Immunotherapy in pancreatic adenocarcinoma—overcoming barriers to response
Introduction
Pancreatic adenocarcinoma (PAC) remains one of the most lethal of human malignancies. It is the fourth leading cause of cancer-related death in the United States (1). Unfortunately, the only curative option is surgical resection. The majority of patients are diagnosed with advanced stage disease, and as such, for the large majority of patients, curative surgery is not an option. Despite significant advances in improvements in cancer therapy, mortality for pancreatic cancer has remained relatively unchanged (2). The mainstay of treatment for patients with advanced PAC remains systemic combination chemotherapy (3). Patient outcomes remain poor, with 5-year survival of less than 10%. Development of new therapies for PAC is greatly needed.
Recently, immunotherapies that boost T-cells to destroy cancer cells have generated much excitement in cancer therapy. In particular, inhibition of programmed death 1 (PD-1) and cytotoxic T lymphocyte antigen-4 (CTLA-4), has demonstrated clinical benefit in a number of malignancies (4) such as melanoma (5,6), lung cancer (7), bladder cancer (8), kidney cancer (9), head and neck cancer (10), hepatocellular cancer (HCC) (11), as well as Hodgkin lymphoma (12). This has generated hope and enthusiasm for a potential effective therapy in pancreatic cancer, however results of early clinical studies utilizing single-agent immune checkpoint inhibition in PAC have been disappointing (13,14). PAC has a unique tumor microenvironment (TME) that promotes immune evasion, and has demonstrated remarkable resistance to immune therapies (15). In this review, we discuss some of the strategies of overcoming barriers to response to immune therapies in PAC, as well as ongoing strategies currently being evaluated in the clinical trial setting.
The TME in PAC
The TME in PAC, which consists of complex and heterogeneous stroma, has been identified as a major contributor to resistance to systemic therapies (16). The stroma in PAC is very dense, fibrotic, and heterogeneous, and consists of fibroblasts, stellate cells, immune cells, and extracellular matrix (16). Furthermore, the immune infiltrate in PAC is unique in that it consists predominantly of macrophages and other myeloid cells, which interestingly are associated with inflammation in pancreatitis which in of itself is a risk factor for PAC (17). Macrophages are an important component of the innate immunity, and higher ratios of M1 macrophages (classically activated macrophages by Th1 cytokines) is associated with longer survival in PAC (17). In contrast, myeloid derived suppressor cells (MDSCs) are immature myeloid cells that that suppress T-cell responses (17,18).
The T-cell infiltrate in PAC is unique in both its location and function within the TME, which may inform our strategies with regards to immune therapies in PAC. The impact of T-cell infiltration on prognosis has demonstrated inconsistent results (19,20). Some studies have shown that increased intratumoral CD3+ T-cells are associated with improved OS (19,20). Other reports have not shown an association between T-cell density and patient survival (21,22). There is a unique distribution of T-cell infiltrates in PAC. In PAC, T-cell infiltrates are found more commonly at the invasive front of the tumor mass, suggesting malignant cell exclusion from the center of the tumor mass (17,22). Additionally, T-cells appear to be trapped within peritumoral tissues, with limited direct contact with tumor cells (22,23). These findings suggest that the TME may limit T-cell interaction with malignant cells.
However, further characterization of T-cells in PAC does seem to indicate that PAC may indeed be immunogenic to some extent. In contrast to immune infiltrate in chronic pancreatitis, in PAC the T-cell infiltrate consists of decreased CD4+ and CD8+ T-cells and increased regulatory T-cells (Tregs) (20), suggesting that immune suppression may play a role in malignant transformation. T-cell clonal expansion and proliferation has been identified in PAC with a T-cell receptor (TCR) repertoire similar to melanoma (24). PAC vaccine therapy can induce the formation of intratumoral tertiary lymphoid aggregates in PAC, in which suppressed Tregs was associated with improved prognosis (25). Another study that characterized the T-cell infiltrate of PAC has shown that increased Tregs are associated with worse prognosis, while higher levels of tumor infiltrating CD4+ and CD8+ T-cells are associated with improved prognosis (17). Taken together, while the T-cell infiltrate of PAC may have immunogenic properties, the TME in PAC seems to limit the immunogenic potential of T-cells in PAC and may act as a barrier to the effectiveness of immunotherapeutic strategies (26).
The question then becomes, how can we overcome the intrinsic resistance presented by PAC and its associated TME to enable T-cell mediated immune attack?
Immunomodulation following chemotherapy and radiation therapy
Chemotherapy and radiotherapy in PAC
In recent years, a number of chemotherapeutic agents have demonstrated efficacy in PAC, which has provided some improvement in patient prognosis in advanced disease (3). Gemcitabine monotherapy demonstrated efficacy in a landmark paper published in 1997 with a clinical benefit response in 23.8% of patients, and a median OS of 5.65 months (27). Subsequent work evaluating fluoropyrimidine based combinations demonstrated the clinical benefit of FOLFIRINOX (infusional FU/leucovorin, oxaliplatin, and irinotecan) in patients with advanced PAC (28). In this trial, FOLFIRINOX was directly compared to gemcitabine monotherapy demonstrating an improvement in median OS of 11.1 versus 6.8 months, with an improvement in ORR of 31.6% versus 9.4% respectively (28). Additional strides were achieved when gemcitabine was combined with nanoparticle albumin-bound paclitaxel (nab-paclitaxel) in which the combination of gemcitabine with nab-paclitaxel compared with gemcitabine alone demonstrated an improvement in median OS of 8.7 versus 6.6 months (29), and an ORR of 23% vs. 7%. With these therapeutic advances, systemic chemotherapy has become the mainstay treatment for metastatic PAC.
Given the technical challenges, limitations, and morbidity of surgery for loco-regional treatment of PAC, radiation with or without systemic chemotherapy has been incorporated as an effective tool for local control of PAC. Radiotherapy has a role with or without chemotherapy in a number of clinical settings in PAC, such as in the neoadjuvant setting for borderline resectable disease, for locally advanced unresectable disease, in the adjuvant setting for resectable disease, and in the palliative or recurrent settings (3). The current use of systemic chemotherapy with or without radiotherapy in the management of advanced PAC has led to great interest in the immunomodulatory effects of these modalities.
Effect of cytotoxic chemotherapy and radiotherapy on immune microenvironment
Although cytotoxic chemotherapy previously had been regarded as immunosuppressive with regards to its effects on anti-tumor immunity, more recently it has been suggested that it may actually increase tumor immunogenicity (30). Chemotherapy can kill malignant cells by immunogenic cell death (Figure 1) (31). It has been suggested that dying cells undergoing immunogenic cell death expose proteins on its surface (DAMPs; damage-associated molecular patterns) to facilitate uptake of dying cells by dendritic cells (DC), which can subsequently prime CD4+ and CD8+ T-cells to trigger an immunogenic, tumor-specific, immune response (32). Furthermore, cytotoxic chemotherapy can also modify host immunity via modulation of a variety of immunoregulatory cells (30). This can occur through decreasing the immunosuppressive effects of Tregs, decreasing MDSCs which inhibit T-cell mediated responses, enhancement of DC responses, and promotion of T-cell lymphocyte proliferation of anti-tumor immune response (30).
Chemotherapy and radiotherapy and immune modulation in solid tumors
The role of cytotoxic chemotherapy and radiotherapy as immune modulators have been explored in a number of solid tumors. Initial combinations of cytotoxic chemotherapy with immune therapies suggested that the addition of chemotherapy does not reduce anti-tumor immunity. In a phase I/II trial in patients with metastatic colorectal cancer (mCRC) and elevated carcinoembryonic antigen (CEA), patients received concurrent chemotherapy with 5-fluorouracil/leucovorin and irinotecan (FOLFIRI) with a CEA derived peptide (CAP-1) vaccine therapy (31). Of seventeen patients, 8 (47%) demonstrated increased CAP-1 specific cytotoxic T-cells. A similar trial randomized 118 patients with mCRC to vaccine therapy before and concomitant with chemotherapy, vaccine therapy before and concomitant with chemotherapy with tetanus toxoid added, and chemotherapy followed by vaccine therapy (33). CEA-specific T-cells were increased in 50%, 37%, and 30% respectively with no statistical differences between the groups, suggesting that combining vaccine therapy with chemotherapy was able to elicit an anti-tumor immune response. In metastatic androgen independent prostate cancer, a study comparing a PSA-based vaccine therapy alone or in combination with docetaxel demonstrated similar increases in T-cell precursors to PSA (3.33-fold increase) in both groups (34), again suggesting that chemotherapy does not inhibit anti-tumor immune responses.
Subsequent clinical trials combining immune therapies with chemotherapy confirm this observation with checkpoint inhibitor therapy. In patients with metastatic melanoma, dacarbazine in combination with ipilimumab demonstrated improved overall survival compared with dacarbazine alone with median OS of 11.2 and 9.1 months respectively (35). Subsequently, a randomized phase II trial in patients with advanced stage non-small cell lung cancer (NSCLC) and extensive stage small cell lung cancer (ES-SCLC) compared a concurrent combination with ipilimumab with carboplatin and paclitaxel for four cycles followed by carboplatin and paclitaxel alone for two cycles, versus a phased combination in which carboplatin and paclitaxel for two cycles followed by ipilimumab with carboplatin and paclitaxel for four cycles, versus carboplatin and paclitaxel alone for six cycles. The phased strategy demonstrated improvement in immune-related PFS (irPFS) in both groups, whereas the concurrent strategy did not demonstrate a statistical improvement in irPFS (36,37). Another example is a large phase II trial in advanced NSCLC comparing pembrolizumab with carboplatin and pemetrexed versus carboplatin and pemetrexed alone, demonstrating an improved PFS of 19.0 versus 8.9 months respectively (38).
Radiation therapy also seems to have immunomodulatory effects that has been explored in solid tumors. It has been previously recognized that ionizing radiation can lead to an abscopal effect, or off-target responses, thought to be mediated by anti-tumor T-cell responses induced by immunogenic cell death (39,40). In a proof-of-principle clinical trial of patients with metastatic solid tumors treated with radiation to 1 of 3 or more metastatic sites with concurrent granulocyte-macrophage colony stimulating factor (GM-CSF), abscopal responses occurred in 11 of 41 patients (41). This suggests that local radiation may induce anti-tumor immunity which leads to off-target efficacy.
Immunomodulatory effects of chemotherapy and radiotherapy in PAC
This has led to the hypothesis that in pancreatic cancer, a strategy of combining cytotoxic chemotherapy and radiotherapy with immune therapy may increase tumor immunogenicity and sensitize pancreatic tumors to immune therapy. Evaluation of the local immune environment in resected PAC treated with neoadjuvant chemoradiotherapy (chemoXRT) suggests that these modalities may enhance the immunogenicity of this disease. In one study, 52 patients who underwent surgical resection for PAC with 22 having received neoadjuvant chemoXRT, demonstrated that there were increased numbers of CD4+ and CD8+ T-lymphocytes in those patients treated with chemoXRT compared with patients resected without neoadjuvant therapy, and that high accumulation of CD8+ cells was associated with improved OS (42). Another study in which 7 of 17 patients received neoadjuvant chemoXRT prior to resection demonstrated no difference in the number of CD4+ and CD8+ T-cell infiltration, but the number of Tregs was significantly lower in the neoadjuvant chemoXRT group, suggesting a sensitizing effect to anti-tumor immunity with this strategy (43). Additionally, in PAC increased T-cell infiltrating lymphocytes (TILs) in patients treated with neoadjuvant chemotherapy was associated with improved disease free survival (DFS) (44), supporting the immunomodulatory role of cytotoxic chemotherapy in this disease. Chemotherapy, radiotherapy, and immune therapies have been combined with vaccine therapy (25,45-49), cytokine therapy (50), and checkpoint blockade (51) in the treatment of PAC.
The combination of chemotherapy and radiation therapy with various immune therapies are being explored in both pre-clinical and clinical settings in the treatment of PAC (52). In a mouse model of pancreatic cancer, combining radiation with dual blockade of PD-(L)1 and CTLA-4 resulted in improved survival and tumor responses than dual blockade without radiation or radiation alone (53). A phase Ib/II trial of neoadjuvant chemoradiotherapy in combination with pembrolizumab is currently ongoing and appears to be safe, yet efficacy data has not yet been reported (54). Interestingly, while some data suggests that radiation may enhance anti-tumor T-cell responses, other pre-clinical data has suggested an immunosuppressive T-cell effect as well. In mouse models, radiation exposure also induced a macrophage immunosuppressive phenotype, as well as a reduction in CD8+ T-cells with increased Tregs (55), suggesting that immune responses to ionizing radiation may be mixed. It has also been suggested that inhibition of macrophage signaling may enhance anti-tumor responses (56), and that this may be a reasonable strategic approach in combination with radiotherapy (26). A number of clinical trials are currently ongoing investigating the role of radiation in combination with immune therapies. A phase II trial evaluating stereotactic body radiation therapy (SBRT) in combination with pembrolizumab and GVAX (GM-CSF secreting allogeneic pancreatic cancer vaccine) is currently ongoing (NCT02648282). Similarly, a pilot study evaluating SBRT in combination with tremilimumab (CTLA-4 monoclonal antibody) and/or MEDI4736 (PD-L1 monoclonal antibody) is also ongoing (NCT02311361). An open label phase II study in metastatic PAC is currently combining radiation with nivolumab with or without ipilimumab (NCT02866383). The true impact of radiation on anti-tumor immunity in-vivo has yet to be determined, as we wait with anticipation for the results of these clinical trials.
At this time, chemotherapy and radiotherapy are being combined with a number of immunomodulatory strategies in order to overcome these challenging barriers to the efficacy in immune therapies in PAC.
Immunomodulatory strategies in pancreatic cancer
Checkpoint inhibitors
Unfortunately, as of yet, the combination of cytotoxic chemotherapy with an immune therapy has not resulted in an overwhelming improvement in effectiveness of immune therapies (summarized in Table 1). Gemcitabine has been combined with CTLA4 blockade in several early phase clinical trials. In a phase I trial combining gemcitabine with tremelimumab, of 28 evaluable patients, two patients received a partial response (PR), and seven patients had stable disease (SD) for >10 weeks (57). In a phase Ib trial combining gemcitabine and ipilimumab, of sixteen evaluable patients, two patients had a PR and five patients had SD (58). This combination was well tolerated, yet objective response rate did not seem to be significantly improved over gemcitabine alone (59,60).

Full table
Gemcitabine in combination with programmed death (ligand) 1 [PD-(L)1] blockade, is also being evaluated. In murine models, gemcitabine and PD-(L)1 blockade demonstrate synergy and resulted in some complete responses (CR) (61). In a phase Ib trial evaluating pembrolizumab in combination with chemotherapy in advanced solid tumors, there were ten evaluable patients who received gemcitabine in combination with pembrolizumab. Of these, two patients had a PR, and six patients had SD (62). Recently, a phase I trial combining nab-paclitaxel with or without gemcitabine with nivolumab reported results (51). The combination was overall well tolerated, with disease control (SD or PR) in 12 of 17 patients with locally advanced or metastatic PAC. Responses were observed in both the second line and upfront setting. This compares favorably with a historical control of chemotherapy alone, in which gemcitabine plus nab-paclitaxel reported a disease control rate was 48% (63). This provides at least a signal regarding combining single-agent checkpoint blockade with chemotherapy. However, larger clinical trials need to be completed to demonstrate a clinical benefit in this setting.
Cancer vaccines
In addition to checkpoint inhibition, cancer vaccine therapy has also been developed in hopes of inducing an anti-tumor immune response in PAC. In addition to evaluating advanced stage disease, a number of vaccine-based studies have also been evaluated in the adjuvant setting, as the low disease burden post-resection may suggest a role for a consolidative anti-tumor immune response (26,64). The most extensively evaluated anti-tumor vaccine is GVAX, an irradiated allogeneic whole tumor cell vaccine that expresses granulocyte-macrophage colony-stimulating factor (GM-CSF) (15). In early phase clinical trials, GVAX demonstrated anti-tumor delayed hypersensitivity responses in PAC (65). A phase II trial of 60 patients evaluating GVAX in combination with chemoradiotherapy in the adjuvant setting for resected PAC demonstrated 17.3 months DFS and 24.8 months OS, was well tolerated, and demonstrated mesothelin-specific CD8+ T-cells which correlated with DFS (25). Mesothelin had been previously demonstrated to be a tumor-associated antigen overexpressed in PAC (66). Subsequently, a GVAX immunization strategy was modified by combining with low dose cyclophosphamide with the goal of inhibiting Tregs, with increased anti-mesothelin CD8+ T-cell responses (67). GVAX was subsequently combined with CRS-207, a recombinant live-attenuated, double-deleted Listeria Monocytogenes, engineered to secrete mesothelin into antigen presenting cells in order to enhance mesothelin-specific CD8+ T-cell activity. A phase II trial was conducted in which patients with metastatic PAC were randomized in a 2:1 fashion to GVAX with cyclophosphamide (Cy/GVAX) followed by CRS-207 or Cy/GVAX alone. The Cy/GVAX plus CRS-207 arm demonstrated an OS benefit of 6.1 months versus 3.9 months in the Cy/GVAX alone arm, and mesothelin-specific CD8+ T-cell responses were associated with longer OS (46).
Unfortunately, several other attempts at vaccine therapy for PAC have not similarly demonstrated improvements in patient outcomes, despite eliciting anti-tumor T-cell immunity. A large, randomized, phase III trial combining chemotherapy with a telomerase vaccine did not demonstrate improvement in patient survival (49). Algenpantucel-L, irradiated allogeneic pancreatic cancer cells transfected to express alpha-1,3-galactosyltransferase, has been evaluated in a phase II adjuvant trial in combination chemoradiotherapy demonstrated a 12-month DFS of 62% and an OS of 86% (45), however a large phase III randomized trial comparing chemoradiotherapy plus algenpantucel-L versus chemoradiotherapy alone in the adjuvant setting demonstrating no difference in OS (68). A phase I/II trial consisting of twenty-three patients who were treated with a mutant RAS peptide vaccine, demonstrated some long-term T-cell immune responses, with four of twenty evaluable patients demonstrating 10-year survival (69). Peptide vaccines consisting of VEGFR1 and VEGFR2 (antiangiogenic) and KIF20A have been evaluated in a phase 2 setting of locally advanced and metastatic PAC in combination with chemotherapy, suggesting that patients who mount a cytotoxic T-cell immune response seemed to have improved OS (47). Gemcitabine was combined with elpamotide, a VEGFR2 vaccine, in a randomized phase II/III trial in advanced and metastatic PAC with no difference in OS (48). Other peptide vaccines have been attempted including personalized peptide vaccines (70), KIF20A-66 (member of kinesin super family protein 20A that is transactivated in PAC) peptide vaccine (70), and RAS peptide vaccine (71-73), demonstrating that these peptides can illicit variable immune responses.
Chemotherapy in combination with immune vaccines has been evaluated without much success. An investigation pancreatic cancer vaccine, algenpantucel-L, was evaluated in a phase III trial with standard of care chemotherapy, demonstrating an overall survival of 27.3 months versus 30.4 months with chemotherapy alone (45). An additional phase III trial investigating chemotherapy (gemcitabine and capecitabine) with either sequential or concomitant telomerase cancer vaccination also demonstrated no difference in overall survival (49). Gemcitabine was combined with IMM-101, a systemic immune modulator containing heat-killed Mycobacterium obuense, in a phase II randomized trial which demonstrated a non-significant improvement in OS of 6.7 vs. 5.6 months (P=0.074) when IMM-101 was combined with gemcitabine compared with gemcitabine alone, and was well tolerated.
Taken together, clinical data suggests that vaccines in PAC can elicit an anti-tumor T-cell response. While some of these trials provide a signal of clinical benefit, others demonstrating no clinically meaningful endpoints, which suggests that additional barriers to effective anti-tumor immune therapy are at play (Table 2).
Full table
Cytokines
The use of cytokines as an immune therapy has been evaluated in the clinical setting both alone and in combination with other systemic therapies for PAC in advanced and adjuvant settings. In a large phase III trial of resected PAC, chemotherapy (5-fluorouracil; 5-FU) alone was compared with combination chemotherapy (5-FU plus cisplatin) with interferon alfa-2b. The combination was more toxic than 5-FU alone and did not demonstrate an improvement in overall survival (74). An adjuvant trial comparing surgical resection alone with combination chemotherapy with or without IL-2 therapy directly injected into superior mesenteric artery (SMA) demonstrated an improvement in survival (31.0, 25.0, and 18.8 months respectively for adjuvant chemoimmunotherapy, adjuvant chemotherapy alone, or no adjuvant therapy respectively) (50), however this was a small trial, and the rationale behind injection of adjuvant therapy into the SMA was not adequately explained. In a phase Ib trial, FOLFOX was combined with AM0010, PEGylated human IL-10. Of nineteen evaluable patients, two patients had a CR, 1 patient had a PR, and 11 patients had SD. Treatment resulted in increased serum cytokine levels and expansion of novel T-cell clones (75). Similar to vaccines therapies, while some evidence of eliciting anti-tumor immunity was demonstrated in some studies, significant strides in improving clinical outcomes has not been demonstrated.
Oncolytic viral therapy
Oncolytic viral therapy is a strategy that can induce tumor responses through direct tumor cell lysis by infecting tumor cells, replicating, and eventually lysing the cell. However, cell lysis also releases damage associated molecular pattern molecules (DAMPs) which can trigger innate and adaptive immune responses (76). Of these, adenovirus-based oncolytic viruses have been the most extensively evaluated. One of these, ONYX-015, is an E1B-55kDa region-deleted adenovirus that selectively replicates in and lyses tumor cells with p53 abnormalities. In a phase I dose escalation study in which ONYX-015 was directly injected into locally advanced unresectable pancreatic cancer, but resulted in no responses, and no evidence of viral replication (77). A phase I/II study of direct tumor injection of ONYX-015 followed by gemcitabine in localized PAC demonstrated PRs in 4 of 11 patients after gemcitabine, but no objective responses after treatment of the oncolytic virus alone (75). Another oncolytic viral therapy has utilized a reovirus that preferentially replicates in cells with activated RAS pathways, reolysin. In a phase II study, of 29 evaluable patients with advanced or metastatic PAC, one patient demonstrated a PR. Interestingly however, there was upregulation of PD-L1 in reolysin treated patients, again suggesting the potential immunomodulatory impact of oncolytic viral therapy in PAC (78). While these strategies seem to be limited by anti-tumor potency and immune neutralization, follow-up clinical studies utilizing reolysin in combination with anti-PD1 therapy (pembrolizumab) are ongoing(NCT02620423) (76).
Adoptive T-cell therapy
Adoptive T-cell therapy involving genetically engineered T-cells expanded ex-vivo and re-infused into patients to target malignancies has been an exciting advancement in cancer therapy and is being explored in PAC. Adoptive T-cells utilizing a chimeric antigen receptor (CAR) has demonstrated impressive results in a number hematologic malignancies such as acute lymphoblastic leukemia (ALL) (79) which eventually has led to its Food and Drug Administration (FDA) approval (80). This has led to investigation and hope for an adoptive T-cell strategy in solid tumor malignancies as well (81). CAR T-cells that target mesothelin have demonstrated an anti-tumor immune response in patients with metastatic PAC (82). Furthermore, in one patient treated with mesothelin-specific CAR T cells with metastatic PAC, there was resolution of an FDG-avid liver metastasis after one month of therapy (83). Despite only small numbers of patients with metastatic PAC that have been treated thus far with adoptive T-cell therapy, this at least suggests a signal of immune-mediated anti-tumor effects, yet with variable effects in metastatic sites versus primary tumor (22). Mixed responses of metastatic and primary lesions have also been described in the setting of 20 patients treated with MUC-1 specific cytotoxic T-cells with a MUC-1 dendritic cell vaccine therapy, including one patient who experienced resolution of multiple pulmonary metastases (22,84). It therefore begs to reason that perhaps despite antitumor efficacy of adoptive T-cell therapy, selective barriers to efficacy based on primary versus metastatic sites may explain these variable responses. Currently, adoptive T-cell therapies under investigation include an anti-mesothelin CAR-T in the metastatic setting that is currently recruiting patients (NCT01583686), as well as prostate stem cell antigen (PSCA)-specific CAR T-cells (NCT02744287).
Targeted therapies to amplify T-cell mediated immunity
The biology of PAC and genomic makeup demonstrates a number of driver mutations, yet at this time no targeted therapies have made significant inroads into improving patient prognosis with this disease. The current model of oncogenic transformation suggests a stepwise progression from polyp to adenocarcinoma, similar to what is seen in colon cancer, with pancreatic intraepithelial neoplasia (PIN) as the precursor lesion (85). In fact, over 90% of PINs of various grades demonstrate KRAS mutations, while mutations in CDKN2A, p53, and SMAD4 are seen more frequently in higher grade PINs, suggesting that these are cumulative events in the malignant transformation of PAC (85). PACs are heterogeneous with regards to its genomic mutational pattern, which is notable for mutational frequencies of >90% KRAS, 60–70% p53, >50% CDKN2A, ~50% SMAD4, and other less frequent mutations (85). Other emerging drivers include mutations in BRCA1/2, which has a prevalence of 4–5% in unselected patients with PAC, with BRCA2 as the more common variant (86). This is of particular importance in light of mounting evidence that these patients may have enhanced sensitivity to platinum-based chemotherapy as well as poly(ADP-ribose) polymerase (PARP) inhibition (87). Other less common mutations which may have clinical relevance is human epidermal growth factor receptor 2 (Her2), which has a prevalence of approximately 2% in unselected patients with PAC (88). Although as of yet, Her2 targeting in PAC has not yielded significant improvements in clinical outcomes (89,90). Mutational patterns of PAC may also have prognostic significance, and long-term survivors of resected PAC tend to have lower rates of KRAS, p53, and SMAD4 mutations (91). Despite this work however, targeted therapies in PAC has proved challenging, in large part due to tumor heterogeneity (92). With the exception of erlotinib (93), other attempts at targeted therapies have failed to yield positive results such as bevacizumab (94,95), cetuximab (96), axitinib (97,98), sorafenib (99), and aflibercept (100).
Recently, targeted therapies as a means of modulating and enhancing T-cell immunity, is being explored as a potential partner with immunotherapies such as checkpoint inhibitors. Specifically, research is focusing on targeted strategies that are able to transform “cold” tumors, or noninflamed tumors, into “hot” tumors that demonstrate T-cell infiltration and may enhance the efficacy of checkpoint inhibitor therapies. A number of targeted therapies appear to affect tumor cells directly, as well as modulation of immune cells, such as BRAF and MEK inhibition in melanoma (101). This has sparked interest that combining immunotherapies with targeted therapies may overcome some of the barriers in efficacy found with single-agent checkpoint inhibitor therapies, especially in malignancies such as PAC with poor responses to single-agent checkpoint inhibition.
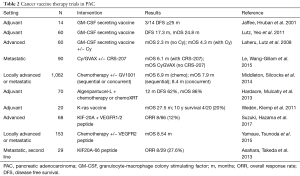
Targeted therapies that enhance T-cell mediated immunity is a strategy that has been evaluated in PAC as well. Some of these strategies have included blockade of additional immune inhibitory pathways, stimulation of activating pathways, epigenetic modifications, which include not only lymphocytes but also macrophages, natural killer (NK) cells, and stromal cells (102). Many different targets have been and are currently being evaluated in the immune activation cascade including T-cells, myeloid cells, and stromal tissue in order to both amplify T-cell immune response and sensitize pancreatic tumors to anti-tumor immunity (Table 3).

Full table
MAP-ERK kinase (MEK) inhibition
Other targets have been suggested as potential mechanisms of synergism with immune therapy include inhibition of MEK pathway and epigenetic targeting (22). PAC is characterized by KRAS mutations present in >90% of cases, which has generated much interest in targeting downstream pathways such as MEK in this disease (103). For example, MEK inhibition has been shown to upregulate MHC I on tumor cells and induce T-cell infiltration into tumors (104), and enhance the activity of PD-(L)1 blockade. Combination MEK inhibition with checkpoint blockade is being evaluated in colorectal cancer and appears to be safe and demonstrates some responses (105). However in PAC, these have not yet reached the clinical trial arena in combination with immune therapy.
CD40 agonist
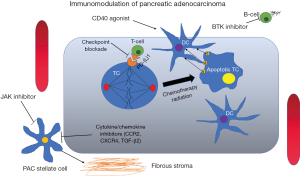
CD40 is broadly expressed on immune cells such as B-cells, dendritic cells, and monocytes, and CD40 agonists can mediate both T-cell dependent and independent mechanisms of tumor regression in PAC (Figure 1) (106). Of the strategies utilized to activate myeloid cells as a means of enhancing anti-tumor immunity, CD40 are the most extensively evaluated target. CD40 activates APCs and enhances immune responses (107). CD40 agonist monoclonal antibody, has demonstrated some responses in solid tumors (108), leading to interest in investigating this strategy in PAC. Gemcitabine in combination with a CD40 agonist therapy demonstrated that out of 21 evaluable patients with 90% having metastatic PAC, four had a PR, and eleven had SD (109). Additionally, CD40 agonist therapy resulted in cytokine release syndrome (CRS), increased inflammatory cytokines, and an increase in B-cell co-stimulatory molecules (110). Importantly however, extratumoral macrophages regulate infiltration of T-cell into PAC, suggesting that reversal of immune privilege may increase efficacy of T-cell immunotherapy in PAC (22,111).
JAK-STAT pathway inhibition
The PAC TME is composed of fibrotic extracellular matrix, produced by pancreatic stellate cells within the TME (112), which may limit the therapeutic efficacy of immune therapies in PAC (Figure 1). The JAK/STAT pathway plays an important role in activation of pancreatic stellate cells (113), and inhibition of this pathway may have a favorable impact on the efficacy of immune therapies. Targeted therapies that target the unique PAC microenvironment have also been investigated in combination with immune therapy (26). In mouse models of PAC, JAK pathway blockade results in immune-mediated inhibition of tumor growth (114). However, in two large phase III trials, ruxolitinib in combination with capecitabine did not improve outcomes of metastatic PAC over capecitabine alone (115). (NCT02117479 and NCT02119663).
Bruton’s tyrosine kinase (BTK) inhibition
Another target that is being investigated in PAC is inhibition of BTK. BTK is a Tec family non-receptor tyrosine kinase that is required for B-cell receptor (BCR) signaling, and has been developed primarily for B-cell malignancies such as chronic lymphocytic leukemia (CLL), mantle cell lymphoma, and Waldenstrom’s macroglobulinemia (116). In PAC, BTK regulates B-cell and macrophage mediated suppression of T-cells (Figure 1) (117). This has led to the evaluation of ibrutinib as an immunomodulatory agent in PAC. In pre-clinical models of PAC, ibrutinib demonstrates reduction in stromal fibrosis and inhibition of tumor progression (118), suggesting that it may have the potential to sensitize tumors to checkpoint blockade. This combination is currently being investigated in a phase Ib/II, multicenter, study in combination with durvalumab (NCT02403271) (119).
Colony stimulating factor 1 receptor (CSF1R)
Myeloid lineage immune cells are also being targeted through inhibition of colony stimulating factor 1 receptor (CSF1R) with a goal of amplifying checkpoint inhibitor efficacy. Tumor associated macrophages exposed to CSF1 enhances a tumor promoting and immune suppressive macrophage phenotype (120), which has led to this as an attractive target for immune modulation in PAC. Preclinical data has demonstrated that inhibiting CSF1R can reprogram macrophages and thereby enhance antigen presentation to increase anti-tumor T-cell responses (56). CSF1R blockade is being evaluated in combination with PD-1 blockade and vaccine therapy (NCT03153410 and NCT03153410).
Cytokine and chemokine pathway inhibition
A number of cytokines and chemokines involved in recruitment of immunosuppressive myeloid cells have also gained interest as immunomodulatory targets in PAC. The CCL2/CCR2 is a chemokine receptor pathway that is involved in recruitment of immunosuppressive monocytes into the TME (Figure 1), and has been evaluated as a target for inhibition in combination with chemotherapy, demonstrating some responses (121). Chemokine receptor type 4 (CXCR4) with its chemokine ligand CXCL12 is a major player in the immunosuppressive TME in PAC, and contributes to chemotherapy resistance and poor outcomes in this disease (122). An early phase clinical trial evaluating CXCR4 blockade in combination with PD-1 blockade in patients with metastatic PAC is currently recruiting (NCT02826486). Another immunosuppressive target that has been investigated in PAC is transforming growth factor-β2 (TGF-β2). TGF-β2 plays an important role in both development of pancreatic cancer stem cells as well as mediating the interaction between pancreatic stellate cells and cancer cells (123). Trabedersen, an anti-sense peptide which inhibits biosynthesis of transforming growth factor-β2 (TGF-β2) (75). This has been evaluated in a phase I/II trial including patients with advanced PAC (76). One PAC patient was reported to have had a CR of liver metastasis, but no other efficacy data was reported (76).
Cyclin-dependent kinases 4 and 6 (CDK4/6) inhibition
Cyclin-dependent kinase 4 and 6 are involved in cell cycle progression and are required for malignant transformation in solid tumors, such as breast cancer (124). This has led to the development of CDK4/6 inhibitors that act through inhibition of the phosphorylation of the retinoblastoma (RB) tumor suppressor gene, resulting in cell cycle arrest in tumor cells (125). In breast cancer, inhibitors of CDK4/6 demonstrated efficacy in combination with endocrine therapy for hormone-responsive breast cancer in the metastatic setting (126,127). Recently, work in CDK4/6 inhibition has suggested that in addition to direct tumor cell cytotoxicity, it may also have immunomodulatory properties. Enhanced anti-tumor immunity may be related to tumor cell expression of endogenous retroviral elements leading to increased tumor antigen presentation and suppression of regulatory T-cell proliferation (125).
This data has led to the hypothesis that CDK4/6 inhibition in PAC may have a role, and may enhance antitumor immunity in combination with immunotherapeutic interventions. In PAC, inactivation of CDKN2A is found in approximately 95% of cases, which encodes the tumor suppressor p16INK4A whose role is inhibition cyclin dependent kinases 4 and 6 (128). In previous work evaluating CDK4/6 suppression in patient-derived xenographs of PAC, tumor proliferation was completely suppressed (129). In another study of patient-derived xenograft models of pancreatic cancer, palbociclib as a single-agent demonstrated greater than 50% tumor growth, and the combination with gemcitabine and nab-paclitaxel increased the degree of tumor response as well (130). Currently, CDK4/6 inhibitors are being evaluated in combination with anti-PD-L1 antibody in multiple solid tumors, including PAC (NCT02791334).
Wnt inhibitor therapy
The Wnt/β-catenin signaling pathway has been implicated in carcinogenesis, including gastrointestinal cancers. In colorectal cancer the loss of the APC gene is an early pathogenic occurrence and is a major driver of Wnt/β-catenin signaling with accumulation of β-catenin leading to promotion of cellular proliferation, and this seems to play an important role in tumor maintenance (131). Wnt/β-catenin pathway activation has also been demonstrated in other upper gastrointestinal cancers such as in gastric cancer, HCC, and cholangiocarcinoma (132-134). In PAC, Wnt/β-catenin pathway mutations are rare, however nuclear localization of β-catenin can be found (131,135). Furthermore, RNF43 inhibits Wnt/β-catenin signaling in PAC, and PAC cell lines with RNF43 mutations were sensitive to inhibition of Wnt/β-catenin signaling (136). Upregulation of the Wnt/β-catenin signaling pathway may also have immunomodulatory properties through effects on dendritic cells leading to reduced CD8+ T-cell function, interactions with tumor-associated macrophages, and increased Treg survival (137). Given the suggested role of Wnt/β-catenin in PAC and the immunomodulatory effects, combining Wnt inhibitors with immunotherapeutic interventions may be a rational combination to be explored in the pre-clinical and subsequently clinical setting.
Conclusions
Unfortunately, PAC remains a major cause of cancer-related mortality, with little improvement despite significant strides made in cancer therapy in recent years. Harnessing the immune system to attack cancer is revitalizing progress and hope for a wide range of malignancies, yet these strategies have not yet made significant inroads in the realm of PAC. A number of barriers to immune therapy in PAC include lower levels of neoantigens, the unique immunosuppressive TME, and low levels of intratumoral infiltrating T-lymphocytes. Despite this, a number of pre-clinical and early clinical data suggests that PAC may be more immunogenic than initially thought, however these strategies have yet to make significant strides in terms of clinical benefit. Further investigation into overcoming barriers to immune therapy in PAC must be strategically applied to discover combinations that have the potential to improve outcomes of patients with PAC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Worni M, Guller U, White RR, et al. Modest improvement in overall survival for patients with metastatic pancreatic cancer: a trend analysis using the surveillance, epidemiology, and end results registry from 1988 to 2008. Pancreas 2013;42:1157-63. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Pancreatic Adenocarcinoma (Version 3.2017).
- Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015;348:69-74. [Crossref] [PubMed]
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320-30. [Crossref] [PubMed]
- Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521-32. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017;18:312-22. [Crossref] [PubMed]
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373:1803-13. [Crossref] [PubMed]
- Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med 2016;375:1856-67. [Crossref] [PubMed]
- El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492-502. [Crossref] [PubMed]
- Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015;372:311-9. [Crossref] [PubMed]
- Brahmer JR, Tykodi SS, Chow LQM, et al. Safety and Activity of Anti–PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [Crossref] [PubMed]
- Royal RE, Levy C, Turner K, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother 2010;33:828-33. [Crossref] [PubMed]
- Beatty GL, Gladney WL. Immune escape mechanisms as a guide for cancer immunotherapy. Clin Cancer Res 2015;21:687-92. [Crossref] [PubMed]
- Feig C, Gopinathan A, Neesse A, et al. The pancreas cancer microenvironment. Clin Cancer Res 2012;18:4266-76. [Crossref] [PubMed]
- Ino Y, Yamazaki-Itoh R, Shimada K, et al. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer 2013;108:914-23. [Crossref] [PubMed]
- Gabrilovich DI, Nagaraj S. Myeloid-derived-suppressor cells as regulators of the immune system. Nat Rev Immunol 2009;9:162-74. [Crossref] [PubMed]
- Tewari N, Zaitoun AM, Arora A, et al. The presence of tumour-associated lymphocytes confers a good prognosis in pancreatic ductal adenocarcinoma: an immunohistochemical study of tissue microarrays. BMC Cancer 2013;13:436. [Crossref] [PubMed]
- Helm O, Mennrich R, Petrick D, et al. Comparative characterization of stroma cells and ductal epithelium in chronic pancreatitis and pancreatic ductal adenocarcinoma. PLoS One 2014;9:e94357. [Crossref] [PubMed]
- Ryschich E, Nötzel T, Hinz U, et al. Control of T-cell–mediated immune response by HLA class i in human pancreatic carcinoma. Clin Cancer Res 2005;11:498-504. [PubMed]
- Beatty GL, Eghbali S, Kim R. Deploying immunotherapy in pancreatic cancer: defining mechanisms of response and resistance. Am Soc Clin Oncol Educ Book 2017;37:267-78. [Crossref] [PubMed]
- von Bernstorff W, Voss M, Freichel S, et al. Systemic and local immunosuppression in pancreatic cancer patients. Clin Cancer Res 2001;7:925s-32s. [PubMed]
- Poschke I, Faryna M, Bergmann F, et al. Identification of a tumor-reactive T-cell repertoire in the immune infiltrate of patients with resectable pancreatic ductal adenocarcinoma. Oncoimmunology 2016;5:e1240859. [Crossref] [PubMed]
- Lutz E, Yeo CJ, Lillemoe KD, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma: a phase ii trial of safety, efficacy, and immune activation. Ann Surg 2011;253:328-35. [Crossref] [PubMed]
- Guo S, Contratto M, Miller G, et al. Immunotherapy in pancreatic cancer: Unleash its potential through novel combinations. World J Clin Oncol 2017;8:230-40. [Crossref] [PubMed]
- Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403-13. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Goldstein D, El-Maraghi RH, Hammel P, et al. nab-paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III Trial. J Natl Cancer Inst 2015;107:dju413. [Crossref] [PubMed]
- Chen G, Emens LA. Chemoimmunotherapy: reengineering tumor immunity. Cancer Immunol Immunother 2013;62:203-16. [Crossref] [PubMed]
- Weihrauch MR, Ansén S, Jurkiewicz E, et al. Phase I/II Combined Chemoimmunotherapy with Carcinoembryonic Antigen–Derived HLA-A2–Restricted CAP-1 peptide and irinotecan, 5-fluorouracil, and leucovorin in patients with primary metastatic colorectal cancer. Clin Cancer Res 2005;11:5993-6001. [Crossref] [PubMed]
- Green DR, Ferguson T, Zitvogel L, et al. Immunogenic and tolerogenic cell death. Nat Rev Immunol 2009;9:353-63. [Crossref] [PubMed]
- Kaufman HL, Lenz H-J, Marshall J, et al. Combination Chemotherapy and ALVAC-CEA/B7.1 vaccine in patients with metastatic colorectal cancer. Clin Cancer Res 2008;14:4843-9. [Crossref] [PubMed]
- Arlen PM, Gulley JL, Parker C, et al. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res 2006;12:1260-9. [Crossref] [PubMed]
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus Dacarbazine for Previously Untreated Metastatic Melanoma. N Engl J Med 2011;364:2517-26. [Crossref] [PubMed]
- Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in Combination With Paclitaxel and Carboplatin As First-Line Treatment in Stage IIIB/IV non–small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol 2012;30:2046-54. [Crossref] [PubMed]
- Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial†. Ann Oncol 2013;24:75-83. [Crossref] [PubMed]
- Borghaei H, Langer CJ, Gadgeel S, et al. LBA49Updated results from KEYNOTE-021 cohort G: A randomized, phase 2 study of pemetrexed and carboplatin (PC) with or without pembrolizumab (pembro) as first-line therapy for advanced nonsquamous NSCLC. Ann Oncol 2017;28:mdx440.052.
- Ng J, Dai T. Radiation therapy and the abscopal effect: a concept comes of age. Ann Transl Med 2016;4:118. [Crossref] [PubMed]
- Park B, Yee C, Lee K-M. The effect of radiation on the immune response to cancers. Int J Mol Sci 2014;15:927-43. [Crossref] [PubMed]
- Golden EB, Chhabra A, Chachoua A, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol 2015;16:795-803. [Crossref] [PubMed]
- Homma Y, Taniguchi K, Murakami T, et al. Immunological Impact of Neoadjuvant Chemoradiotherapy in Patients with Borderline Resectable Pancreatic Ductal Adenocarcinoma. Ann Surg Oncol 2014;21:670-6. [Crossref] [PubMed]
- Tsuchikawa T, Hirano S, Tanaka E, et al. Novel aspects of preoperative chemoradiation therapy improving anti-tumor immunity in pancreatic cancer. Cancer Sci 2013;104:531-5. [Crossref] [PubMed]
- Goldstein JB, Chatterjee D, Zaid M, et al. 735PThe prognostic significance of infiltrating lymphocytes in resectable pancreatic ductal adenocarcinoma in untreated versus neoadjuvant treated patients. Ann Oncol 2017;28:mdx369.118.
- Hardacre JM, Mulcahy M, Small W, et al. Addition of algenpantucel-l immunotherapy to standard adjuvant therapy for pancreatic cancer: a phase 2 study. J Gastrointest Surg 2013;17:94-100; discussion 100-1. [Crossref] [PubMed]
- Le DT, Wang-Gillam A, Picozzi V, et al. Safety and Survival With GVAX Pancreas Prime and Listeria Monocytogenes–Expressing Mesothelin (CRS-207) Boost Vaccines for Metastatic Pancreatic Cancer. J Clin Oncol 2015;33:1325-33. [Crossref] [PubMed]
- Suzuki N, Hazama S, Iguchi H, et al. Phase II clinical trial of peptide cocktail therapy for patients with advanced pancreatic cancer: VENUS‐PC study. Cancer Sci 2017;108:73-80. [Crossref] [PubMed]
- Yamaue H, Tsunoda T, Tani M, et al. Randomized phase II/III clinical trial of elpamotide for patients with advanced pancreatic cancer: PEGASUS-PC Study. Cancer Sci 2015;106:883-90. [Crossref] [PubMed]
- Middleton G, Silcocks P, Cox T, et al. Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): an open-label, randomised, phase 3 trial. Lancet Oncol 2014;15:829-40. [Crossref] [PubMed]
- Lygidakis NJ, Sgourakis G, Georgia D, et al. Regional Targeting Chemoimmunotherapy in Patients Undergoing Pancreatic Resection in an Advanced Stage of Their Disease: A Prospective Randomized Study. Ann Surg 2002;236:806-13. [Crossref] [PubMed]
- Wainberg ZA, Hochster HS, George B, et al. Phase I study of nivolumab (nivo) + nab-paclitaxel (nab-P) ± gemcitabine (Gem) in solid tumors: Interim results from the pancreatic cancer (PC) cohorts. J Clin Oncol 2017;35:412. [Crossref] [PubMed]
- Robin TP, Goodman KA. Radiation therapy in the management of pancreatic adenocarcinoma: review of current evidence and future opportunities. Chin Clin Oncol 2017;6:28. [Crossref] [PubMed]
- Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and Dual Checkpoint Blockade Activates Non-Redundant Immune Mechanisms in Cancer. Nature 2015;520:373-7. [Crossref] [PubMed]
- Katz MHG, Varadhachary GR, Bauer TW, et al. Preliminary safety data from a randomized multicenter phase Ib/II study of neoadjuvant chemoradiation therapy (CRT) alone or in combination with pembrolizumab in patients with resectable or borderline resectable pancreatic cancer. J Clin Oncol 2017;35:4125.
- Seifert L, Werba G, Tiwari S, et al. Radiation Therapy Induces Macrophages to Suppress Immune Responses Against Pancreatic Tumors in Mice. Gastroenterology 2016;150:1659-72.e5. [Crossref] [PubMed]
- Zhu Y, Knolhoff BL, Meyer MA, et al. CSF1/CSF1R Blockade Reprograms Tumor-Infiltrating Macrophages and Improves Response to T-cell Checkpoint Immunotherapy in Pancreatic Cancer Models. Cancer Res 2014;74:5057-69. [Crossref] [PubMed]
- Aglietta M, Barone C, Sawyer MB, et al. A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann Oncol 2014;25:1750-5. [Crossref] [PubMed]
- Kalyan A, Kircher SM, Mohindra NA, et al. Ipilimumab and gemcitabine for advanced pancreas cancer: A phase Ib study. J Clin Oncol 2016;34:e15747.
- Casper ES, Green MR, Kelsen DP, et al. Phase II trial of gemcitabine (2,2'-difluorodeoxycytidine) in patients with adenocarcinoma of the pancreas. Invest New Drugs 1994;12:29-34. [Crossref] [PubMed]
- Carmichael J, Fink U, Russell RC, et al. Phase II study of gemcitabine in patients with advanced pancreatic cancer. Br J Cancer 1996;73:101-5. [Crossref] [PubMed]
- Nomi T, Sho M, Akahori T, et al. Clinical Significance and Therapeutic Potential of the Programmed Death-1 Ligand/Programmed Death-1 Pathway in Human Pancreatic Cancer. Clin Cancer Res 2007;13:2151-7. [Crossref] [PubMed]
- Weiss GJ, Waypa J, Blaydorn L, et al. A phase Ib study of pembrolizumab plus chemotherapy in patients with advanced cancer (PembroPlus). Br J Cancer 2017;117:33-40. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Gulley JL, Madan RA, Schlom J. Impact of tumour volume on the potential efficacy of therapeutic vaccines. Curr Oncol 2011;18:e150-7. [Crossref] [PubMed]
- Jaffee EM, Hruban RH, Biedrzycki B, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor–secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol 2001;19:145-56. [Crossref] [PubMed]
- Thomas AM, Santarsiero LM, Lutz ER, et al. Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med 2004;200:297-306. [Crossref] [PubMed]
- Laheru D, Lutz E, Burke J, et al. Allogeneic granulocyte macrophage colony-stimulating factor–secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res 2008;14:1455-63. [Crossref] [PubMed]
- NewLink Genetics Announces Results from Phase 3 IMPRESS Trial of Algenpantucel-L for Patients with Resected Pancreatic Cancer. NewLink Genetics Corporation AMES, Iowa. 2016. Available online: http://investors.linkp.com/releasedetail.cfm?releaseid=969978
- Wedén S, Klemp M, Gladhaug IP, et al. Long-term follow-up of patients with resected pancreatic cancer following vaccination against mutant K-ras. Int J Cancer 2011;128:1120-8. [Crossref] [PubMed]
- Asahara S, Takeda K, Yamao K, et al. Phase I/II clinical trial using HLA-A24-restricted peptide vaccine derived from KIF20A for patients with advanced pancreatic cancer. J Transl Med 2013;11:291. [Crossref] [PubMed]
- Gjertsen MK, Bakka A, Breivik J, et al. Ex vivo ras peptide vaccination in patients with advanced pancreatic cancer: results of a phase I/II study. Int J Cancer 1996;65:450-3. [Crossref] [PubMed]
- Toubaji A, Achtar M, Provenzano M, et al. Pilot study of mutant ras peptide-based vaccine as an adjuvant treatment in pancreatic and colorectal cancers. Cancer Immunol Immunother 2008;57:1413-20. [Crossref] [PubMed]
- Gjertsen MK, Buanes T, Rosseland AR, et al. Intradermal ras peptide vaccination with granulocyte-macrophage colony-stimulating factor as adjuvant: Clinical and immunological responses in patients with pancreatic adenocarcinoma. Int J Cancer 2001;92:441-50. [Crossref] [PubMed]
- Schmidt J, Abel U, Debus J, et al. Open-Label, Multicenter, Randomized Phase III Trial of Adjuvant Chemoradiation Plus Interferon Alfa-2b versus fluorouracil and folinic acid for patients with resected pancreatic adenocarcinoma. J Clin Oncol 2012;30:4077-83. [Crossref] [PubMed]
- Hecht JR, Naing A, Falchook G, et al. 744POverall survival and immunologic responses in metastatic pancreatic adenocarcinoma (PDAC) on PEGylated human IL-10 (AM0010) with 5-FU/LV and oxaliplatin (FOLFOX). Ann Oncol 2017;28:mdx369.127.
- Rahal A, Musher B. Oncolytic viral therapy for pancreatic cancer. J Surg Oncol 2017;116:94-103. [Crossref] [PubMed]
- Mulvihill S, Warren R, Venook A, et al. Safety and feasibility of injection with an E1B-55 kDa gene-deleted, replication-selective adenovirus (ONYX-015) into primary carcinomas of the pancreas: a phase I trial. Gene Therapy 2001;8:308. [Crossref] [PubMed]
- Mahalingam D, Goel S, Coffey M, et al. P-175Oncolytic Virus Therapy in Pancreatic Cancer: Clinical Efficacy and Pharmacodynamic Analysis of REOLYSIN in Combination with Gemcitabine in Patients with Advanced Pancreatic Adenocarcinoma. Ann Oncol 2015;26:iv51. [Crossref]
- Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 2013;5:177ra38. [Crossref] [PubMed]
- FDA news release: FDA approval brings first gene therapy to the United States. U.S. Food and Drug Administration. 2017. Available online: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm574058.htm. Accessed October 16 2017.
- Beatty GL, O’Hara M. Chimeric antigen receptor-modified T cells for the treatment of solid tumors: Defining the challenges and next steps Pharmacol Ther 2016;166:30-9. [Crossref] [PubMed]
- Beatty GL, Haas AR, Maus MV, et al. Mesothelin-specific Chimeric Antigen Receptor mRNA-Engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res 2014;2:112-20. [Crossref] [PubMed]
- Beatty GL, O'Hara MH, Nelson AM, et al. Safety and antitumor activity of chimeric antigen receptor modified T cells in patients with chemotherapy refractory metastatic pancreatic cancer. J Clin Oncol 2015;33:3007.
- Kondo H, Hazama S, Kawaoka T, et al. Adoptive immunotherapy for pancreatic cancer using MUC1 peptide-pulsed dendritic cells and activated T lymphocytes. Anticancer Res 2008;28:379-87. [PubMed]
- Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014;371:1039-49. [Crossref] [PubMed]
- Holter S, Borgida A, Dodd A, et al. Germline BRCA mutations in a large clinic-based cohort of patients with pancreatic adenocarcinoma. J Clin Oncol 2015;33:3124-9. [Crossref] [PubMed]
- Luo G, Lu Y, Jin K, et al. Pancreatic cancer: BRCA mutation and personalized treatment. Expert Rev Anticancer Ther 2015;15:1223-31. [Crossref] [PubMed]
- Chou A, Waddell N, Cowley MJ, et al. Clinical and molecular characterization of HER2amplified-pancreatic cancer. Genome Med 2013;5:78. [Crossref] [PubMed]
- Harder J, Ihorst G, Heinemann V, et al. Multicentre phase II trial of trastuzumab and capecitabine in patients with HER2 overexpressing metastatic pancreatic cancer. Br J Cancer 2012;106:1033-8. [Crossref] [PubMed]
- Geissler M, Hofheinz R, Moehler MH, et al. Trastuzumab and capecitabine in patients with HER2-expressing metastatic pancreatic cancer: A multicenter phase II study of the German AIO Pancreatic Cancer Group (AIO PK-0204). J Clin Oncol 2010;28:4070. [Crossref]
- Masetti M, Acquaviva G, Visani M, et al. Long-term survivors of pancreatic adenocarcinoma show low rates of genetic alterations in KRAS, TP53 and SMAD4. Cancer Biomark 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Mahalingam D, Giles F. Challenges in developing targeted therapy for pancreatic adenocarcinoma. Expert Opin Ther Targets 2008;12:1389-401. [Crossref] [PubMed]
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III Trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:1960-6. [Crossref] [PubMed]
- Cutsem EV, Vervenne WL, Bennouna J, et al. Phase III Trial of Bevacizumab in Combination With Gemcitabine and Erlotinib in Patients With Metastatic Pancreatic Cancer. J Clin Oncol 2009;27:2231-7. [Crossref] [PubMed]
- Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III Trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol 2010;28:3617-22. [Crossref] [PubMed]
- Philip PA, Benedetti J, Corless CL, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group–Directed Intergroup Trial S0205. J Clin Oncol 2010;28:3605-10. [Crossref] [PubMed]
- Spano JP, Chodkiewicz C, Maurel J, et al. Efficacy of gemcitabine plus axitinib compared with gemcitabine alone in patients with advanced pancreatic cancer: an open-label randomised phase II study. Lancet 2008;371:2101-8. [Crossref] [PubMed]
- Kindler HL, Ioka T, Richel DJ, et al. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol 2011;12:256-62. [Crossref] [PubMed]
- Gonçalves A, Gilabert M, François E, et al. BAYPAN study: a double-blind phase III randomized trial comparing gemcitabine plus sorafenib and gemcitabine plus placebo in patients with advanced pancreatic cancer. Ann Oncol 2012;23:2799-805. [Crossref] [PubMed]
- Rougier P, Riess H, Manges R, et al. Randomised, placebo-controlled, double-blind, parallel-group phase III study evaluating aflibercept in patients receiving first-line treatment with gemcitabine for metastatic pancreatic cancer. Eur J Cancer 2013;49:2633-42. [Crossref] [PubMed]
- Ott PA, Hodi FS, Kaufman HL, et al. Combination immunotherapy: a road map. J Immunother Cancer 2017;5:16. [Crossref] [PubMed]
- Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov 2015;14:561. [Crossref] [PubMed]
- Alagesan B, Contino G, Guimaraes AR, et al. Combined MEK and PI3K inhibition in a mouse model of pancreatic cancer. Clin Cancer Res 2015;21:396-404. [Crossref] [PubMed]
- Ebert PJR, Cheung J, Yang Y, et al. MAP Kinase Inhibition Promotes T Cell and Anti-tumor Activity in Combination with PD-L1 Checkpoint Blockade. Immunity 2016;44:609-21. [Crossref] [PubMed]
- Bendell JC, Kim TW, Goh BC, et al. Clinical activity and safety of cobimetinib (cobi) and atezolizumab in colorectal cancer (CRC). J Clin Oncol 2016;34:3502. [PubMed]
- Vonderheide RH, Bajor DL, Winograd R, et al. CD40 immunotherapy for pancreatic cancer. Cancer Immunol Immunother: CII 2013;62:949-54. [Crossref] [PubMed]
- Beatty GL, Li Y, Long KB. Cancer immunotherapy: activating innate and adaptive immunity through CD40 agonists. Expert Rev Anticancer Ther 2017;17:175-86. [Crossref] [PubMed]
- Vonderheide RH, Flaherty KT, Khalil M, et al. Clinical Activity and Immune Modulation in Cancer Patients Treated With CP-870,893, a Novel CD40 Agonist Monoclonal Antibody. J Clin Oncol 2007;25:876-83. [Crossref] [PubMed]
- Beatty GL, Chiorean EG, Fishman MP, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011;331:1612-6. [Crossref] [PubMed]
- Beatty GL, Torigian DA, Chiorean EG, et al. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res 2013;19:6286-95. [Crossref] [PubMed]
- Beatty GL, Winograd R, Evans RA, et al. Exclusion of T cells from pancreatic carcinomas in mice is regulated by Ly6C(low) F4/80(+) Extra-tumor Macrophages. Gastroenterology 2015;149:201-10. [Crossref] [PubMed]
- Phillips P. Pancreatic stellate cells and fibrosis. In: Grippo PJ, Munshi HG, editors. Pancreatic Cancer and Tumor Microenvironment. Trivandrum (India): Transworld Research Network, 2012.
- Komar HM, Serpa G, Kerscher C, et al. Inhibition of Jak/STAT signaling reduces the activation of pancreatic stellate cells in vitro and limits caerulein-induced chronic pancreatitis in vivo. Sci Rep 2017;7:1787. [Crossref] [PubMed]
- Koblish HK, Hansbury M, Wang LCS, et al. Abstract 1336: Novel immunotherapeutic activity of JAK and PI3Kδ inhibitors in a model of pancreatic cancer. Cancer Res 2015;75:1336. [Crossref]
- Hurwitz H, Cutsem EV, Bendell JC, et al. Two randomized, placebo-controlled phase 3 studies of ruxolitinib (Rux) + capecitabine (C) in patients (pts) with advanced/metastatic pancreatic cancer (mPC) after failure/intolerance of first-line chemotherapy: JANUS 1 (J1) and JANUS 2 (J2). J Clin Oncol 2017;35:343. [Crossref] [PubMed]
- Massó-Vallés D, Jauset T, Soucek L. Ibrutinib repurposing: from B-cell malignancies to solid tumors. Oncoscience 2016;3:147-8. [PubMed]
- Gunderson AJ, Kaneda MM, Tsujikawa T, et al. Bruton Tyrosine Kinase–Dependent Immune Cell Cross-talk Drives Pancreas Cancer. Cancer Discov 2016;6:270-85. [Crossref] [PubMed]
- Massó-Vallés D, Jauset T, Serrano E, et al. Ibrutinib exerts potent antifibrotic and antitumor activities in mouse models of pancreatic adenocarcinoma. Cancer Res 2015;75:1675-81. [Crossref] [PubMed]
- Borazanci EH, Hong DS, Gutierrez M, et al. Ibrutinib + durvalumab (MEDI4736) in patients (pts) with relapsed or refractory (R/R) pancreatic adenocarcinoma (PAC): A phase Ib/II multicenter study. J Clin Oncol 2016;34:TPS484. [Crossref]
- Cannarile MA, Weisser M, Jacob W, et al. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J Immunother Cancer 2017;5:53. [Crossref] [PubMed]
- Nywening TM, Wang-Gillam A, Sanford DE, et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol 2016;17:651-62. [Crossref] [PubMed]
- Sleightholm RL, Neilsen BK, Li J, et al. Emerging roles of the CXCL12/CXCR4 axis in pancreatic cancer progression and therapy. Pharmacol Ther 2017;179:158-70. [Crossref] [PubMed]
- Shen W, Tao GQ, Zhang Y, et al. TGF-β in pancreatic cancer initiation and progression: two sides of the same coin. Cell Biosci 2017;7:39. [Crossref] [PubMed]
- Yu Q, Sicinska E, Geng Y, et al. Requirement for CDK4 kinase function in breast cancer. Cancer Cell 2006;9:23-32. [Crossref] [PubMed]
- Goel S, DeCristo MJ, Watt AC, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 2017;548:471. [Crossref] [PubMed]
- Finn RS, Martin M, Rugo HS, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med 2016;375:1925-36. [Crossref] [PubMed]
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N Engl J Med 2016;375:1738-48. [Crossref] [PubMed]
- Cowan RW, Maitra A. Genetic progression of pancreatic cancer. Cancer J 2014;20:80-4. [Crossref] [PubMed]
- Witkiewicz AK, Borja NA, Franco J, et al. Selective impact of CDK4/6 suppression on patient-derived models of pancreatic cancer. Oncotarget 2015;6:15788-801. [Crossref] [PubMed]
- Hidalgo M, Menendez C, Yuan J, et al. Abstract A42: Palbociclib potentiates nab-paclitaxel efficacy in pancreatic ductal adenocarcinoma. Mol Cancer Ther 2015;14:A42. [Crossref]
- Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene 2017;36:1461. [Crossref] [PubMed]
- Clements WM, Wang J, Sarnaik A, et al. beta-Catenin mutation is a frequent cause of wnt pathway activation in gastric cancer. Cancer Res 2002;62:3503-6. [PubMed]
- Laurent-Puig P, Legoix P, Bluteau O, et al. Genetic alterations associated with hepatocellular carcinomas define distinct pathways of hepatocarcinogenesis. Gastroenterology 2001;120:1763-73. [Crossref] [PubMed]
- Boulter L, Guest RV, Kendall TJ, et al. WNT signaling drives cholangiocarcinoma growth and can be pharmacologically inhibited. J Clin Invest 2015;125:1269-85. [Crossref] [PubMed]
- Zeng G, Germinaro M, Micsenyi A, et al. Aberrant Wnt/β-Catenin Signaling in Pancreatic Adenocarcinoma. Neoplasia 2006;8:279-89. [Crossref] [PubMed]
- Jiang X, Hao HX, Growney JD, et al. Inactivating mutations of RNF43 confer Wnt dependency in pancreatic ductal adenocarcinoma. Proc Natl Acad Sci U S A 2013;110:12649-54. [Crossref] [PubMed]
- Pai SG, Carneiro BA, Mota JM, et al. Wnt/beta-catenin pathway: modulating anticancer immune response. J Hematol Oncol 2017;10:101. [Crossref] [PubMed]


