Overexpressed genes in malignant pleural mesothelioma: implications in clinical management
Overexpressed genes identified in malignant pleural mesothelioma (MPM)
Global gene expression profiling studies have allowed quantifying gene expression in cancer cell lines as well as in human tissues, leading to the identification of several overexpressed genes in MPM. In recent years, a number of aberrantly expressed genes were suggested, but with a poor consistency among studies. In 2012, in order to find a consensus among nine independent transcriptome studies carried out between 2000 and 2010 (1-9), it has been performed a review and a data mining (10) and it has been identified a group of 96 overexpressed genes in MPM compared to non-malignant tissues and cell lines. This group of genes have been further evaluated in 2015 through a gene expression study performed on an independent cohort of mesothelioma tissues compared to non-malignant tissues: the overexpression of 51 genes (Table 1) have been confirmed (11).

Full table
Clinical implications of overexpressed genes in MPM
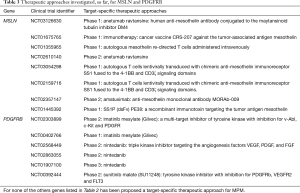
The identification of the overexpressed genes may lead to a deeper knowledge of the main pathways involved in mesothelioma carcinogenesis and could reveal also novel prognostic and diagnostic marker. Among the 51 overexpressed genes identified, only 14 have been further studied in MPM and they have been proposed as predictor of survival in mesothelioma patients or as marker able to differentiate MPM from other types of cancers. Moreover, they have been proposed as potential therapeutic targets: several in vitro and in vivo studies reported their role in MPM progression and carcinogenesis, showing a decrement of the malignant phenotype after target-inhibition. Here are summarized the last findings describing the clinical implications of these 14 overexpressed genes in MPM (Table 2).

Full table
The gene MSLN codifies a preproprotein that is proteolytically processed to generate two protein products, megakaryocyte potentiating factor and mesothelin. Normal mesothelial cells show low levels of MSLN and it is undetectable in most normal tissues. On the contrary, mesothelin is overexpressed in several human cancers, including MPM (10,11). In the last years mesothelin has become an active topic of investigation in MPM. It has been proposed as a promising candidate for tumour-specific therapy, given its limited expression in normal tissues and high expression in mesothelioma tissues (31). Three main anti-mesothelin therapeutic strategies have been developed including the monoclonal antibody amatuximab (MORAb-009), an antibody-drug conjugates with the fully human anti-mesothelin antibody (for example the anetumab ravtansine), and recombinant immunotoxins. Although there were no objective tumour response to amatuximab, it was well tolerated and disease stabilization was observed in some patients (33,37). In a phase II study the addiction of amatuximab to cisplatin and pemetrexed did not prolong progression free survival longer than historical controls, but an extension of the median overall survival (OS) was observed (34). A phase II double-blind study, which involved 49 sites, is still ongoing (clinicaltrials.gov NCT02357147). The antibody-drug conjugate anetumab ravtansine selectively binds to mesothelin allowing the internalization of the conjugated tubulin inhibitor DM4 into MPM cells. Recently a phase I study showed that 31% of patients treated with anetumab ravtansine had a partial response and 44% of patients have stable disease for an overall disease control rate of 75% (38). A phase II trial in 2nd-line metastatic pleural mesothelioma (clinicaltrials.gov NCT02610140) is still ongoing. Two recombinant immunotoxins have been engineered for the targeted elimination of cancer cells that express mesothelin: SS1P and RG7787 (39). The efficacy of these agents have been demonstrated in vitro (32,36) and this preclinical potential has been confirmed in 24 MPM patients chemotherapy-naïve, treated with SS1P in combination with standard recommend doses of cisplatin/pemetrexed (35).
Worth to note that, so far, mesothelin is the only Food and Drug Administration (FDA) approved (HDE id: H060004) biomarker for MPM (25). In 2007, serum mesothelin has been reported, for the first time, as a prognostic marker in MPM: high soluble mesothelin level significantly correlates with shorter survival, leading to a poor prognosis (12). Its prognostic value has been strongly debated: many following studies confirmed this association (13-15) but others did not endorse serum mesothelin prognostic significance (26,87). While a recent meta-analysis performed on 579 MPM patients further corroborates the inverse association of serum SMRP concentration with the OS (16). The mixed results observed are due to the small samples size and to the heterogeneity of the treatment among the studies and lead to the necessity of further investigations.
In 2003 serum mesothelin was declared, for the first time, as a diagnostic marker for MPM (17) and it resulted strongly associated with tumour volume (18,20). Serum soluble mesothelin related peptides (SMRPs) levels have been proposed for the differentiation of MPM patients from patients with pleural metastases of different types of carcinomas (18,19). The diagnostic accuracy of mesothelin has been evaluated in several studies (21,23-25) and the systematic review and meta-analysis recently performed showed a high specificity (around 89% and 96%) but a low sensitivity (between 32% and 47%) of mesothelin as a diagnostic marker; thus although SMRPs may help to discriminate the MPM from the non-MPM subjects, the sensitivity of the assay is still inadequate (88). In order to better characterize the sensitivity and specificity of SMRP as a biomarker for MPM it should take in consideration that SMRP performance as diagnostic biomarker could be influenced by genetics variants, as show in the recent work by De Santi et al. (30) and SNP located in the promoter or in the 3’UTR of MSLN gene could affect protein expression levels (89). SMRPs diagnostic value has been evaluated also in pleural effusion (25,26,29) and it has been observed a higher diagnostic performance in pleural effusion than in serum assessment (22). The need to detect the MPM at the early stages led several authors to investigate whether mesothelin can contribute towards the evaluation of the carcinogenic risk in populations exposed to asbestos: high level of SMRPs have been proposed as a marker for early diagnosis in combination with two epigenetic marker (27) or alone (28). Interestingly higher levels of SMRP have been found in the asbestos exposure group than in the control group and the increment observed was gradual among the controls, the asbestos exposed and mesothelioma patients (28).
PDGFRB encodes for a cell surface tyrosine kinase receptor for the platelet-derived growth factor beta; this receptor specifically binds the B isoform of PDGF (PDGF-BB). Preferentially MPM cell lines express PDGF beta-chain and PDGF beta-receptor transcripts, whereas normal mesothelial cell lines do not express PDGF B-chain mRNA and little or no PDGF beta-receptor mRNA; in contrast normal mesothelial cell lines were found to express PDGF alpha-receptor mRNA, not detected in mesothelioma cell lines and in non-neoplastic mesothelium (90,91). PDGFRB has been recognized as an attractive therapeutic target for several cancers (92) due to its involvement in increased proliferation, dissemination and metastasis of cancer cells (93-95) and to its overexpression in cancer cells and tissues, as compared to the non-malignant counterpart (94-96). For this reason, a plethora of PDGF/PDGFR pathway inhibitors have been developed in the last years and assayed in clinical trials for leukemia, gastrointestinal stromal tumors, and glioma (
Baculoviral IAP repeat-containing 5 (BIRC5) encodes the well-known protein survivin. Survivin overexpression in MPM has been confirmed in different independent cohort of mesothelioma patients (7-9,11,47,53). Survivin is a multifunctional protein that plays critical roles in several crucial cell processes: several studies described its anti-apoptotic function (49,98,99), a role in microtubule dynamics, in cell proliferation, in cell migration and control bipolar spindle formation (100). It has been demonstrated that BIRC5 inhibition in mesothelioma cells decreases cell growth and enhances the rate of spontaneous and drug-induced apoptosis (53), induces mitotic cell arrest and strong cytoxicity (51). For these reasons, in the last years, several research groups attempt the development of survivin-based cancer therapeutics (51,56). Interestingly the vaccine strategy proposed in 2013 (55) effectively suppresses MPM tumor growth in vivo without induction of autoimmune response and the cytotoxic activity induced by this vaccine proved to be specific for MPM cells (57). Furthermore survivin expression proved to be linked to resistance to chemotherapeutic agents, including vincristine, cisplatin, bortezomib, tamoxifen, paclitaxel, TNF-a and TRAIL in tumour cells (52,54) and it is also responsible of the suppression of radiation-induced apoptosis (50). Thus survivin-targeting in combination with anti-cancer drug could be useful to enhance the effect of chemotherapeutic agents and may be promising for mesothelioma treatment. Survivin expression alone did not seem to show any prognostic value but it has been proposed as a predictive marker of treatment response (47). A more recent study confirmed these results reporting that serum survivin levels before and during chemotherapy could be useful in the prediction of MPM treatment response (48).
CALB2 gene encodes an intracellular calcium-binding protein called calretinin. Several studies on the value of this marker were published since 1996 (63). Among the current immunomarkers, calretinin appear to be the most valuable in differentiating MPM from lung and breast adenocarcinoma (64). Calretinin expression resulted highly specific for MPM in fact 97% of mesothelioma samples compared to only 3% of adenocarcinomas were positive for calretinin (66). This specificity has been confirmed in independent sets of mesothelioma samples (67,68) and calretinin proved to be 96% sensitive and 100% specific (P<0.01) for identifying mesothelial differentiation (mesothelioma and benign reactive effusions) from adenocarcinoma (69). Calretinin is considered the most sensitive and specific positive mesothelioma marker, above all for epithelioid mesothelioma subtype (65). Also the prognostic role of calretinin has been explored: in several studies higher calretinin expression was observed in tumours with more favourable prognosis (58-61). Lately the correlation of increased calretinin expression with a better survival has been confirmed and its higher expression has been also associated with epithelioid histology subtype (62).
The protein encoded by MKI67 gene is widely used as a marker of proliferation in routine pathological investigations and the nuclear protein Ki67 is an established prognostic marker in cancer (101-105). The prognostic value of Ki67 in mesothelioma is known since 1998 when it has been reported a statistically significant difference between the survival of patients having a low and high Ki67 index (P<0.001) (106); subsequently other studies confirmed its prognostic significance (70,71). Recently it has been reported that patients with high Ki67 expression had significantly (P<0.001) shorter median OS (7.5 months) than those with low Ki67 (19.1 months) (72). In particular Ki67 proved to be an independent prognostic factor in epithelioid but not in non-epithelioid MPM (72). Interestingly ki67 index was lower in patients who received the induction chemotherapy (n=33, mean Ki67 index: 10.5±8.5) as compared to patients who had not received chemotherapy before sample collection (n=124, mean Ki67 index: 18.3±13.9, P<0.001), giving evidence of decrease of the MPM proliferative capacity after induction chemotherapy (72). Ki67 may be also a promising molecular candidate for the diagnosis of MPM: used in combination with repp86 (also called TPX2, Microtubule Nucleation Factor) showed a significant ability in differentiating MPM from benign reactive mesothelial hyperplasia (73).
KIF23 overexpression has been recently confirmed in a tissue microarray of 53 mesothelioma samples and a shorter overall survival has been observed in patients who received curative resection with tumors displaying high KIF23 expression (P=0.0194 by a log-rank test) (74). This suggests a potential value as a prognostic marker that needs to be validated in a different set of mesothelioma patients, due to the small sample size in this study. KIF23 gene encodes for a member of kinesin protein involved in the regulation of cytokinesis (107) and its inhibition suppresses midbody formation, hence the completion of cytokinesis (108) hampering cancer cells proliferation in vitro and in vivo (109). The critical role of this gene in proliferation and survival of mesothelioma cells suggests the possibility to consider this gene in the development of future therapeutic approaches in mesothelioma.
PKM2 gene encodes for a pyruvate kinase involved in glycolysis and many studies report its role in the achievement of the nutrient demands of proliferating cancer cells (110,111). RNA inhibition on mesothelioma cell lines did not produce significant change in apoptosis and mitosis (112) thus its overexpression in mesothelioma does not seem to influence the progression of this cancer. In 2009 PKM2 has been proposed as a predictor of MPM outcome in a four-gene expression ratio test: this test resulted able to predict survival in multivariable analysis [hazard ratio for death =2.09; 95% confidence interval (CI), 1.27–3.45; P=0.004] (75).
THBS2 has been found overexpressed in 3 independent transcriptome studies on mesothelioma tissues (10) and, previously, the titers of the antibody against THBS2 has been proposed as a tumor marker for the diagnosis and follow up of patients with MPM. THBS2 antibody has been detected in the 88.9% of the mesothelioma patients sera analysed and interestingly, the serum antibody titers decreased after surgical treatment of MPM and increased after recurrence of the disease (76).
RAN gene encodes for a small GTP binding protein that is abundantly expressed in many human cancers; its overexpression in mesothelioma has been observed in three independent transcriptome studies. RAN overexpression could be involved in the development of chemoresistance, as suggested by Roe (9): in particular RAN has been suggested as an antitubulin (the taxanes and vinca alkaloids) resistance related gene. Until now little is known about its role in malignant pleural mesothelioma but emerging evidences show that RAN signalling is a dominant pathway for tumour cell maintenance (77,78) and it has been proposed as a novel drug co-target against mesothelioma (9).
The gene CHEK1 encodes for a kinase required for checkpoint mediated cell cycle arrest in response to DNA damage or in the presence of unreplicated DNA. CHEK1 expression in MPM samples has been found correlated with tumour progression (P=0.0362) and appear to be a predictive marker for platin-response in the adjuvant-treated patients, showing a significant correlation to the overall survival (P=0.0162) (79). Moreover CHEK1 expression seems to be involved in chemoresistance (113): loss of CHEK1 enhances camptotecins toxicity and sensitized mesothelioma cell-lines for pemetrexed (114) suggesting that CHEK1 could be a putative co-drug target for mesothelioma (9). Worth to note is that CHEK1 appears to be selectively expressed in mesothelioma cells (9) and not expressed in most of the normal tissues (115), making it a suitable target for mesothelioma treatment.
HEG1 gene codifies for a mucin-like membrane protein, the sialylated protein HEG homolog 1 that recently proved to be a highly specific marker for MPM (80). The monoclonal antibody used for the identification of HEG1 protein (SKM9-2), is able to recognize both HEG1 peptide and its sialylated O-glycosylation and reached 99% of specificity and 92% of sensitivity in 130 MPM cases compared to 310 cases of non-mesothelioma tumours. These characteristics make SKM9-2 antibody a suitable marker for pathological diagnosis (80). HEG1 silencing on mesothelioma cell-lines revealed a survival role of this gene in MPM suggesting HEG1 as a novel putative therapeutic target (80).
ASS1 encodes for the argininosuccinate synthase 1 that catalyzes the penultimate step of the arginine biosynthetic pathway. ASS1 loss, observed in different cancers, results in an intrinsic dependence on extracellular arginine due to an inability to synthesise arginine for growth. For this reason arginine deprivation has been proposed as a promising therapeutic strategy in ASS1-negative tumours. In the multicenter phase 2 randomized clinical trial, conducted between March 2011 and May 2013, has been observed that arginine deprivation with ADI-PEG20 in ASS1-negative mesothelioma patients improved the progression free survival (116). About 50% of mesothelioma appeared to be ASS1-negative (117) but ASS1 has been found overexpressed in a significant number of mesothelioma patients (10,11,82). ASS1 overexpression is linked with chloroquine and cisplatin resistance in mesothelioma: high ASS1 expression in mesothelioma cells decreased their sensitivity to chloroquine toxicity (118) and ASS1 silencing in MPM spheroids increased the apoptotic response to cisplatin plus pemetrexed (82), rather suggesting an important contribution to the onset of chemo-resistance in MPM.
EFEMP1 codifies for epidermal growth factor containing fibulin like extracellular matrix protein 1, best known as Fibulin-3. It has been found overexpressed in MPM (81) and it is expressed at low levels in normal tissues (83). In 2012 Fibulin-3 has been declared, for the first time, a highly promising diagnostic and prognostic marker for MPM, able to distinguish MPM patients from asbestos-exposed persons without MPM with 94% specificity and 100% sensitivity (83). Surprisingly in the external validation cohort, the diagnostic accuracy decreased (AUC, 0.87) (119) but later, in the study performed by Creaney et al. (25) pleural effusion fibulin-3 was proven a more potent prognostic marker than mesothelin. Afterwards the potential prognostic value of pleural effusion FBLN3 has been confirmed in two independent mesothelioma cohorts (120). The first evidence of the diagnostic value of fibulin-3 appeared in 2012 (83), but in 2014 these data were not confirmed and soluble mesothelin related peptide was declared a more potent diagnostic marker in serum than fibulin-3 (25). In 2015 its diagnostic value has been claimed again with the observation that MPM patients had significantly higher serum levels of fibulin-3 than controls (84). Two subsequent studies in 2015 and 2017, did not confirmed this result and declared FBLN3 detection in pleural effusion not useful as a biomarker for the diagnosis of MPM (29). The discordant results obtained until now do not lead to a final judgement on the relation between fibulin-3 and MPM, thus more studies are needed to clarify its clinical significance.
CDC2 codifies for a cyclin dependent kinase 1 (CDK1) and it has been found overexpressed in mesothelioma in three independent transcriptome studies (10). CDK1 knockdown could provide a novel therapeutic approach to arrest cell-cycle progression in MPM cells: in 2009 it has been observed that CDC2 silencing increased the apoptotic fraction of MPM cells (85) and in 2014, the RNA-interference screening performed on MPM cell-lines confirmed its role in cell-cycle and in the apoptosis (86). CDC2 inhibition with roscovitine reduced MPM cells growth and sensitised cells to cisplatin by 2.5 to 4 fold (86). These results revealed an interesting therapeutic potential of this target, alone or in combination with cisplatin-based therapy.
Conclusions
MPM is a highly aggressive disease with a poor prognosis. In the last years many efforts have been done to find new therapeutic strategies and to improve the clinical management of MPM through the detection of new potential markers. The identification of overexpressed genes is an important starting point for the comprehension of the main pathways involved in MPM carcinogenesis and progression, that may help to achieve a customized therapy in MPM (Table 3). The development of antibody-conjugated drugs or of monoclonal antibody and inhibitors, that directly suppress the activity of the overexpressed genes, is an attractive therapeutic approach (Table 3). Several biomarkers of diagnostic and prognostic significance have been analyzed but, to date, there are no solidly established markers for MPM. At the present time, among the selected genes described in this review, MSLN seems to be the most promising and unique FDA approved, marker for MPM.
Full table
The prognostic and diagnostic value of some of these genes (i.e., fibulin3) is still inconclusive due to the conflicting results observed; to overcome this problem it could be useful to analyze, with the same method and using the same patient stratification, the expression of these candidate markers in a larger sample size cohort. Due to the heterogeneity of the disease, it could be more effective the evaluation of a panel of markers instead of one independent marker, as recently suggested for the differential diagnosis of MPM (121). Moreover, the identification of reliable markers for early diagnosis of asymptomatic MPM is a very interesting research field because advances in therapy for patients with MPM may result in an improved outcome if they are applied to stage I disease.
It is plausible that a combination of the most relevant markers validated by the ongoing studies will allow a more specific MPM diagnosis and earlier detection in the next future.
The knowledge of driver genes of mesothelioma tumorigenesis and of the molecular interaction between the overexpressed genes hopefully will result in a personalized and more effective therapeutic approach in which several target-specific agents will be combined with chemotherapeutic regimes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rihn BH, Mohr S, McDowell SA, et al. Differential gene expression in mesothelioma. FEBS Lett 2000;480:95-100. [Crossref] [PubMed]
- Kettunen E, Nissen AM, Ollikainen T, et al. Gene expression profiling of malignant mesothelioma cell lines: cDNA microarray study. Int J Cancer 2001;91:492-6. [Crossref] [PubMed]
- Singhal S, Wiewrodt R, Malden LD, et al. Gene expression profiling of malignant mesothelioma. Clin Cancer Res 2003;9:3080-97. [PubMed]
- Hoang CD, D’Cunha J, Kratzke MG, et al. Gene expression profiling identifies matriptase overexpression in malignant mesothelioma. Chest 2004;125:1843-52. [Crossref] [PubMed]
- Mohr S, Keith G, Galateau-Salle F, et al. Cell protection, resistance and invasiveness of two malignant mesotheliomas as assessed by 10K-microarray. Biochim Biophys Acta 2004;1688:43-60.
- Gordon GJ, Rockwell GN, Jensen RV, et al. Identification of novel candidate oncogenes andtuomor suppressors in malignant pleural mesothelioma using large-scale transcriptional profiling. Am J Pathol 2005;166:1827-40. [Crossref] [PubMed]
- Crispi S, Calogero RA, Santini M, et al. Global gene expression profiling of human pleural mesotheliomas: identification of matrix metalloproteinase 14 (MMP-14) as potential tumor target. PLoS One 2009;4:e7016. [Crossref] [PubMed]
- Røe OD, Anderssen E, Helge E, et al. Genome-wide profile of pleural mesothelioma versus parietal and visceral pleura: the emerging gene portrait of the mesothelioma phenotype. PLoS One 2009;4:e6554. [Crossref] [PubMed]
- Røe OD, Anderssen E, Sandeck H, et al. Malignant pleural mesothelioma: genome-wide expression patterns reflecting general resistance mechanisms and a proposal of novel targets. Lung Cancer 2010;67:57-68. [Crossref] [PubMed]
- Melaiu O, Cristaudo A, Melissari E, et al. A review of transcriptome studies combined with data mining reveals novel potential markers of malignant pleural mesothelioma. Mutat Res 2012;750:132-40. [Crossref] [PubMed]
- Melaiu O, Melissari E, Mutti L, et al. Expression status of candidate genes in mesothelioma tissues and cell lines. Mutat Res 2015;771:6-12. [Crossref] [PubMed]
- Cristaudo A, Foddis R, Vivaldi A, et al. Clinical significance of serum mesothelin in patients with mesothelioma and lung cancer. Clin Cancer Res 2007;13:5076-81. [Crossref] [PubMed]
- Grigoriu BD, Scherpereel A, Devos P, et al. Utility of osteopontin and serum mesothelin in malignant pleural mesothelioma diagnosis and prognosis assessment. Clin Cancer Res 2007;13:2928-35. [Crossref] [PubMed]
- Schneider J, Hoffmann H, Dienemann H, et al. Diagnostic and prognostic value of soluble mesothelin-related proteins in patients with malignant pleural mesothelioma in comparison with benign asbestosis and lung cancer. J Thorac Oncol 2008;3:1317-24. [Crossref] [PubMed]
- Linch M, Gennatas S, Kazikin S, et al. A serum mesothelin level is a prognostic indicator for patients with malignant mesothelioma in routine clinical practice. BMC Cancer 2014;14:674. [Crossref] [PubMed]
- Tian L, Zeng R, Wang X, et al. Prognostic significance of soluble mesothelin in malignant pleural mesothelioma: a meta-analysis. Oncotarget 2017;8:46425-35. [Crossref] [PubMed]
- Robinson BW, Creaney J, Lake R, et al. Mesothelin-family proteins and diagnosis of mesothelioma. Lancet 2003;362:1612-6. [Crossref] [PubMed]
- Hassan R, Remaley AT, Sampson ML, et al. Detection and quantitation of serum mesothelin, a tumor marker for patients with mesothelioma and ovarian cancer. Clin Cancer Res 2006;12:447-53. [Crossref] [PubMed]
- Scherpereel A, Grigoriu B, Conti M, et al. Soluble mesothelin-related peptides in the diagnosis of malignant pleural mesothelioma. Am J Respir Crit Care Med 2006;173:1155-60. [Crossref] [PubMed]
- Creaney J, Francis RJ, Dick IM, et al. Serum soluble mesothelin concentrations in malignant pleural mesothelioma: relationship to tumor volume, clinical stage and changes in tumor burden. Clin Cancer Res 2011;17:1181-9. [Crossref] [PubMed]
- Creaney J, Sneddon S, Dick IM, et al. Comparison of the diagnostic accuracy of the MSLN gene products, mesothelin and megakaryocyte potentiating factor, as biomarkers for mesothelioma in pleural effusions and serum. Dis Markers 2013;35:119-27. [Crossref] [PubMed]
- Ferro P, Canessa PA, Battolla E, et al. Mesothelin is more useful in pleural effusion than in serum in the diagnosis of pleural mesothelioma. Anticancer Res 2013;33:2707-13. [PubMed]
- Bayram M, Dongel I, Akbaş A, et al. Serum biomarkers in patients with mesothelioma and pleural plaques and healthy subjects exposed to naturally occurring asbestos. Lung 2014;192:197-203. [Crossref] [PubMed]
- Felten MK, Khatab K, Knoll L, et al. Changes of mesothelin and osteopontin levels over time in formerly asbestos-exposed power industry workers. Int Arch Occup Environ Health 2014;87:195-204. [Crossref] [PubMed]
- Creaney J, Dick IM, Meniawy TM, et al. Comparison of fibulin-3 and mesothelin as markers in malignant mesothelioma. Thorax 2014;69:895-902. [Crossref] [PubMed]
- Creaney J, Dick IM, Robinson BW. Comparison of mesothelin and fibulin-3 in pleural fluid and serum as markers in malignant mesothelioma. Curr Opin Pulm Med 2015;21:352-6. [Crossref] [PubMed]
- Santarelli L, Staffolani S, Strafella E, et al. Combined circulating epigenetic markers to improve mesothelin performance in the diagnosis of malignant mesothelioma. Lung Cancer 2015;90:457-64. [Crossref] [PubMed]
- Demir M, Kaya H, Taylan M, et al. Evaluation of new biomarkers in the prediction of malignant mesothelioma in subjects with environmental asbestos exposure. Lung 2016;194:409-17. [Crossref] [PubMed]
- Battolla E, Canessa PA, Ferro P, et al. Comparison of the Diagnostic Performance of Fibulin-3 and Mesothelin in Patients with Pleural Effusions from Malignant Mesothelioma. Anticancer Res 2017;37:1387-91. [Crossref] [PubMed]
- De Santi C, Pucci P, Bonotti A, et al. Mesothelin promoter variants are associated with increased soluble mesothelin-related peptide levels in asbestos-exposed individuals. Occup Environ Med 2017;74:456-63. [Crossref] [PubMed]
- Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res 2004;10:3937-42. [Crossref] [PubMed]
- Zhang Y, Xiang L, Hassan R, et al. Pastan I Synergistic antitumor activity of taxol and immunotoxin SS1P in tumor-bearing mice. Clin Cancer Res 2006;12:4695-701. [Crossref] [PubMed]
- Hassan R, Cohen SJ, Phillips M, et al. Phase I clinical trial of the chimeric anti-mesothelin monoclonal antibody MORAb-009 in patients with mesothelin-expressing cancers. Clin Cancer Res 2010;16:6132-8. [Crossref] [PubMed]
- Hassan R, Kindler HL, Jahan T, et al. Phase II clinical trial of amatuximab, a chimeric antimesothelin antibody with pemetrexed and cisplatin in advanced unresectable pleural mesothelioma. Clin Cancer Res 2014;20:5927-36. [Crossref] [PubMed]
- Hassan R, Sharon E, Thomas A, et al. Phase 1 study of the antimesothelin immunotoxin SS1P in combination with pemetrexed and cisplatin for front-line therapy of pleural mesothelioma and correlation of tumor response with serum mesothelin, megakaryocyte potentiating factor, and cancer antigen 125. Cancer 2014;120:3311-9. [Crossref] [PubMed]
- Hollevoet K, Mason-Osann E, Liu XF, et al. In vitro and in vivo activity of the low-immunogenic antimesothelin immunotoxin RG7787 in pancreatic cancer. Mol Cancer Ther 2014;13:2040-9. [Crossref] [PubMed]
- Fujisaka Y, Kurata T, Tanaka K, et al. Phase I study of amatuximab, a novel monoclonal antibody to mesothelin, in Japanese patients with advanced solid tumors. Invest New Drugs 2015;33:380-8. [Crossref] [PubMed]
- Blumenschein George R., Hassan Raffit, et al. Phase I study of anti-mesothelin antibody drug conjugate anetumab ravtansine (AR). J Clin Oncol 2016;34:2509. [PubMed]
- Zhao XY, Subramanyam B, Sarapa N, et al. Novel Antibody Therapeutics Targeting Mesothelin In Solid Tumors. Clin Cancer Drugs 2016;3:76-86. [Crossref] [PubMed]
- Buikhuisen WA, Scharpfenecker M, Griffioen AW, et al. Randomized Phase II Study Adding Axitinib to Pemetrexed-Cisplatin in Patients with Malignant Pleural Mesothelioma: A Single-Center Trial Combining Clinical and Translational Outcomes. J Thorac Oncol 2016;11:758-68. [Crossref] [PubMed]
- Bertino P, Porta C, Barbone D, et al. Preliminary data suggestive of a novel translational approach to mesothelioma treatment: imatinib mesylate with gemcitabine or pemetrexed. Thorax 2007;62:690-5. [Crossref] [PubMed]
- Buckstein R, Meyer RM, Seymour L, et al. Phase II testing of sunitinib: the National Cancer Institute of Canada Clinical Trials Group IND Program Trials IND.182-185. Curr Oncol 2007;14:154-61. [Crossref] [PubMed]
- Bertino P, Piccardi F, Porta C, et al. Imatinib mesylate enhances therapeutic effects of gemcitabine in human malignant mesothelioma xenografts. Clin Cancer Res 2008;14:541-8. [Crossref] [PubMed]
- Laurie SA, Gupta A, Chu Q, et al. Brief report: a phase II study of sunitinib in malignant pleural mesothelioma. the NCIC Clinical Trials Group. J Thorac Oncol 2011;6:1950-4. [Crossref] [PubMed]
- Tsao AS, Harun N, Lee JJ, et al. Phase I trial of cisplatin, pemetrexed, and imatinib mesylate in chemonaive patients with unresectable malignant pleural mesothelioma. Clin Lung Cancer 2014;15:197-201. [Crossref] [PubMed]
- Melaiu O, Catalano C, De Santi C, et al. Inhibition of the platelet-derived growth factor receptor beta (PDGFRB) using gene silencing, crenolanib besylate, or imatinib mesylate hampers the malignant phenotype of mesothelioma cell lines. Genes Cancer 2017;8:438-52. [PubMed]
- Hmeljak J, Erčulj N, Dolžan V, et al. Is survivin expression prognostic or predictive in malignant pleural mesothelioma? Virchows Arch 2013;462:315-21. [Crossref] [PubMed]
- Goričar K, Kovač V, Franko A, et al. Serum Survivin Levels and Outcome of Chemotherapy in Patients with Malignant Mesothelioma. Dis Markers 2015;2015:316739.
- Xia C, Xu Z, Yuan X, et al. Induction of apoptosis in mesothelioma cells by antisurvivin oligonucleotides. Mol Cancer Ther 2002;1:687-94. [PubMed]
- Rödel C, Haas J, Groth A, et al. Spontaneous and radiation-induced apoptosis in colorectal carcinoma cells with different intrinsic radiosensitivities: Survivin as a radioresistance factor. Int J Radiat Oncol Biol Phys 2003;55:1341-7. [Crossref] [PubMed]
- Zhu ZB, Makhija SK, Lu B, et al. Targeting mesothelioma using an infectivity enhanced survivin-conditionally replicative adenoviruses. J Thorac Oncol 2006;1:701-11. [PubMed]
- Zhang MC, Hu CP, Chen Q. Effect of down-regulation of survivin gene on apoptosis and cisplatin resistance in cisplatin resistant human lung adenocarcinoma A549/CDDP cells. Zhonghua Zhong Liu Za Zhi 2006;28:408-12. [PubMed]
- Zaffaroni N, Costa A, Pennati M, et al. Survivin is highly expressed and promotes cell survival in malignant peritoneal mesothelioma. Cell Oncol 2007;29:453-66. [PubMed]
- Cheung CH, Sun X, Kanwar JR, et al. A cell-permeable dominant-negative survivin protein induces apoptosis and sensitizes prostate cancer cells to TNF alpha therapy. Cancer Cell Int 2010;10:36. [Crossref] [PubMed]
- Bertino P, Panigada M, Soprana E, et al. Fowlpox-based survivin vaccination for malignant mesothelioma therapy. Int J Cancer 2013;133:612-23. [Crossref] [PubMed]
- Soleimanpour E, Babaei E. Survivin as a Potential Target for Cancer Therapy. Asian Pac J Cancer Prev 2015;16:6187-91. [Crossref] [PubMed]
- Hoffmann PR, Panigada M, Soprana E, et al. Preclinical development of HIvax: Human survivin highly immunogenic vaccines. Hum Vaccin Immunother 2015;11:1585-95. [Crossref] [PubMed]
- Takeshima Y, Inai K, Ishikawa Y, et al. The trial of differentiation grading of epithelioid mesothelioma with reference to its clinicopathological significance. The trial of differentiation grading of epithelioid mesothelioma with reference to its clinicopathological significance (Abstr P10–1). In: 10th International Conference of the International Mesothelioma Interest Group, 2010;175. Available online: http://imig.org/wp-content/uploads/2011/01/IMIG2010Abstractfinal101227.pdf
- Kao SC, Lee K, Armstrong NJ, et al. Validation of tissue microarray technology in malignant pleural mesothelioma. Pathology 2011;43:128-32. [Crossref] [PubMed]
- Kao SC, Klebe S, Henderson DW, et al. Low calretinin expression and high neutrophil-to-lymphocyte ratio are poor prognostic factors in patients with malignant mesothelioma undergoing extrapleural pneumonectomy. J Thorac Oncol 2011;6:1923-9. [Crossref] [PubMed]
- Linton A, Pavlakis N, O’Connell R, et al. Factors associated with survival in a large series of patients with malignant pleural mesothelioma in New South Wales. Br J Cancer 2014;111:1860-9. [Crossref] [PubMed]
- Thapa B, Walkiewicz M, Murone C, et al. Calretinin but not caveolin-1 correlates with tumour histology and survival in malignant mesothelioma. Pathology 2016;48:660-5. [Crossref] [PubMed]
- Doglioni C, Dei A, Tos P, et al. Calretinin: a novel immunocytochemical marker for mesothelioma. Am J Surg Pathol 1996;20:1037-46. [Crossref] [PubMed]
- King JE, Thatcher N, Pickering CAC, et al. Sensitivity and specificity of immunohistochemical markers used in the diagnosis of epithelioid mesothelioma: a detailed systematic analysis using published data. Histopathology 2006;48:223-32. [Crossref] [PubMed]
- Ordóñez NG. What are the current best immunohistochemical markers for the diagnosis of epithelioid mesothelioma? A review and update. Hum Pathol 2007;38:1-16. [Crossref] [PubMed]
- Shield PW, Koivurinne K. The value of calretinin and cytokeratin 5/6 as markers for mesothelioma in cell block preparations of serous effusions. Cytopathology 2008;19:218-23. [Crossref] [PubMed]
- Dinu M, Ciurea RN, Stefan M, et al. The role of immunohistochemistry in the diagnosis of neoplastic pleural effusions. Rom J Morphol Embryol 2012;53:817-20. [PubMed]
- Mohammad T, Garratt J, Torlakovic E, et al. Utility of a CEA, CD15, calretinin, and CK5/6 panel for distinguishing between mesotheliomas and pulmonary adenocarcinomas in clinical practice. Am J Surg Pathol 2012;36:1503-8. [Crossref] [PubMed]
- Hyun TS, Barnes M, Tabatabai ZL. The diagnostic utility of D2-40, calretinin, CK5/6, desmin and MOC-31 in the differentiation of mesothelioma from adenocarcinoma in pleural effusion cytology. Acta Cytol 2012;56:527-32. [Crossref] [PubMed]
- Beer TW. Immunohistochemical MIB-1 and p27 as prognostic factors in pleural mesothelioma. Pathol Res Pract 2001;197:859. [PubMed]
- Leonardo E, Zanconati F, Bonifacio D, et al. Immunohistochemical MIB-1 and p27kip1 as prognostic factors in pleural mesothelioma. Pathol Res Pract 2001;197:253-6. [Crossref] [PubMed]
- Ghanim B, Klikovits T, Hoda MA. Ki67 index is an independent prognostic factor in epithelioid but not in non-epithelioid malignant pleural mesothelioma: a multicenter study. Br J Cancer 2015;112:783-92. [Crossref] [PubMed]
- Taheri ZM, Mehrafza M, Mohammadi F, Khoddami M, Bahadori M, Masjedi MR. The diagnostic value of Ki-67 and repp86 in distinguishing between benign and malignant mesothelial proliferations. Arch Pathol Lab Med 2008;132:694-7. [PubMed]
- Kato T, Lee D, Wu L, et al. Kinesin family members KIF11 and KIF23 as potential therapeutic targets in malignant pleural mesothelioma. Int J Oncol 2016;49:448-56. [Crossref] [PubMed]
- Gordon GJ, Dong L, Yeap BY, et al. Four-gene expression ratio test for survival in patients undergoing surgery for mesothelioma. J Natl Cancer Inst 2009;101:678-86. [Crossref] [PubMed]
- Shigematsu Y, Hanagiri T, Kuroda K, et al. Malignant mesothelioma-associated antigens recognized by tumor-infiltrating B cells and the clinical significance of the antibody titers. Cancer Sci 2009;100:1326-34. [Crossref] [PubMed]
- Xia F, Lee CW, Altieri DC. Tumour cell dependence on Ran-GTP-directed mitosis. Cancer Res 2008;68:1826-33. [Crossref] [PubMed]
- Ly TK, Wang J, Pereira R, et al. Activation of the Ran GTPase is subject to growth factor regulation and can give rise to cellular transformation. J Biol Chem 2010;285:5815-26. [Crossref] [PubMed]
- Walter RF, Vollbrecht C, Werner R, et al. Screening of Pleural Mesotheliomas for DNA-damage Repair Players by Digital Gene Expression Analysis Can Enhance Clinical Management of Patients Receiving Platin-Based Chemotherapy. J Cancer 2016;7:1915-25. [Crossref] [PubMed]
- Tsuji S, Washimi K, Kageyama T, et al. HEG1 is a novel mucin-like membrane protein that serves as a diagnostic and therapeutic target for malignant mesothelioma. Sci Rep 2017;7:45768. [Crossref] [PubMed]
- Gordon GJ, Rockwell GN, Jensen RV, et al. Identification of novel candidate oncogenes and tumor suppressors in malignant pleural mesothelioma using large-scale transcriptional profiling. Am J Pathol 2005;166:1827-40. [Crossref] [PubMed]
- Barbone D, Van Dam L, Follo C, Jithesh PV, Zhang SD, Richards WG, Bueno R, Fennell DA, Broaddus VC. Analysis of Gene Expression in 3D Spheroids Highlights a Survival Role for ASS1 in Mesothelioma. PLoS One 2016;11:e0150044. [Crossref] [PubMed]
- Pass HI, Levin SM, Harbut MR, et al. Fibulin-3 as a blood and effusion biomarker for pleural mesothelioma. N Engl J Med 2012;367:1417-27. [Crossref] [PubMed]
- Kaya H, Demir M, Taylan M, et al. Fibulin-3 as a diagnostic biomarker in patients with malignant mesothelioma. Asian Pac J Cancer Prev 2015;16:1403-7. [Crossref] [PubMed]
- Romagnoli S, Fasoli E, Vaira V, et al. Identification of potential therapeutic targets in malignant mesothelioma using cell-cycle gene expression analysis. Am J Pathol 2009;174:762-70. [Crossref] [PubMed]
- Linton A, Cheng YY, Griggs K, et al. An RNAi-based screen reveals PLK1, CDK1 and NDC80 as potential therapeutic targets in malignant pleural mesothelioma. Br J Cancer 2014;110:510-9. [Crossref] [PubMed]
- Hollevoet K, Nackaerts K, Thas O, et al. The effect of clinical covariates on the diagnostic and prognostic value of soluble mesothelin and megakaryocyte potentiating factor. Chest 2012;141:477-84. [Crossref] [PubMed]
- Chen Z, Gaudino G, Pass HI, et al. Diagnostic and prognostic biomarkers for malignant mesothelioma: an update. Transl Lung Cancer Res 2017;6:259-69. [Crossref] [PubMed]
- Garritano S, De Santi C, Silvestri R, et al. A common polymorphism within MSLN affects miR-611 binding site and soluble mesothelin levels in healthy people. J Thorac Oncol 2014;9:1662-8. [Crossref] [PubMed]
- Versnel MA, Claesson-Welsh L, Hammacher A, et al. Human malignant mesothelioma cell lines express PDGF beta-receptors whereas cultured normal mesothelial cells express predominantly PDGF alpha-receptors. Oncogene 1991;6:2005-11. [PubMed]
- Ramael M, Buysse C, van den Bossche J, et al. Immunoreactivity for the beta chain of the platelet-derived growth factor receptor in malignant mesothelioma and non-neoplastic mesothelium. J Pathol 1992;167:1-4. [Crossref] [PubMed]
- Heinrich MC, Blanke CD, Corless CL, et al. PDGF Receptors as Therapeutic Targets. In: Kufe DW, Pollock RE, Weichselbaum RR, et al. editors. Holland-Frei Cancer Medicine, 6th edition. Hamilton: BC Decker, 2003.
- Wehler TC, Frerichs K, Graf C, et al. PDGFRalpha/beta expression correlates with the metastatic behavior of human colorectal cancer: a possible rationale for a molecular targeting strategy. Oncol Rep 2008;19:697-704. [PubMed]
- Yokoyama Y, Mori S, Hamada Y, et al. Platelet-derived growth factor regulates breast cancer progression via β-catenin expression. Pathobiology 2011;78:253-60. [Crossref] [PubMed]
- Wang W, Qi L, Tan M, et al. Effect of platelet-derived growth factor-B on renal cell carcinoma growth and progression. Urol Oncol 2015;33:168.e17-27. [Crossref] [PubMed]
- Tsao AS, Wei W, Kuhn E, et al. Immunohistochemical overexpression of platelet-derived growth factor receptor-beta (PDGFR-β) is associated with PDGFRB gene copy number gain in sarcomatoid non-small-cell lung cancer. Clin Lung Cancer 2011;12:369-74. [Crossref] [PubMed]
- George D. Platelet-derived growth factor receptors: a therapeutic target in solid tumors. Semin Oncol 2001;28:27-33. [Crossref] [PubMed]
- Falleni M, Pellegrini C, Marchetti A, et al. Quantitative evaluation of the apoptosis regulating genes Survivin, Bcl-2 and Bax in inflammatory and malignant pleural lesions. Lung Cancer 2005;48:211-6. [Crossref] [PubMed]
- Jin L, Amatya VJ, Takeshima Y, et al. Evaluation of apoptosis and immunohistochemical expression of the apoptosis-related proteins in mesothelioma. Hiroshima J Med Sci 2010;59:27-33. [PubMed]
- Altieri DC. Targeting surviving in cancer. Cancer Lett 2013;332:225-8. [Crossref] [PubMed]
- Brown DC, Gatter KC. Ki67 protein: the immaculate deception? Histopathology 2002;40:2-11. [Crossref] [PubMed]
- Yerushalmi R, Woods R, Ravdin PM, et al. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol 2010;11:174-83. [Crossref] [PubMed]
- Park JY, Kim KR, Nam JH. Immunohistochemical analysis for therapeutic targets and prognostic markers in low-grade endometrial stromal sarcoma. Int J Gynecol Cancer 2013;23:81-9. [Crossref] [PubMed]
- Nielsen PS, Riber-Hansen R, Jensen TO, et al. Proliferation indices of phosphor-histone H3 and Ki67: strong prognostic markers in a consecutive cohort with stage I/II melanoma. Mod Pathol 2013;26:404-13. [Crossref] [PubMed]
- Penault-Llorca F, Radosevic-Robin N. Ki67 assessment in breast cancer: an update. Pathology 2017;49:166-71. [Crossref] [PubMed]
- Beer TW, Buchanan R, Matthews AW, et al. Prognosis in malignant mesothelioma related to MIB 1 proliferation index and histological subtype. Hum Pathol 1998;29:246-51. [Crossref] [PubMed]
- Neef R, Klein UR, Kopajtich R, et al. Cooperation between mitotic kinesins controls the late stages of cytokinesis. Curr Biol 2006;16:301-7. [Crossref] [PubMed]
- Zhu C, Bossy-Wetzel E, Jiang W. Recruitment of MKLP1 to the spindle midzone/midbody by INCENP is essential for midbody formation and completion of cytokinesis in human cells. Biochem J 2005;389:373-81. [Crossref] [PubMed]
- Takahashi S, Fusaki N, Ohta S, et al. Downregulation of KIF23 suppresses glioma proliferation. J Neurooncol 2012;106:519-29. [Crossref] [PubMed]
- Wong N, Ojo D, Yan J, Tang D. PKM2 contributes to cancer metabolism. Cancer Lett 2015;356:184-91. [Crossref] [PubMed]
- Dayton TL, Jacks T, Vander Heiden MG. PKM2, cancer metabolism, and the road ahead. EMBO Rep 2016;17:1721-30. [Crossref] [PubMed]
- Gordon GJ, Bueno R, Sugarbaker DJ. Genes associated with prognosis after surgery for malignant pleural mesothelioma promote tumor cell survival in vitro. BMC Cancer 2011;11:169. [Crossref] [PubMed]
- Bucher N, Britten CD. G2 checkpoint abrogation and checkpoint kinase-1 targeting in the treatment of cancer. Br J Cancer 2008;98:523-8. [Crossref] [PubMed]
- Min SH, Goldman ID, Zhao R. Caffeine markedly sensitizes human mesothelioma cell lines to pemetrexed. Cancer Chemother Pharmacol 2008;61:819-27. [Crossref] [PubMed]
- Berglund L, Bjorling E, Oksvold P, et al. A gene-centric human protein atlas for expression profiles based on antibodies. Mol Cell Proteomics 2008;7:2019-27. [Crossref] [PubMed]
- Szlosarek PW, Steele JP, Nolan L, et al. Arginine Deprivation With Pegylated Arginine Deiminase in Patients With Argininosuccinate Synthetase 1-Deficient Malignant Pleural Mesothelioma: A Randomized Clinical Trial. JAMA Oncol 2017;3:58-66. [Crossref] [PubMed]
- Szlosarek PW, Klabatsa A, Pallaska A, et al. In vivo loss of expression of argininosuccinate synthetase in malignant pleural mesothelioma is a biomarker for susceptibility to arginine depletion. Clin Cancer Res 2006;12:7126-31. [Crossref] [PubMed]
- Battisti S, Valente D, Albonici L, et al. Nutritional stress and arginine auxotrophy confer high sensitivity to chloroquine toxicity in mesothelioma cells. Am J Respir Cell Mol Biol 2012;46:498-506. [Crossref] [PubMed]
- Pass HI, Goparaju C. Fibulin-3 as a biomarker for pleural mesothelioma. N Engl J Med 2013;368:190. [PubMed]
- Kirschner MB, Pulford E, Hoda MA, et al. Fibulin-3 levels in malignant pleural mesothelioma are associated with prognosis but not diagnosis Br J Cancer 2015;113:963-9. [Crossref] [PubMed]
- Bruno R, Alì G, Giannini R, et al. Malignant pleural mesothelioma and mesothelial hyperplasia: A new molecular tool for the differential diagnosis. Oncotarget 2017;8:2758-70. [Crossref] [PubMed]


