T, Zen Y, Yamashita T, Kaneko S, Nakanuma Y. The uptake transporter OATP8 expression decreases during multistep hepatocarcinogenesis: correlation with gadoxetic acid enhanced MR imaging. Eur Radiol. 2011;21:2056-2066. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 192] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 101. | Kitao A, Zen Y, Matsui O, Gabata T, Kobayashi S, Koda W, Kozaka K, Yoneda N, Yamashita T, Kaneko S, Nakanuma Y. Hepatocellular carcinoma: signal intensity at gadoxetic acid-enhanced MR Imaging--correlation with molecular transporters and histopathologic features. Radiology. 2010;256:817-826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 284] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 102. | Yamashita T, Kitao A, Matsui O, Hayashi T, Nio K, Kondo M, Ohno N, Miyati T, Okada H, Yamashita T, Mizukoshi E, Honda M, Nakanuma Y, Takamura H, Ohta T, Nakamoto Y, Yamamoto M, Takayama T, Arii S, Wang X, Kaneko S. Gd-EOB-DTPA-enhanced magnetic resonance imaging and alpha-fetoprotein predict prognosis of early-stage hepatocellular carcinoma. Hepatology. 2014;60:1674-1685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 103. | Ueno A, Masugi Y, Yamazaki K, Komuta M, Effendi K, Tanami Y, Tsujikawa H, Tanimoto A, Okuda S, Itano O, Kitagawa Y, Kuribayashi S, Sakamoto M. OATP1B3 expression is strongly associated with Wnt/β-catenin signalling and represents the transporter of gadoxetic acid in hepatocellular carcinoma. J Hepatol. 2014;61:1080-1087. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 104. | Yoneda N, Matsui O, Ikeno H, Inoue D, Yoshida K, Kitao A, Kozaka K, Kobayashi S, Gabata T, Ikeda H, Nakamura K, Ohta T. Correlation between Gd-EOB-DTPA-enhanced MR imaging findings and OATP1B3 expression in chemotherapy-associated sinusoidal obstruction syndrome. Abdom Imaging. 2015;40:3099-3103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 105. | Doi N, Tomiyama Y, Kawase T, Nishina S, Yoshioka N, Hara Y, Yoshida K, Korenaga K, Korenaga M, Moriya T, Urakami A, Nakashima O, Kojiro M, Hino K. Focal nodular hyperplasia-like nodule with reduced expression of organic anion transporter 1B3 in alcoholic liver cirrhosis. Intern Med. 2011;50:1193-1199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 106. | Alam K, Farasyn T, Ding K, Yue W. Characterization of Liver- and Cancer-type-Organic Anion Transporting Polypeptide (OATP) 1B3 Messenger RNA Expression in Normal and Cancerous Human Tissues. Drug Metab Lett. 2018;12:24-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 107. | Tweedie D, Polli JW, Berglund EG, Huang SM, Zhang L, Poirier A, Chu X, Feng B; International Transporter Consortium. Transporter studies in drug development: experience to date and follow-up on decision trees from the International Transporter Consortium. Clin Pharmacol Ther. 2013;94:113-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 108. | Lee HH, Leake BF, Teft W, Tirona RG, Kim RB, Ho RH. Contribution of hepatic organic anion-transporting polypeptides to docetaxel uptake and clearance. Mol Cancer Ther. 2015;14:994-1003. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 109. | Yamaguchi H, Kobayashi M, Okada M, Takeuchi T, Unno M, Abe T, Goto J, Hishinuma T, Mano N. Rapid screening of antineoplastic candidates for the human organic anion transporter OATP1B3 substrates using fluorescent probes. Cancer Lett. 2008;260:163-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 110. | Lancaster CS, Sprowl JA, Walker AL, Hu S, Gibson AA, Sparreboom A. Modulation of OATP1B-type transporter function alters cellular uptake and disposition of platinum chemotherapeutics. Mol Cancer Ther. 2013;12:1537-1544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 111. | Zimmerman EI, Hu S, Roberts JL, Gibson AA, Orwick SJ, Li L, Sparreboom A, Baker SD. Contribution of OATP1B1 and OATP1B3 to the disposition of sorafenib and sorafenib-glucuronide. Clin Cancer Res. 2013;19:1458-1466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 112. | Vasuri F, Golfieri R, Fiorentino M, Capizzi E, Renzulli M, Pinna AD, Grigioni WF, D'Errico-Grigioni A. OATP 1B1/1B3 expression in hepatocellular carcinomas treated with orthotopic liver transplantation. Virchows Arch. 2011;459:141-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 113. | Cui Y, König J, Nies AT, Pfannschmidt M, Hergt M, Franke WW, Alt W, Moll R, Keppler D. Detection of the human organic anion transporters SLC21A6 (OATP2) and SLC21A8 (OATP8) in liver and hepatocellular carcinoma. Lab Invest. 2003;83:527-538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 114. | Durnez A, Verslype C, Nevens F, Fevery J, Aerts R, Pirenne J, Lesaffre E, Libbrecht L, Desmet V, Roskams T. The clinicopathological and prognostic relevance of cytokeratin 7 and 19 expression in hepatocellular carcinoma. A possible progenitor cell origin. Histopathology. 2006;49:138-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 289] [Cited by in F6Publishing: 317] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 115. | Klein K, Jüngst C, Mwinyi J, Stieger B, Krempler F, Patsch W, Eloranta JJ, Kullak-Ublick GA. The human organic anion transporter genes OAT5 and OAT7 are transactivated by hepatocyte nuclear factor-1α (HNF-1α). Mol Pharmacol. 2010;78:1079-1087. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 116. | Kikuchi R, Kusuhara H, Hattori N, Shiota K, Kim I, Gonzalez FJ, Sugiyama Y. Regulation of the expression of human organic anion transporter 3 by hepatocyte nuclear factor 1alpha/beta and DNA methylation. Mol Pharmacol. 2006;70:887-896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 117. | Kikuchi R, Kusuhara H, Hattori N, Kim I, Shiota K, Gonzalez FJ, Sugiyama Y. Regulation of tissue-specific expression of the human and mouse urate transporter 1 gene by hepatocyte nuclear factor 1 alpha/beta and DNA methylation. Mol Pharmacol. 2007;72:1619-1625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 118. | Ogasawara K, Terada T, Asaka J, Katsura T, Inui K. Hepatocyte nuclear factor-4{alpha} regulates the human organic anion transporter 1 gene in the kidney. Am J Physiol Renal Physiol. 2007;292:F1819-F1826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 119. | Popowski K, Eloranta JJ, Saborowski M, Fried M, Meier PJ, Kullak-Ublick GA. The human organic anion transporter 2 gene is transactivated by hepatocyte nuclear factor-4 alpha and suppressed by bile acids. Mol Pharmacol. 2005;67:1629-1638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 120. | Sladek FM, Zhong WM, Lai E, Darnell JE. Liver-enriched transcription factor HNF-4 is a novel member of the steroid hormone receptor superfamily. Genes Dev. 1990;4:2353-2365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 761] [Cited by in F6Publishing: 843] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 121. | Cereghini S. Liver-enriched transcription factors and hepatocyte differentiation. FASEB J. 1996;10:267-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 385] [Cited by in F6Publishing: 382] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 122. | Hayhurst GP, Lee YH, Lambert G, Ward JM, Gonzalez FJ. Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol Cell Biol. 2001;21:1393-1403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 842] [Cited by in F6Publishing: 842] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 123. | Tian JM, Schibler U. Tissue-specific expression of the gene encoding hepatocyte nuclear factor 1 may involve hepatocyte nuclear factor 4. Genes Dev. 1991;5:2225-2234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 151] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 124. | Meier PJ, Stieger B. Bile salt transporters. Annu Rev Physiol. 2002;64:635-661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 434] [Cited by in F6Publishing: 392] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 125. | Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, Lehmann JM. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365-1368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1717] [Cited by in F6Publishing: 1670] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 126. | Ohtsuka H, Abe T, Onogawa T, Kondo N, Sato T, Oshio H, Mizutamari H, Mikkaichi T, Oikawa M, Rikiyama T, Katayose Y, Unno M. Farnesoid X receptor, hepatocyte nuclear factors 1alpha and 3beta are essential for transcriptional activation of the liver-specific organic anion transporter-2 gene. J Gastroenterol. 2006;41:369-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 127. | Seol W, Choi HS, Moore DD. An orphan nuclear hormone receptor that lacks a DNA binding domain and heterodimerizes with other receptors. Science. 1996;272:1336-1339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 419] [Cited by in F6Publishing: 404] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 128. | Lee YK, Dell H, Dowhan DH, Hadzopoulou-Cladaras M, Moore DD. The orphan nuclear receptor SHP inhibits hepatocyte nuclear factor 4 and retinoid X receptor transactivation: two mechanisms for repression. Mol Cell Biol. 2000;20:187-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 235] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 129. | Eloranta JJ, Kullak-Ublick GA. Coordinate transcriptional regulation of bile acid homeostasis and drug metabolism. Arch Biochem Biophys. 2005;433:397-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 193] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 130. | Claudel T, Inoue Y, Barbier O, Duran-Sandoval D, Kosykh V, Fruchart J, Fruchart JC, Gonzalez FJ, Staels B. Farnesoid X receptor agonists suppress hepatic apolipoprotein CIII expression. Gastroenterology. 2003;125:544-555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 193] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 131. | Claudel T, Sturm E, Duez H, Torra IP, Sirvent A, Kosykh V, Fruchart JC, Dallongeville J, Hum DW, Kuipers F, Staels B. Bile acid-activated nuclear receptor FXR suppresses apolipoprotein A-I transcription via a negative FXR response element. J Clin Invest. 2002;109:961-971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 132. | Jung D, Hagenbuch B, Gresh L, Pontoglio M, Meier PJ, Kullak-Ublick GA. Characterization of the human OATP-C (SLC21A6) gene promoter and regulation of liver-specific OATP genes by hepatocyte nuclear factor 1 alpha. J Biol Chem. 2001;276:37206-37214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 132] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 133. | He YJ, Zhang W, Tu JH, Kirchheiner J, Chen Y, Guo D, Li Q, Li ZY, Chen H, Hu DL, Wang D, Zhou HH. Hepatic nuclear factor 1alpha inhibitor ursodeoxycholic acid influences pharmacokinetics of the organic anion transporting polypeptide 1B1 substrate rosuvastatin and bilirubin. Drug Metab Dispos. 2008;36:1453-1456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 134. | Colletti M, Cicchini C, Conigliaro A, Santangelo L, Alonzi T, Pasquini E, Tripodi M, Amicone L. Convergence of Wnt signaling on the HNF4alpha-driven transcription in controlling liver zonation. Gastroenterology. 2009;137:660-672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 135. | Kitao A, Matsui O, Yoneda N, Kozaka K, Kobayashi S, Koda W, Minami T, Inoue D, Yoshida K, Yamashita T, Yamashita T, Kaneko S, Takamura H, Ohta T, Ikeda H, Sato Y, Nakanuma Y, Harada K, Kita R, Gabata T. Gadoxetic acid-enhanced magnetic resonance imaging reflects co-activation of β-catenin and hepatocyte nuclear factor 4α in hepatocellular carcinoma. Hepatol Res. 2018;48:205-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 136. | Maeda K, Sugiyama Y. Impact of genetic polymorphisms of transporters on the pharmacokinetic, pharmacodynamic and toxicological properties of anionic drugs. Drug Metab Pharmacokinet. 2008;23:223-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 137. | Ieiri I, Higuchi S, Sugiyama Y. Genetic polymorphisms of uptake (OATP1B1, 1B3) and efflux (MRP2, BCRP) transporters: implications for inter-individual differences in the pharmacokinetics and pharmacodynamics of statins and other clinically relevant drugs. Expert Opin Drug Metab Toxicol. 2009;5:703-729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 161] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 138. | Niemi M, Pasanen MK, Neuvonen PJ. Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol Rev. 2011;63:157-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 463] [Cited by in F6Publishing: 463] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 139. | Nakanishi T, Tamai I. Genetic polymorphisms of OATP transporters and their impact on intestinal absorption and hepatic disposition of drugs. Drug Metab Pharmacokinet. 2012;27:106-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 140. | International Transporter Consortium. Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2418] [Cited by in F6Publishing: 2385] [Article Influence: 170.4] [Reference Citation Analysis (0)] |
| 141. | Li W, Yan B, Wang R, Jia Z. [Role of drug transporters in rational use of drugs at high altitude area]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2018;43:327-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 142. | Watanabe T, Kusuhara H, Maeda K, Shitara Y, Sugiyama Y. Physiologically based pharmacokinetic modeling to predict transporter-mediated clearance and distribution of pravastatin in humans. J Pharmacol Exp Ther. 2009;328:652-662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 272] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 143. | Hirano M, Maeda K, Hayashi H, Kusuhara H, Sugiyama Y. Bile salt export pump (BSEP/ABCB11) can transport a nonbile acid substrate, pravastatin. J Pharmacol Exp Ther. 2005;314:876-882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 144. | Marin JJ, Briz O, Monte MJ, Blazquez AG, Macias RI. Genetic variants in genes involved in mechanisms of chemoresistance to anticancer drugs. Curr Cancer Drug Targets. 2012;12:402-438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 145. | Nozawa T, Minami H, Sugiura S, Tsuji A, Tamai I. Role of organic anion transporter OATP1B1 (OATP-C) in hepatic uptake of irinotecan and its active metabolite, 7-ethyl-10-hydroxycamptothecin: in vitro evidence and effect of single nucleotide polymorphisms. Drug Metab Dispos. 2005;33:434-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 232] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 146. | van de Steeg E, van Esch A, Wagenaar E, van der Kruijssen CM, van Tellingen O, Kenworthy KE, Schinkel AH. High impact of Oatp1a/1b transporters on in vivo disposition of the hydrophobic anticancer drug paclitaxel. Clin Cancer Res. 2011;17:294-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 147. | Briz O, Serrano MA, Rebollo N, Hagenbuch B, Meier PJ, Koepsell H, Marin JJ. Carriers involved in targeting the cytostatic bile acid-cisplatin derivatives cis-diammine-chloro-cholylglycinate-platinum(II) and cis-diammine-bisursodeoxycholate-platinum(II) toward liver cells. Mol Pharmacol. 2002;61:853-860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 108] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 148. | Zollner G, Fickert P, Zenz R, Fuchsbichler A, Stumptner C, Kenner L, Ferenci P, Stauber RE, Krejs GJ, Denk H, Zatloukal K, Trauner M. Hepatobiliary transporter expression in percutaneous liver biopsies of patients with cholestatic liver diseases. Hepatology. 2001;33:633-646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 244] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 149. | Campbell SD, de Morais SM, Xu JJ. Inhibition of human organic anion transporting polypeptide OATP 1B1 as a mechanism of drug-induced hyperbilirubinemia. Chem Biol Interact. 2004;150:179-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 150. | Keppler D. The roles of MRP2, MRP3, OATP1B1, and OATP1B3 in conjugated hyperbilirubinemia. Drug Metab Dispos. 2014;42:561-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 151. | Noguchi S, Nishimura T, Mukaida S, Benet LZ, Nakashima E, Tomi M. Cellular Uptake of Levocetirizine by Organic Anion Transporter 4. J Pharm Sci. 2017;106:2895-2898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 152. | Kimura H, Takeda M, Narikawa S, Enomoto A, Ichida K, Endou H. Human organic anion transporters and human organic cation transporters mediate renal transport of prostaglandins. J Pharmacol Exp Ther. 2002;301:293-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 127] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 153. | Russel FG, Masereeuw R, van Aubel RA. Molecular aspects of renal anionic drug transport. Annu Rev Physiol. 2002;64:563-594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 191] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 154. | Suzuki T, Onogawa T, Asano N, Mizutamari H, Mikkaichi T, Tanemoto M, Abe M, Satoh F, Unno M, Nunoki K, Suzuki M, Hishinuma T, Goto J, Shimosegawa T, Matsuno S, Ito S, Abe T. Identification and characterization of novel rat and human gonad-specific organic anion transporters. Mol Endocrinol. 2003;17:1203-1215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 155. | Nakanishi T, Tamai I. Roles of Organic Anion Transporting Polypeptide 2A1 (OATP2A1/SLCO2A1) in Regulating the Pathophysiological Actions of Prostaglandins. AAPS J. 2017;20:13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 156. | Lee SY, Williamson B, Caballero OL, Chen YT, Scanlan MJ, Ritter G, Jongeneel CV, Simpson AJ, Old LJ. Identification of the gonad-specific anion transporter SLCO6A1 as a cancer/testis (CT) antigen expressed in human lung cancer. Cancer Immun. 2004;4:13. [PubMed] [Cited in This Article: ] |