- 1Division of Aivan Infectious Diseases, State Key Laboratory of Veterinary Biotechnology, Harbin Veterinary Research Institute of Chinese Academy of Agricultural Sciences, Harbin, China
- 2Jiangsu Co-innovation Center for Prevention and Control of Important Animal Infectious Disease and Zoonoses, Yangzhou, China
A hypervirulent fowl adenovirus serotype 4 (FAdV-4) has caused hepatitis-hydropericardium syndrome (HHS) with mortalities that range from 30 to 80% in outbreaks across China since 2015. The FAdV-4 strain was characterized as a novel genotype based on the specific genome characteristics. However, our understanding of the dynamic distribution, tissue tropism, and pathogenesis of the novel FAdV-4 is incomplete. In this study, a new, sensitive and FAdV-4-specific real-time PCR was developed and applied to detect the dynamic distribution of the duck origin, novel FAdV-4 strain HLJDAd15 in experimentally infected special-pathogen free (SPF) chickens and ducks. Notably, the pathogenicity and replication pattern of HLJDAd15 were completely different between chickens and ducks. Severe hydropericardium and 10% mortality were induced in chickens, whereas no clinical signs were observed in any duck. The virus replicated was detected throughout the study in both chickens and ducks. However, only one replication peak with a high virus concentration appeared in chickens at 5 days post infection (dpi), whereas two peaks with relatively low virus titres appeared in ducks at 7 and 21 dpi. Thus, ducks could be a natural reservoir of the novel FAdV-4 absent of clinical signs, and a new transmission route from ducks shedding FAdV-4 continually to chickens was revealed, which might aggravate the outbreak of HHS in chickens. This study provides the first accurate quantitative data for the replication kinetics of the novel FAdV-4 in different hosts. The different pathogenicity, dynamic distribution and replication pattern in chickens and ducks provide a foundation for further clarification of the pathogenesis of the novel FAdV-4.
Introduction
Adenoviruses, family Adenoviridae, are non-enveloped and double-stranded DNA viruses (Stewart et al., 1993). The International Committee on Taxonomy of Viruses (Harrach and Kaján, 2011) separates the Adenoviridae into five genera: Mastadenovirus, Aviadenovirus, Siadenovirus, Atadenovirus, and Ichtadenovirus. Fowl adenoviruses, members of the genus Aviadenovirus, are further separated into either five species (designated FAdV-A to FAdV-E) based largely on molecular criteria and restriction enzyme digest pattern or twelve serotypes (designated FAdV-1 to 8a and -8b to 11) based on the results of serum cross-neutralization tests (Hess, 2000). The FAdVs are associated with several notable diseases in chickens and other birds, such as inclusion body hepatitis (IBH), hepatitis-hydropericardium syndrome (HHS), and gizzard erosion (GE) (Domanska-Blicharz et al., 2011; Marek et al., 2012).
Hydropericardium-hepatitis syndrome was first reported in Pakistan in 1987 (Khawaja et al., 1988), and subsequent outbreaks have also been reported worldwide in recent years, including in America, Mexico, Hungary, Poland, Peru, Ecuador, Chile, Japan, Korea, India, and China, resulting in significant economic losses to the poultry industry (Hess et al., 1999; Griffin and Nagy, 2011; Grgić et al., 2013; Kaján et al., 2013; Niczyporuk, 2016). HHS appears with high frequency in chickens, particularly in broilers, with a mortality that ranges from 10 to 100% (Vera-Hernández et al., 2016; Pan et al., in press). However, the disease has rarely emerged in other hosts with severe clinical signs and high mortality. Fowl adenovirus serotype 4 (FAdV-4) plays a primary role in the etiology of HHS based on epidemiological studies, and many hypervirulent strains have been isolated from cases of HHS (Vera-Hernández et al., 2016; Ye et al., 2016; Pan et al., in press). For diagnosis of FAdVs infection, several routine PCR methods have been reported based on serotype-specific hexon gene (Steer et al., 2009; Kaján et al., 2013) and a SYBR Green based real-time PCR within 52K gene was developed for detection and quantitation of all FAdV species (Günes et al., 2012). However, a sensitive serotype 4 specific quantitate PCR has not been available and urgently needed for diagnosis and deeper investigation of FAdV-4, especially the emerged novel FAdV-4.
Since June 2015, severe outbreaks of HHS with high mortalities from 30 to 100% have emerged in several different areas of China. Many hypervirulent FAdV-4 strains have been isolated and identified from different cases, and a reproduction experiment revealed high mortality in SPF chickens (Zhao et al., 2015; Li H. et al., 2016; Niu et al., 2016). Given the complete genome sequence analysis, Chinese hypervirulent FAdV-4 strains show many new characters, such as the natural large deletions of ORF19 and ORF27, and are identified as a novel genotype (Li L. et al., 2016; Liu et al., 2016; Ye et al., 2016). Although many studies on chicken origin FAdV-4 have been conducted, less is known about the duck origin FAdV-4 (Chen et al., 2016). In this study, a new, FAdV-4-specific real-time PCR was developed and applied to detect the dynamic distribution of a duck origin FAdV-4 in experimentally infected SPF chickens and ducks, which revealed different replication patterns of the novel FAdV-4 between chickens and ducks, helping to further understand the pathogenicity and transmission mechanism between different hosts.
Materials and Methods
Ethics Statement
The animal experiments with chickens and ducks were approved by the Animal Care and Use Committee of Harbin Veterinary Research Institute (Harbin, China) and performed in accordance with the ‘Guidelines for Experimental Animals’ of the Ministry of Science and Technology (Beijing, China). All SPF chickens and ducks were cared for in accordance with humane procedures.
Virus
Duck origin FAdV-4 (HLJDAd15) was isolated from the field in 2015 in Heilongjiang Province, China (Pan et al., in press), and is a novel genotype of FAdV-4 based on the complete genome sequence (GenBank No. KX538980).
Animal Infection
Forty of each 1-day-old specific pathogen free (SPF) chickens and ducks were purchased from the Experimental Animal Center of Harbin Veterinary Research Institute (HVRI) of the Chinese Academy of Agricultural Sciences (CAAS), China. Thirty SPF chickens and ducks were orally inoculated with 103.0 ELD50 doses of the homogenates of HLJDAd15 in 0.2 ml of phosphate-buffered saline (PBS) at 35 days old, whereas the 10 other SPF chickens and ducks served as negative controls. The challenged and control groups were separately housed in different negative-pressure isolators and monitored daily for 30 days.
Sample Collection
At 1, 3, 5, 7, 10, 14, 21, 28, 35, and 42 days post infection (dpi), three infected birds and one control bird were sacrificed by intraperitoneal injection of sodium pentobarbital. Tissue samples of heart, liver, spleen, lung, kidney, thymus, bursa, caecal tonsi, proventriculus, and rectum were collected. The homogenates of 10 to 100 mg of tissue samples in 200 μl of PBS were subjected to DNA extraction or stored at -80°C. Three serum samples were also collected at each dpi and stored at -80°C until used for antibody assays.
Viral DNA Extraction
The viral DNA of tissue samples was extracted from the tissue homogenates using a DNeasy Tissue kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions, whereas the positive DNA or proviral DNA of FAdV-1, Marek’s disease virus (MDV) (Lv et al., 2016), chicken infectious anaemia virus (CAV) (Wang et al., 2017), avian gyrovirus 2 (AGV2) (Yao et al., 2017), reticuloendotheliosis virus (REV) (Jiang et al., 2013), and avian leukosis virus subgroup A, B, and J (ALV-A, ALV-B, ALV-J) (Gao et al., 2014) were maintained in our laboratory.
Routine PCR
The primers used in this study to detect the genotype of FAdV were designed based on the L1 region of the Hexon gene (FAd-F: 5′-AACTTCGACCCCATGTCGCGTCAGG-3′ and FAd-R: 5′-TGGCGAAAGGCGTACGGAAGTAAGC-3′). The PCR products were subjected to electrophoresis in a 1% (w/v) agarose gel. The PCR procedure consisted of an initial incubation for 5 min at 95°C; 35 cycles of 30 s at 95°C, annealing for 60 s at 55°C, and extension for 60 s at 72°C; and a final extension for 5 min at 72°C.
Real-Time PCR
Real-time PCR was performed with a LightCycler 480 real-time thermocycler (Roche Instrument Center, Switzerland). The primers were designed based on the FAdV-4 specific L1 region of the Hexon gene (Ganesh et al., 2001; Steer et al., 2009; Park et al., 2017) as follow: forward primer 5′-CAGTTCATTTCCGCCACC-3′ and reverse primer 5′-GCAGCCGTTGAGCCTTTT-3′. The relative TaqMan probe was a 23 bp oligonucleotide, 5′FAM-TCTGTCGTGACATTTCGGGTGGG-3′TAMRA, which was labeled with FAM at the 5′ end and TAMRA at the 3′ end. The reactions were conducted with a predenaturation step at 95°C for 5 min and amplification for 40 cycles at a melting temperature of 95°C for 10 s and an elongation at 65°C for 40 s (Qin et al., 2013; Gaudreault et al., 2015). The fluorescent signal was collected during the elongation step. A 341 bp fragment of the L1 region of Hexon was cloned into the pEASY-T1 vector (TransGen, Beijing, China) to prepare a standard recombinant plasmid pAD341. Serial dilutions of the standard plasmid from 1 × 101 to 1 × 1011 copies/μl were used to produce a standard curve.
Histopathology
Tissue samples were fixed in 10% formalin for 48 h at room temperature (RT), and then routinely processed, embedded in paraffin wax, and cut into 5-μm sections. The sections were stained with hematoxylin and eosin (HE) and examined using light microscopy.
Antibody Production
Antisera against the viral isolate HLJFAd15 were collected from 5-week-old SPF chickens at 1, 3, 5, 7, 10, 14, 21, 28, 35, and 42 days post infection. Serum samples were tested for FAdV-specific antibodies using a commercial enzyme-linked immunosorbent assay (ELISA) kit (BioChek, Scarborough, ME, United States).
Results
Development of Real-Time PCR
To generate a standard curve for the real-time PCR, the standard recombinant plasmid pAD341 was serially diluted from 1 × 101 to 1 × 1011 copies/μl, and the serial-diluted plasmids were detected by the real-time PCR assay. Threshold cycle values (CT) were plotted against the known copy numbers of the standard controls. The good correlation (error = 0.085, efficiency = 2.152) between CT values and copy numbers is shown in Figure 1A.
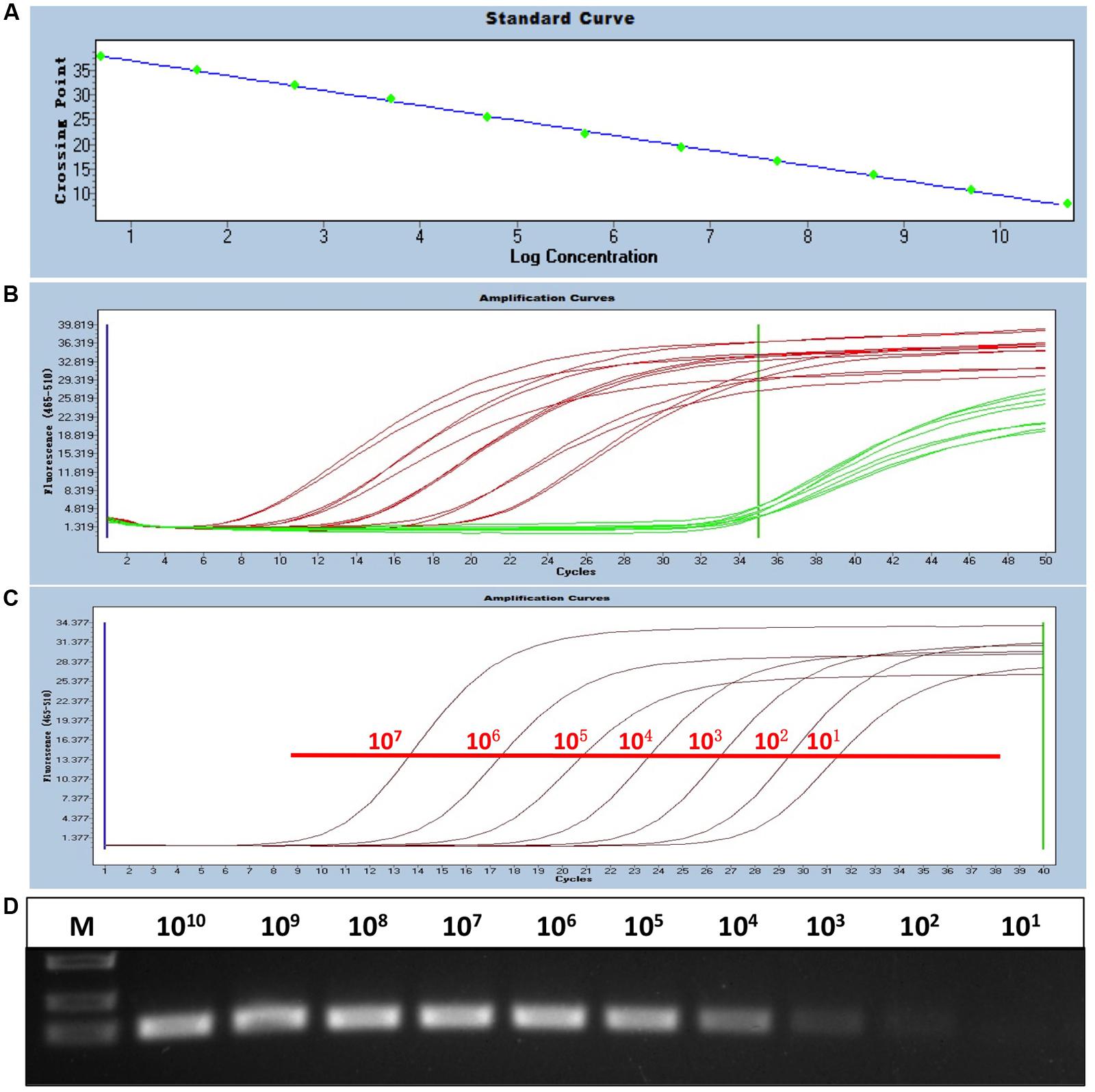
FIGURE 1. Establishment of the sensitive, FAdV-4-specific real-time PCR. (A) Standard curve of the real-time PCR. The standard plasmid was serially diluted from 1 × 101 to 1 × 1011 copies/μl, and a good correlation (error = 0.085, efficiency = 2.152) between CT values and copy numbers was produced. (B) Specificity of the real-time PCR for FAdV-4. Red curves (HLJDAd15 and 11 other FAdV-4 isolates identified by routine PCR) were considered positive, and green curves (FAdV-1, MDV, CAV, AGV2, REV, ALV-A, ALV-B, and ALV-J) were considered negative. (C,D) Sensitivity of the real-time PCR. The detection limit of the real-time PCR (101 copies/μl) was at least 10-fold higher than that of routine PCR (above 102 copies/μl).
Specificity of Real-Time PCR
Viral DNA from 11 chicken origin and 1 duck origin FAdV-4 isolates was subjected to the established real-time PCR and was well detected, whereas fluorescent signals from viral DNA of FAdV-1, MDV, CAV, and AGV2 and proviral DNA of REV, ALV-A, ALV-B, and ALV-J were not detected, indicating the specificity of the real-time PCR for FAdV-4 (Figure 1B).
Sensitivity of Real-Time PCR
The sensitivity of the real-time PCR was compared with that of routine PCR. The recombinant plasmid pAD341 was used as a template. The results showed that the detection limit of the real-time PCR established in this study was fewer than 101 copies/μl (Figure 1C), whereas the detection limit of routine PCR was more than 102 copies/μl (Figure 1D), demonstrating that the sensitivity of our real-time PCR was at least 10-fold higher than that of routine PCR.
Dynamic Distribution in SPF Chickens of HLJDAd15
For chickens in the experimentally infected group, 1 chicken and 2 chickens died on 3 and 5 dpi, respectively. The dead chickens showed severe hydropericardium and IBH. The dynamic distributions of the duck origin FAdV-4 in different tissues of SPF chickens were detected by real-time PCR (Figure 2A). The virus DNA was detectable throughout the experiment, and the peak of virus replication appeared at 5 dpi in all tissues except in caecal tonsi with the peak at 3 dpi. After 14 dpi, the concentration of virus in all tissues decreased and remained at a low level without another replication peak until 42 dpi at the end of the experiment. The highest number of virus copies in different tissues was compared among the heart, liver, spleen, lung, kidney, thymus, bursa, proventriculus, and rectum at 5 dpi and in the caecal tonsi at 3 dpi (Figure 2B). The virus loads in all tissues were higher than 105 copies mg-1, with the concentration in livers significantly higher (p < 0.01) than that in any other tissue. All chickens in the control group were alive and healthy at the end of the experiment, and viral DNA was not detected by either routine PCR or the real-time PCR.
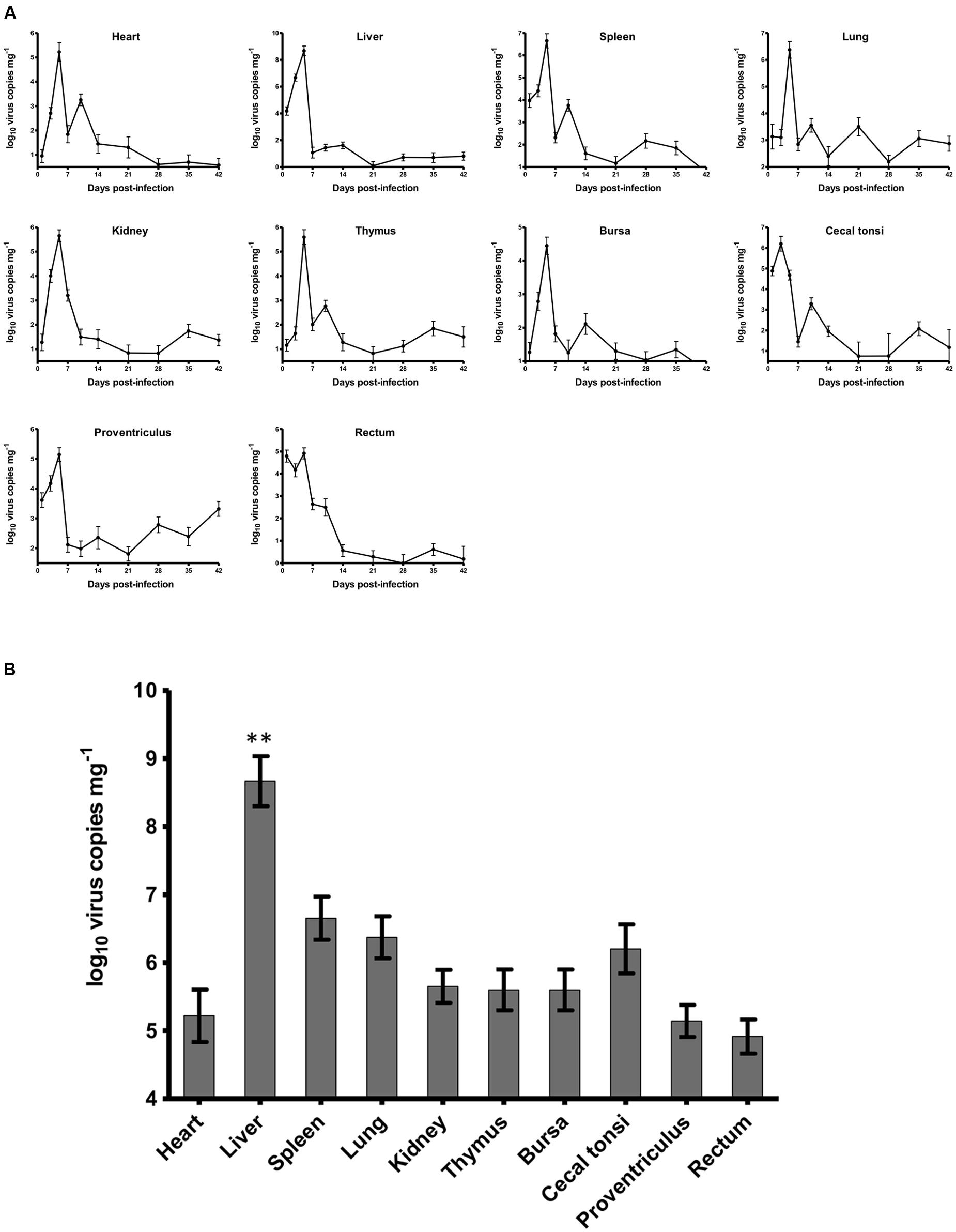
FIGURE 2. Dynamic distribution of the novel FAdV-4 in experimentally infected SPF chickens. (A) One replication peak occurred in the heart, liver, spleen, lung, kidney, thymus, bursa, proventriculus, and rectum at 5 dpi and in caecal tonsi at 3 dpi. (B) The peak concentration of virus replication in the liver was significantly higher than that in other tissues (∗∗p < 0.01).
Histopathology in Different Tissues
Tissues of the heart, liver, spleen, kidney, thymus, and bursa of chickens in different groups were collected 5 days post challenge and fixed, cut into sections, and stained with HE (Figure 3). Generally, there was no significant histopathology damages presented in chickens of control group, while massive pathological damages were observed in various tissues of chickens in infection group: degeneration, vacuolar necrosis and basophilic inclusion bodies presented in liver cells (Figure 3D); large numbers of vacuolar necrosis showed in kidney cells (Figure 3J); large numbers of macrophages proliferation and cortical lymphocyte depletion appeared in thymus (Figure 3L); severe reduction and necrosis of lymphocytes showed in spleen (Figure 3F) and bursa (Figure 3N).
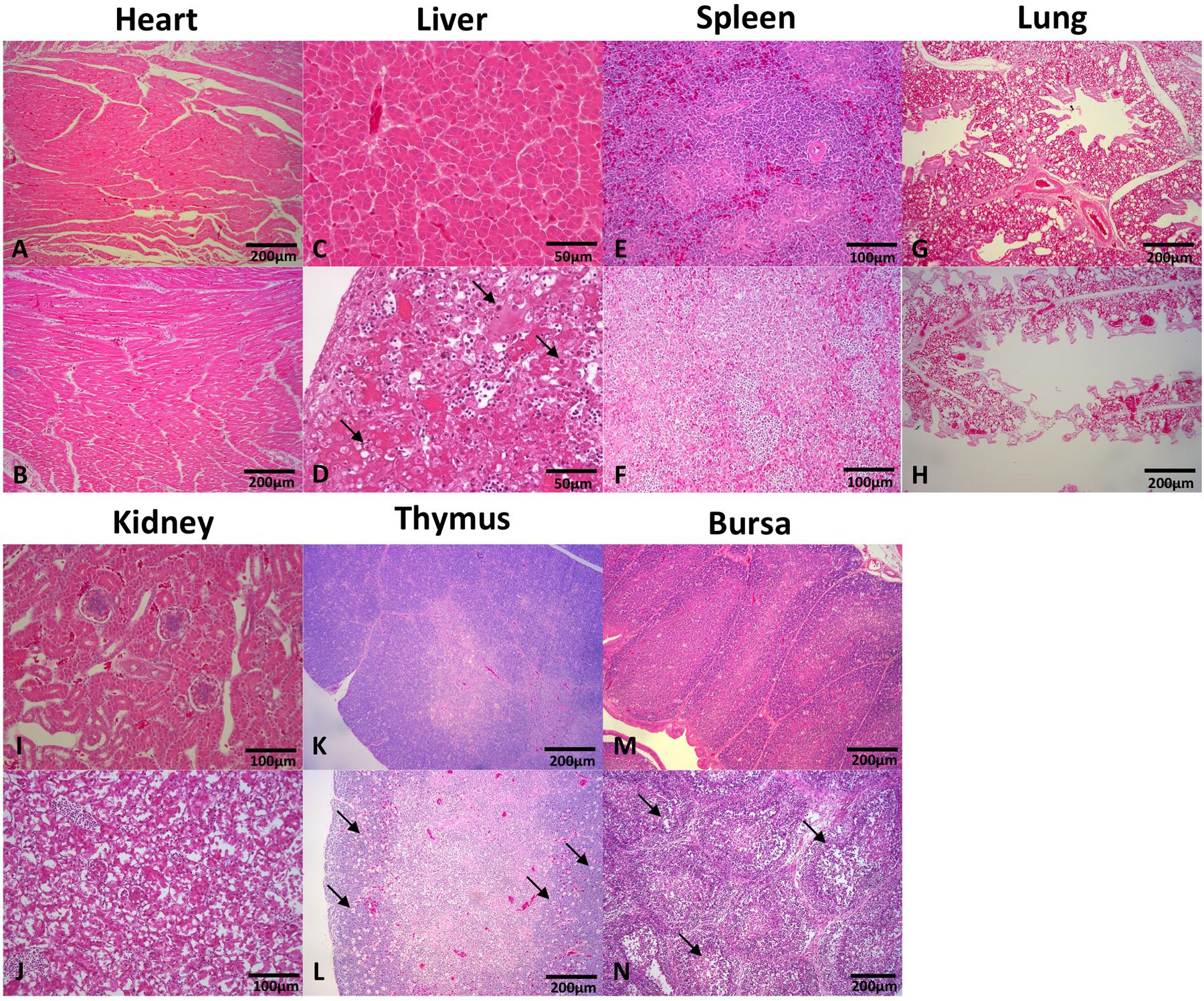
FIGURE 3. Histopathology in tissues from dead chickens inoculated with HLJDAd15. Tissues in infection and control groups were collected, fixed, cut into sections, and stained with HE. Generally, there was no significant histopathology damages presented in the heart (A), liver (C), spleen (E), lung (G), kidney (I), thymus (K), and bursa (M) of chickens in control group, while massive pathological damages were observed in various tissues of chickens in infection group: (D) degeneration, vacuolar necrosis and basophilic inclusion bodies presented in liver cells; (J) large numbers of vacuolar necrosis showed in kidney cells; (L) large numbers of macrophages proliferation and cortical lymphocyte depletion appeared in thymus; severe reduction and necrosis of lymphocytes showed in spleen (F) and bursa (N); no significant differences were observed in heart (B) and lung (H) compared with the control group.
Dynamic Distribution in SPF Ducks of HLJDAd15
All ducks in both experimentally infected and control groups remained alive and did not show visible clinical signs by 42 dpi at the end of the experiment. The dynamic changes of HLJDAd15 in different tissues of SPF ducks were detected by real-time PCR (Figure 4A). The virus DNA was detectable throughout the experiment, and two peaks of virus replication appeared at 7 and 21 dpi (28 dpi for spleen). The virus copy peaks at both 7 dpi (Figure 4B) and 21 dpi (Figure 4C) in the heart, liver, spleen, lung, kidney, thymus, bursa, proventriculus, rectum and caecal tonsi were analyzed. The results showed that the virus concentrations in all tissues were between 102 and 105 copies mg-1 tissue, with the exceptions of concentrations higher than 105 in livers at 7 dpi and lower than 102 in spleens from 5 dpi. Furthermore, the trends between the two virus replication peaks in the different tissues were similar.
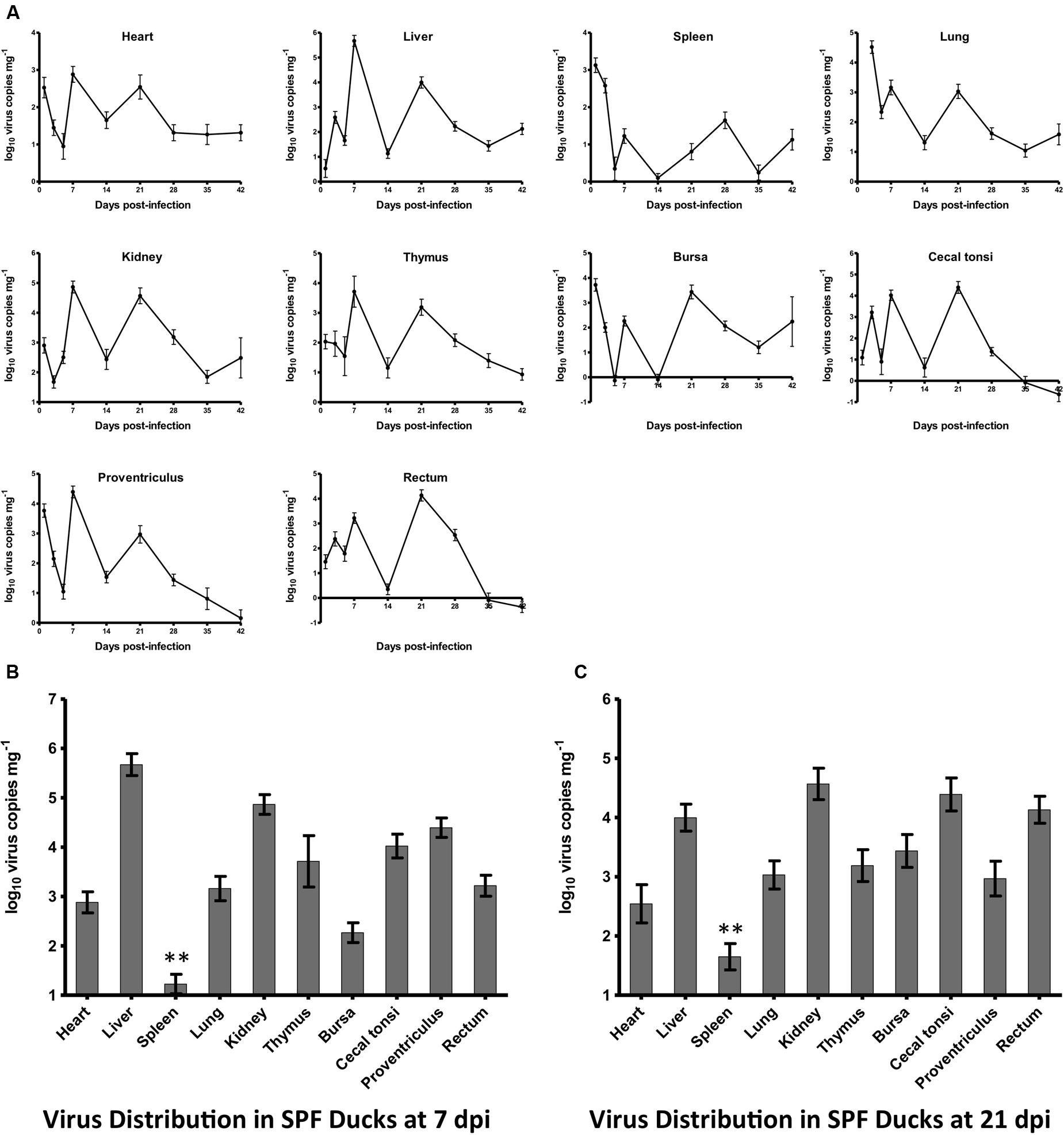
FIGURE 4. Dynamic distribution of the novel FAdV-4 in experimentally infected SPF ducks. (A) Double replication peaks occurred in the heart, liver, spleen, lung, kidney, thymus, bursa, caecal tonsi, proventriculus, and rectum at 7 and 21 dpi. The peak concentrations of virus replication at 7 dpi (B) and 21 dpi (C) were compared, and the virus load in the spleen at both times was significantly lower than that in other tissues (∗∗p < 0.01).
Comparison of Virus Concentrations between Chickens and Ducks
Based on the dynamic distributions of the duck origin FAdV-4, the virus replications in different tissues of SPF chickens and ducks were compared at 5 dpi (Figure 5A), 7 dpi (Figure 5B), and 21 dpi (Figure 5C), respectively. The results showed that the virus copies in different tissues of chickens were significantly higher (p < 0.01) than ducks at 5 dpi, when the unique replication peak showed in chickens. However, the virus concentrations in chickens rapidly decreased after 5 dpi and lower than ducks at 21 dpi, when the second replication peak appeared in ducks. Moreover, the peak concentrations in different tissues of different hosts were also analyzed (Figure 5D). The data showed that the virus peak concentrations in all tissues of chickens were significantly higher (p < 0.01) than those at both peaks in ducks, and particularly in the liver, heart, spleen, lung, thymus, bursa, and caecal tonsi, the virus copies were 100-fold higher in chickens than in ducks. Notably, the highest concentration of more than 108 copies mg-1 in livers was significantly higher (p < 0.01) than that in any other tissues in chickens and ducks at any other times, and the concentration of virus copies in the spleen of ducks was significantly lower (p < 0.01) than that in other tissues of ducks.
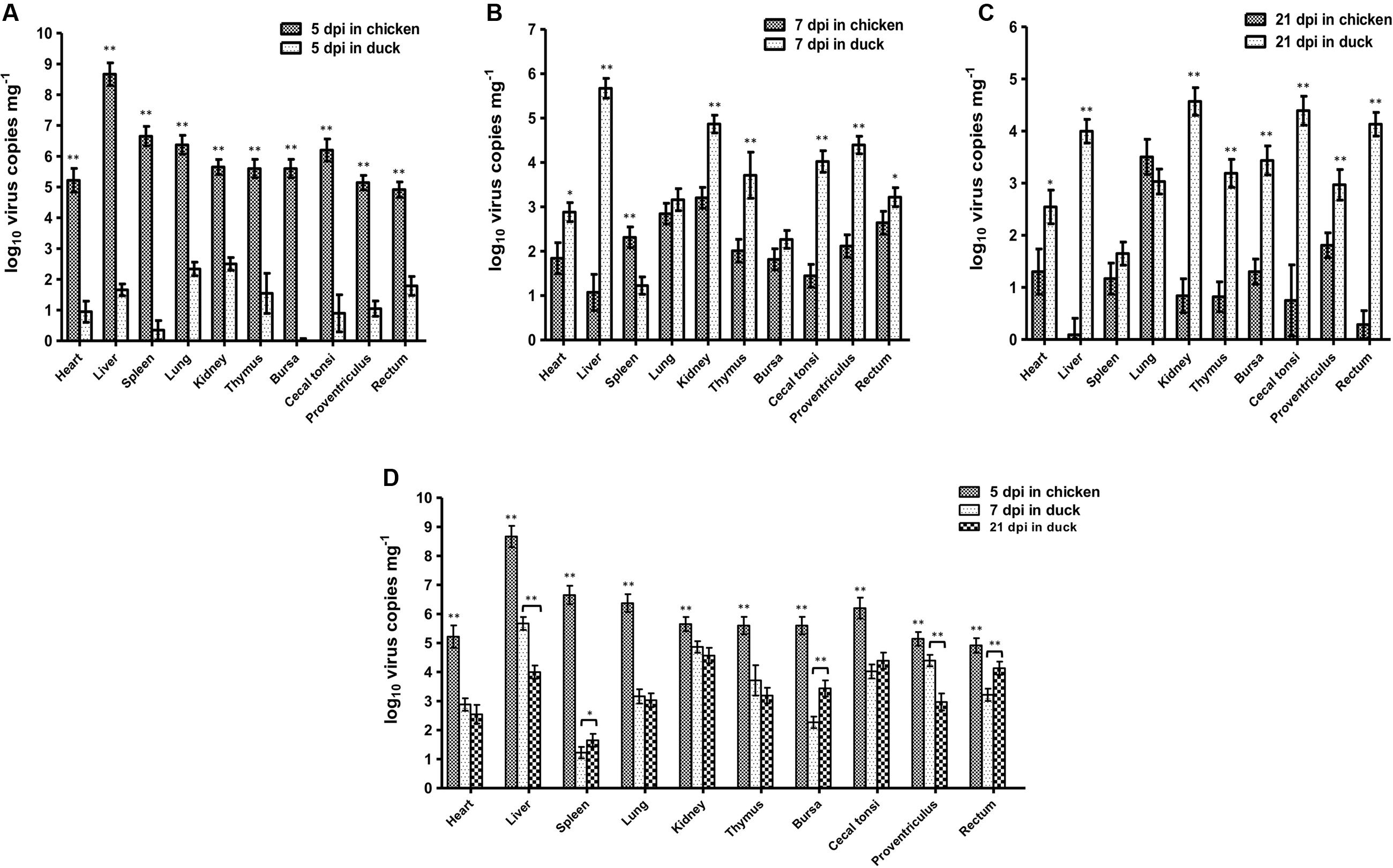
FIGURE 5. Comparison of Virus Peak Concentration between Chickens and Ducks. The peak concentrations of duck origin FAdV-4 showed at 5 dpi in SPF chickens and 7 and 21 dpi in SPF ducks. The comparisons of virus titres between chickens and ducks at 5 dpi (A), 7 dpi (B), and 21 dpi (C) were separately analyzed. Furthermore, the highest virus titres in chickens at 5 dpi and in ducks at 7 and 21 dpi were compared (D). The peak concentrations of FAdV-4 in all tissues of chickens were significantly higher (∗∗p < 0.01) than those in ducks. The highest concentration was in the livers of chickens (∗∗p < 0.01), whereas the lowest concentration of virus copies appeared in the spleens of ducks (∗∗p < 0.01).
Antibody Production
An antibody response of SPF chickens and ducks against FAdV was detected at 10 different times (Figure 6) post infection. The FAdV-specific antibody response was negative in all control birds at all times. From 7 dpi, the antibody titres of chickens continued to increase until reaching a peak at 35 dpi and then began to decrease to 42 dpi. The antibody titres of ducks were completely different from those of chickens and increased from 7 dpi, with the first peak 3 weeks earlier than that of chickens at 14 dpi and the second one at 35 dpi. The peak antibody titre was almost equal between chickens and ducks.
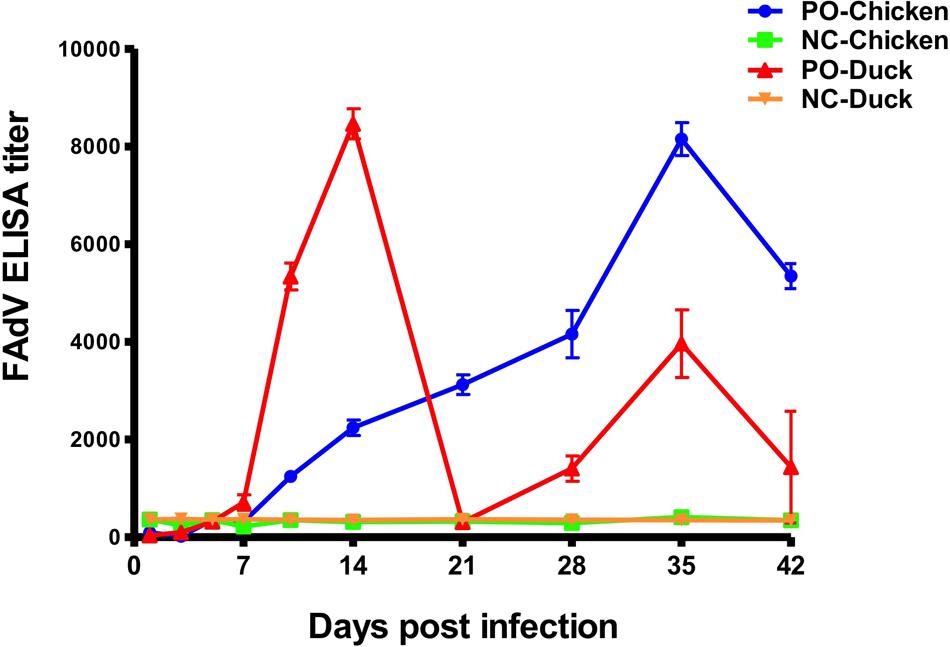
FIGURE 6. Antibody responses of experimentally infected SPF chickens and ducks. The dynamic curves of antibody response showed only one peak in chickens (Blue curve) but two peaks in ducks (Red curve), and the peak in chickens occurred later than the early peak of ducks and approximately at the same time as the late peak in ducks. All birds in the control groups showed negative antibody responses.
Discussion
The HHS associated with FAdV-4 infection has emerged since 2015 and is reported in different provinces of China, inducing huge economic losses for poultry production (Li H. et al., 2016; Niu et al., 2016). The FAdV-4 that caused a recent epidemic in China was isolated and characterized as a novel genotype of FAdV-4 (Liu et al., 2016; Ye et al., 2016). Although many studies have been conducted on virus isolation, characterization and pathogenicity to chickens of the novel FAdV-4, less is known about the replicator dynamics in vivo, which is critical for enclosing the pathogenesis of the emerged novel FAdV-4. As previous reported, Several routine PCR methods have been reported based on hexon gene (Steer et al., 2009; Kaján et al., 2013) and a SYBR Green based real-time PCR within 52K gene was developed for detection and quantitation of all FAdV species (Günes et al., 2012). However, a sensitive serotype 4 specific quantitate PCR has not been available and urgently needed for diagnosis and deeper investigation of the emerged novel FAdV-4. In this study, a novel TaqMan-based, FAdV-4-specific quantitative real-time PCR within serotype-specific hexon gene was developed and applied to detect the dynamic distribution of the novel FAdV-4 not only in chickens but also in ducks. The real-time PCR showed high specificity and sensitivity for FAdV-4, providing a helpful tool for intensive study of the emerged, novel virus.
Chickens were usually considered to the most susceptible natural hosts, including broilers, layers, breeder broilers, but some other hosts were also the potential infection hosts for FAdVs, such as falcons have been reported as the natural host and primary reservoir for FAdV, although the FAdV was characterized as a novel species (Oaks et al., 2005; Schrenzel et al., 2005). The novel FAdV-4 recently emerged in China is hypervirulent to chickens (Niu et al., 2016), particularly broilers, and can be transmitted from chicken to chicken. However, less is reported on the pathogenicity of the novel FAdV-4 to ducks, with the exception that an intracerebral injection induces a mortality of 15% (Chen et al., 2016), although the infection route was not one of natural transmission. Furthermore, the transmission mechanism of novel FAdV-4 between different hosts is poorly understood. Therefore, we chose a duck origin strain HLJDAd15 to evaluate the pathogenicity and potential transmission route of the novel FAdV-4. The results of our study showed that the duck origin FAdV-4 induced severe HHS and a mortality of 10% to chickens, whereas no visible clinical signs and mortality were found in ducks. Moreover, the replication patterns in chickens and ducks were completely different. The virus persisted throughout the experiment in both chickens and ducks, but only one stronger replication peak was detected in chickens, whereas two mildly peaks were observed in ducks. Given the continuous virus shedding occurred in the infected ducks without visible clinical signs, ducks might be an intermediate host or natural virus reservoir of the novel FAdV-4. Thus, a new transmission mechanism from ducks to chickens was revealed, which might aggravate the outbreak of HHS in chickens. Furthermore, the antibody titres were highly negatively correlated with the virus replication, which suggested that the antibody might play a critical role in inhibiting the proliferation of the virus in vivo and might be a key index for evaluation of vaccine development; however, the detailed functions of the antibody in virus infection require further investigation.
Conclusion
Hepatitis-hydropericardium syndrome has been caused by outbreaks of a novel genotype FAdV-4 across China since June 2015, but little is known about this novel FAdV-4. In this study, a new, sensitive and FAdV-4-specific real-time PCR was developed and applied to detect the dynamic distribution of a duck origin FAdV-4 in chickens and ducks. The novel virus showed completely different replication patterns and antibody response in different hosts, which provide a foundation for further clarification of the pathogenesis and the epidemic transmission mechanism of the novel FAdV-4.
Author Contributions
XW and HC conceived and designed the experiments. QP, YY, ZS, and LL performed the experiments. QP and YY analyzed the data. CL and YZ contributed reagents/materials/analysis tools. QP wrote the paper. XQ and YG were involved in the interpretation of the results and critically read the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was partly supported by the National Key R&D Program (2016YFD0500800) and National Natural Science Foundation of China (31602073).
References
Chen, H., Dou, Y., Zheng, X., Tang, Y., Zhang, M., Zhang, Y., et al. (2016). Hydropericardium hepatitis syndrome emerged in cherry valley ducks in China. Transbound. Emerg. Dis. doi: 10.1111/tbed.12500 [Epub ahead of print].
Domanska-Blicharz, K., Tomczyk, G., Smietanka, K., Kozaczynski, W., and Minta, Z. (2011). Molecular characterization of fowl adenoviruses isolated from chickens with gizzard erosions. Poult. Sci. 90, 983–989. doi: 10.3382/ps.2010-01214
Ganesh, K., Suryanarayana, V., Raghavan, R., and Gowda, S. (2001). Nucleotide sequence of L1 and part of P1 of hexon gene of fowl adenovirus associated with hydropericardium hepatitis syndrome differs with the corresponding region of other fowl adenoviruses. Vet. Microbiol. 78, 1–11. doi: 10.1016/S0378-1135(00)00288-1
Gao, Q., Yun, B., Wang, Q., Jiang, L., Zhu, H., Gao, Y., et al. (2014). Development and application of a multiplex PCR method for rapid differential detection of subgroup A, B, and J avian leukosis viruses. J. Clin. Microbiol. 52, 37–44. doi: 10.1128/JCM.02200-13
Gaudreault, N. N., Indran, S. V., Bryant, P. K., Richt, J. A., and Wilson, W. C. (2015). Comparison of Rift Valley fever virus replication in North American livestock and wildlife cell lines. Front. Microbiol. 6:664. doi: 10.3389/fmicb.2015.00664
Grgić, H., Poljak, Z., Sharif, S., and Nagy, É (2013). Pathogenicity and cytokine gene expression pattern of a serotype 4 fowl adenovirus isolate. PLoS ONE 8:e77601. doi: 10.1371/journal.pone.0077601
Griffin, B. D., and Nagy, E. (2011). Coding potential and transcript analysis of fowl adenovirus 4: insight into upstream ORFs as common sequence features in adenoviral transcripts. J. Gen. Virol. 92(Pt 6), 1260–1272. doi: 10.1099/vir.0.030064-0
Günes, A., Marek, A., Grafl, B., Berger, E., and Hess, M. (2012). Real-time PCR assay for universal detection and quantitation of all five species of fowl adenoviruses (FAdV-A to FAdV-E). J. Virol. Methods 183, 147–153. doi: 10.1016/j.jviromet.2012.04.005
Harrach, B., and Kaján, G. (2011). “Aviadenovirus,” in The Springer Index of Viruses, eds C. Tidona and G. Darai (New York, NY: Springer).
Hess, M. (2000). Detection and differentiation of avian adenoviruses: a review. Avian Pathol. 29, 195–206. doi: 10.1080/03079450050045440
Hess, M., Raue, R., and Prusas, C. (1999). Epidemiological studies on fowl adenoviruses isolated from cases of infectious hydropericardium. Avian Pathol. 28, 433–439. doi: 10.1080/03079459994443
Jiang, L., Qi, X., Gao, Y., Hua, Y., Li, K., Deng, X., et al. (2013). Molecular characterization and phylogenetic analysis of the reticuloendotheliosis virus isolated from wild birds in Northeast China. Vet. Microbiol. 166, 68–75. doi: 10.1016/j.vetmic.2013.05.008
Kaján, G. L., Kecskeméti, S., Harrach, B., and Benkõ, M. (2013). Molecular typing of fowl adenoviruses, isolated in Hungary recently, reveals high diversity. Vet. Microbiol. 167, 357–363. doi: 10.1016/j.vetmic.2013.09.025
Khawaja, D. A., Ahmad, S., Rauf, M. A., Zulfiqar, M. Z., Mahmood, S. M., and Hassan, M. (1988). Isolation of an adenovirus from hydropericardium syndrome in broiler chicks. Pak. J. Vet. Res. 1, 2–17.
Li, H., Wang, J., Qiu, L., Han, Z., and Liu, S. (2016). Fowl adenovirus species C serotype 4 is attributed to the emergence of hepatitis-hydropericardium syndrome in chickens in China. Infect. Genet. Evol. 45, 230–241. doi: 10.1016/j.meegid.2016.09.006
Li, L., Luo, L., Luo, Q., Zhang, T., Zhao, K., Wang, H., et al. (2016). Genome sequence of a fowl adenovirus serotype 4 strain lethal to chickens, isolated from China. Genome Announc. 4:e00140-16. doi: 10.1128/genomeA.00140-16
Liu, Y., Wan, W., Gao, D., Li, Y., Yang, X., Liu, H., et al. (2016). Genetic characterization of novel fowl aviadenovirus 4 isolates from outbreaks of hepatitis-hydropericardium syndrome in broiler chickens in China. Emerg. Microbes Infect. 5:e117. doi: 10.1038/emi.2016.115
Lv, H., Zhang, Y., Sun, G., Bao, K., Gao, Y., Qi, X., et al. (2016). Genetic evolution of Gallid herpesvirus 2 isolated in China. Infect. Genet. Evol. 51, 263–274. doi: 10.1016/j.meegid.2016.04.027
Marek, A., Nolte, V., Schachner, A., Berger, E., Schlötterer, C., and Hess, M. (2012). Two fiber genes of nearly equal lengths are a common and distinctive feature of Fowl adenovirus C members. Vet. Microbiol. 156, 411–417. doi: 10.1016/j.vetmic.2011.11.003
Niczyporuk, J. S. (2016). Phylogenetic and geographic analysis of fowl adenovirus field strains isolated from poultry in Poland. Arch. Virol. 161, 33–42. doi: 10.1007/s00705-015-2635-4
Niu, Y. J., Sun, W., Zhang, G. H., Qu, Y. J., Wang, P. F., Sun, H. L., et al. (2016). Hydropericardium syndrome outbreak caused by fowl adenovirus serotype 4 in China in 2015. J. Gen. Virol. 97, 2684–2690. doi: 10.1099/jgv.0.000567
Oaks, J. L., Schrenzel, M., Rideout, B., and Sandfort, C. (2005). Isolation and epidemiology of falcon adenovirus. J. Clin. Microbiol. 43, 3414–3420. doi: 10.1128/JCM.43.7.3414-3420.2005
Pan, Q., Liu, L., Wang, Y., Zhang, Y., Qi, X., Liu, C., et al. (in press). The first whole genome sequence and pathogenicity characterization of a fowl adenovirus 4 isolated from ducks associated with inclusion body hepatitis and hydropericardium syndrome. Avian Pathol.
Park, H. S., Lim, I. S., Kim, S. K., Kim, T. K., Park, C. K., and Yeo, S. G. (2017). Molecular analysis of the hexon, penton base, and fiber-2 genes of Korean fowl adenovirus serotype 4 isolates from hydropericardium syndrome-affected chickens. Virus Genes 53, 111–116. doi: 10.1007/s11262-016-1393-z
Qin, L., Gao, Y., Ni, W., Sun, M., Wang, Y., Yin, C., et al. (2013). Development and application of real-time PCR for detection of subgroup J avian leukosis virus. J. Clin. Microbiol. 51, 149–154. doi: 10.1128/JCM.02030-12
Schrenzel, M., Oaks, J. L., Rotstein, D., Maalouf, G., Snook, E., Sandfort, C., et al. (2005). Characterization of a new species of adenovirus in falcons. J. Clin. Microbiol. 43, 3402–3413. doi: 10.1128/JCM.43.7.3402-3413.2005
Steer, P. A., Kirkpatrick, N. C., O’Rourke, D., and Noormohammadi, A. H. (2009). Classification of fowl adenovirus serotypes by use of high-resolution melting-curve analysis of the hexon gene region. J. Clin. Microbiol. 47, 311–321. doi: 10.1128/JCM.01567-08
Stewart, P. L., Fuller, S. D., and Burnett, R. M. (1993). Difference imaging of adenovirus: bridging the resolution gap between X-ray crystallography and electron microscopy. EMBO J. 12, 2589–2599.
Vera-Hernández, P. F., Morales-Garzón, A., Cortés-Espinosa, D. V., Galiote-Flores, A., García-Barrera, L. J., Rodríguez-Galindo, E. T., et al. (2016). Clinicopathological characterization and genomic sequence differences observed in a highly virulent fowl Aviadenovirus serotype 4. Avian Pathol. 45, 73–81. doi: 10.1080/03079457.2015.1125443
Wang, Y., Song, X., Gao, H., Wang, X., Hu, Y., Gao, Y., et al. (2017). C-terminal region of apoptin affects chicken anemia virus replication and virulence. Virol. J. 14:38. doi: 10.1186/s12985-017-0713-9
Yao, S., Gao, X., Tuo, T., Han, C., Gao, Y., Qi, X., et al. (2017). Novel characteristics of the avian gyrovirus 2 genome. Sci. Rep. 7:41068. doi: 10.1038/srep41068
Ye, J., Liang, G., Zhang, J., Wang, W., Song, N., Wang, P., et al. (2016). Outbreaks of serotype 4 fowl adenovirus with novel genotype, China. Emerg. Microbes Infect. 5:e50. doi: 10.1038/emi.2016.50
Keywords: novel FAdV-4, duck origin, dynamic distribution, real-time PCR, different hosts
Citation: Pan Q, Yang Y, Shi Z, Liu L, Gao Y, Qi X, Liu C, Zhang Y, Cui H and Wang X (2017) Different Dynamic Distribution in Chickens and Ducks of the Hypervirulent, Novel Genotype Fowl Adenovirus Serotype 4 Recently Emerged in China. Front. Microbiol. 8:1005. doi: 10.3389/fmicb.2017.01005
Received: 27 March 2017; Accepted: 19 May 2017;
Published: 06 June 2017.
Edited by:
Akio Adachi, Tokushima University, JapanReviewed by:
Jowita Samanta Niczyporuk, National Veterinary Research Institute (NVRI), PolandSubir Sarker, La Trobe University, Australia
Copyright © 2017 Pan, Yang, Shi, Liu, Gao, Qi, Liu, Zhang, Cui and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongyu Cui, cuihongyu@caas.cn Xiaomei Wang, xmw@hvri.ac.cn
 Qing Pan
Qing Pan Yanchao Yang1
Yanchao Yang1