- 1Marine Bio-products Research Laboratory, Department of Plant, Food and Environmental Sciences, Dalhousie University, Truro, NS, Canada
- 2Research & Development, Acadian Seaplants Limited, Dartmouth, NS, Canada
Abiotic and biotic stresses limit the growth and productivity of plants. In the current global scenario, in order to meet the requirements of the ever-increasing world population, chemical pesticides and synthetic fertilizers are used to boost agricultural production. These harmful chemicals pose a serious threat to the health of humans, animals, plants, and the entire biosphere. To minimize the agricultural chemical footprint, extracts of Ascophyllum nodosum (ANE) have been explored for their ability to improve plant growth and agricultural productivity. The scientific literature reviewed in this article attempts to explain how certain bioactive compounds present in extracts aid to improve plant tolerances to abiotic and/or biotic stresses, plant growth promotion, and their effects on root/microbe interactions. These reports have highlighted the use of various seaweed extracts in improving nutrient use efficiency in treated plants. These studies include investigations of physiological, biochemical, and molecular mechanisms as evidenced using model plants. However, the various modes of action of A. nodosum extracts have not been previously reviewed. The information presented in this review depicts the multiple, beneficial effects of A. nodosum-based biostimulant extracts on plant growth and their defense responses and suggests new opportunities for further applications for marked benefits in production and quality in the agriculture and horticultural sectors.
Introduction
The global effects of negative climatic changes have manifested as desertification, increased atmospheric CO2 and temperature, soil salinization, and nutrient imbalances (e.g., mineral toxicity and deficiency) and have caused dramatic effects on agricultural production and the quality of crops (dos Reis et al., 2012). Such abiotic stresses have reduced the growth, development, productivity, and quality of plants and, in extreme conditions, resulted in death and local extinction of species (Matesanz et al., 2010; Anderson et al., 2011). Abiotic stresses are reported to have led to an average yield loss greater than 50% in most crops (Boyer, 1982; Vinocur and Altman, 2005). Rice yields declined 15% per 1°C rise in mean growing season temperature, measured from 1979 to 2003 (Peng et al., 2004). Additionally, changing climatic conditions can increase plant susceptibility to pathogens (West et al., 2012; Elad and Pertot, 2014), further increasing adverse growing conditions for plants.
The global amount of cultivable land available for agriculture is continuously shrinking due to urbanization and the adverse effects of climate change. In order to meet the ever-increasing demands of the growing human population, world food production must double by the year 2050 (Qin et al., 2011; Voss-Fels and Snowdon, 2016). To address the pressures associated with increasing agricultural productivity to subsequently meet the rising demands for food, producers have turned to excessive applications of synthetic (chemical) fertilizers and pesticides. These harmful chemicals pose both short- and long-term threats to the health of the entire biosphere (Damalas and Koutroubas, 2016). Therefore, an effective, biological-based alternative is required in order to reduce dependency on synthetic fertilizers and pesticides. Plant biostimulants are a new class of crop input, offering a potential alternative to traditional, agro-chemical inputs, and, in most cases, can reduce the application rates of synthetic fertilizers and pesticides by enhancing their efficacy (Calvo et al., 2014; Van Oosten et al., 2017; Yakhin et al., 2017).
According to the European Biostimulants Industry Council (EBIC), “plant biostimulants contain substance(s) and/or micro-organisms whose function when applied to plants or the rhizosphere is to stimulate natural processes to enhance/benefit nutrient uptake, nutrient efficiency, tolerance to abiotic stresses, and crop quality”1. The concept of biostimulants has been researched since 1933 (Yakhin et al., 2017) but has gained attention in more recent years as a potential solution to mitigate the negative impacts of a changing climate on agriculture. It should be noted that seaweed extracts are but one of the inputs that are classed as biostimulants.
Seaweeds are multi-cellular, macroscopic organisms found in coastal, marine ecosystems and are a rich source of polysaccharides, polyunsaturated fatty acids (PUFAs), enzymes, and bioactive peptides among others (Courtois, 2009; De Jesus Raposo et al., 2013; Ahmadi et al., 2015; Shukla et al., 2016; Okolie et al., 2018). In particular, inter-tidal seaweeds may be exposed to unfavorable conditions including extreme variations in temperature, salinity, and light. Seaweeds, as compared to terrestrial organisms, produce different stress-related compounds that are essential for their survival in these environments (Shukla et al., 2016). As such, selected seaweed resources are important sources of plant biostimulants and are widely used to promote agricultural productivity (Khan et al., 2009; Sharma et al., 2014; du Jardin, 2015; Van Oosten et al., 2017). The most widely researched seaweed, used as a source for industrial and commercial plant biostimulants, is the brown, inter-tidal seaweed Ascophyllum nodosum. Various commercial extracts from A. nodosum have been demonstrated to improve plant growth, mitigate some abiotic and biotic stresses while also improving plant defenses by the regulation of molecular, physiological, and biochemical processes. Of all sources of seaweed-based biostimulants, those manufactured from A. nodosum are perhaps the best studied with various modes of action being proposed (Figure 1). This review focuses on accumulating current knowledge of the bioactive compounds presents in A. nodosum extracts and their modes of action in promoting plant growth in the presence of abiotic and biotic stresses.
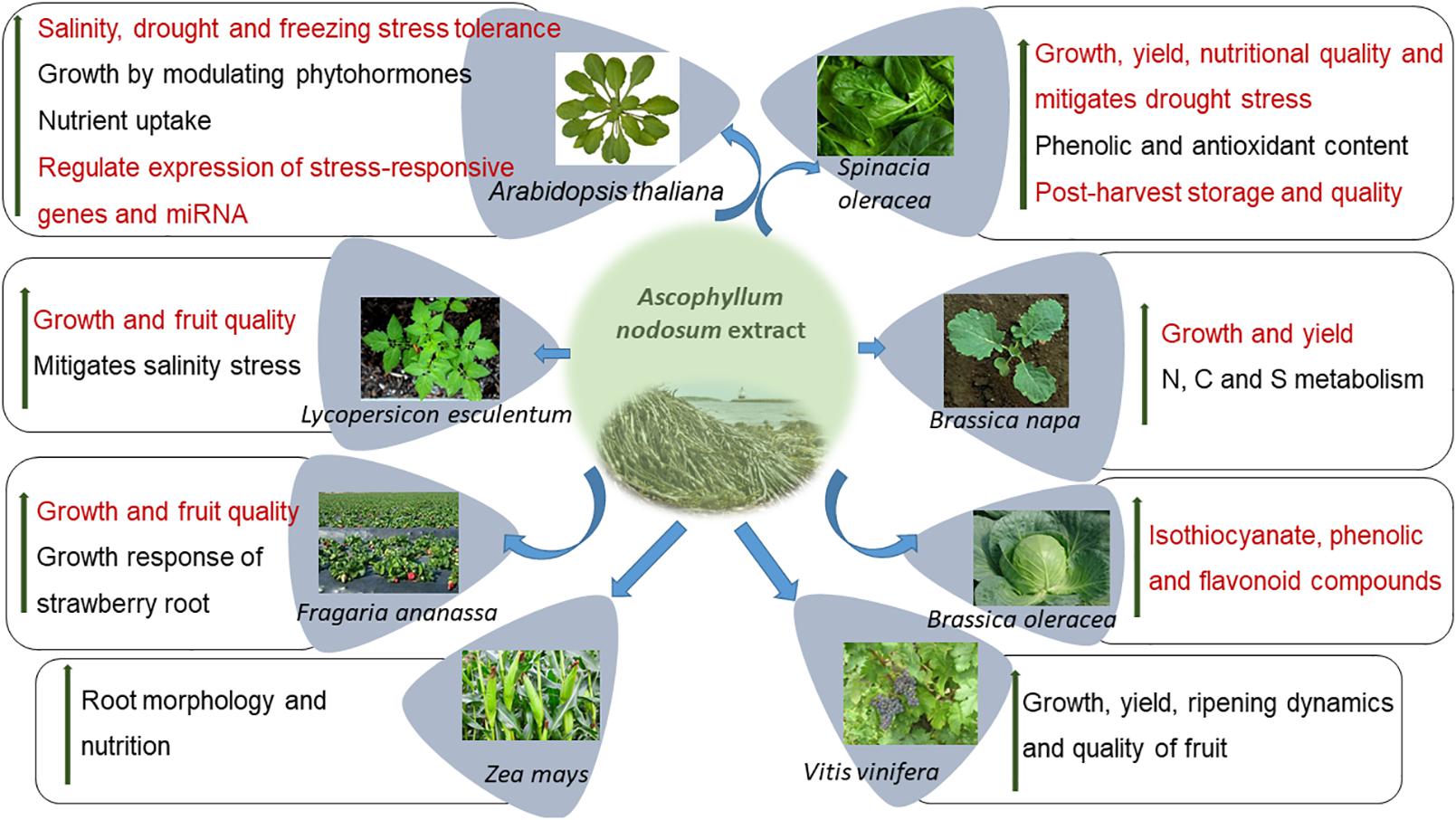
Figure 1. Ascophyllum nodosum extract (ANE) improves the growth of several crops by different modes of action.
Modes of Extraction
Various commercial entities utilize different, proprietary extraction (hydrolysis) procedures for the production of seaweed-based biostimulants in either liquid or soluble powder form (Kadam et al., 2013; Michalak and Chojnacka, 2015). Different extraction methods have been cited in the literature using both dry and wet biomass (Chojnacka et al., 2015; Michalak and Chojnacka, 2015; Bleakley and Hayes, 2017). The bioactivity and composition of A. nodosum biostimulants are not all identical and are indeed dependent on the extraction methods employed (Goñi et al., 2016).
Water-Based Extractions
The name of this extraction method is indicative of the process: biostimulatory compounds are harvested by blending and hydrating dried seaweed meal in the presence of water (Sharma et al., 2014). The solid residues are separated using different filtration methods based on the end use of the biostimulant. Biostimulants prepared using this method are reportedly rich in phytohormone-like activity (Blunden and Wildgoose, 1977; Crouch and van Staden, 1993).
Acid Hydrolysis
In this method, freshly chopped Ascophyllum biomass was treated with sulfuric acid or hydrochloric acid at 40–50°C for 30 min (Sharma et al., 2014). It was reported that acid hydrolysis removed complex phenolic compounds and increased de-polymerization of polysaccharides (Flórez-Fernández et al., 2018). This method is generally used for the extraction of fucose-containing sulfated polysaccharides (Ale et al., 2012; Flórez-Fernández et al., 2018). Sulfated algal polysaccharides are a class of bioactive compounds in algal extracts that promote plant growth (Fry et al., 1993; Paulert et al., 2009; Shukla et al., 2016). Marais and Joseleau (2001) purified fucoidans from A. nodosum by acid hydrolysis. AZAL5® is a commercially available biostimulant manufactured from A. nodosum, which is extracted through acid hydrolysis (Jannin et al., 2013).
Alkaline Hydrolysis
Alkaline hydrolysis is perhaps the most widely used industrial hydrolysis process for the production of an extract from A. nodosum (Craigie, 2011; Sharma et al., 2014; Flórez-Fernández et al., 2018). This method involves extracting A. nodosum biomass in NaOH or KOH solutions, at “relatively low” temperatures, between 70 and 100°C. This process breaks down complex polysaccharides into smaller, lower-molecular-weight oligomers. The alkali treatment of Ascophyllum biomass produces novel compounds that are not initially present within the seaweed biomass. These compounds are a result of the interaction between the hydrolysis chemicals (KOH) and constituents of the brown seaweed tissues—the result of degradation, rearrangement, condensation, and base-catalyzed synthetic reactions (Craigie, 2011). Alkali treatments of brown seaweed biomass also act on polyphenols in the tissue to produce a complex array of reaction products, which are dependent on the hydroxylation pattern of the original polyphenol (Craigie, 2011). Maxicrop® (United States), Seasol® (Australia), and Acadian® (Canada) are major commercial biostimulants that are manufactured using an alkali extraction process of Ascophyllum.
Microwave-Assisted Extraction
Microwave-assisted extraction (MAE) is suggested to be an eco-friendly extraction method for the manufacture of biostimulants from algal biomass, as compared to other solvent-based extraction procedures (Michalak et al., 2015b). In this method, slurry prepared from dried algal biomass, in either water or microwave-supported solvent, is heated by microwave energy to extract bioactive compounds (Magnusson et al., 2017; Flórez-Fernández et al., 2018). Microwave heating is based on dipole polarization and the ionic conduction of the seaweed-derived bioactive compounds into the solvent (Lucchesi et al., 2004; Flórez et al., 2015). This extraction method is favored for its efficient use of time and materials given the resultant selective extraction of carbohydrates, proteins, and other fractions (Eskilsson and Björklund, 2000; Routray and Orsat, 2012). Additionally, this extraction method was found to improve the efficiency of extraction by controlling sub-critical properties of the solvent (Routray and Orsat, 2012; Magnusson et al., 2017). MAE has been used to extract fucoidan, sodium alginate, sugars, and phenolic compounds from A. nodosum (Yuan and Macquarrie, 2015a,b; Yuan et al., 2018).
Ultrasound-Assisted Extraction
Ultrasound-assisted extraction (UAE) is reported as another eco-friendly method for obtaining bioactive compounds from algal biomass. Ultrasound waves are high frequency (greater than 20 kHz), which transmit through solid, liquid, and gas media by rarefactions (largest distance between wave particles) and compression (smallest distance between wave particles) (Kadam et al., 2013). Ultrasound waves were reported to facilitate the release of bioactive compounds from a variety of seaweed biomass by cavitation within the extraction solvent (Kadam et al., 2013, 2015a). When cavitation (i.e., the formation and eventual implosion of empty spaces or bubbles) occurs near seaweed cell walls, the transfer of compounds from the cell to the solvent is facilitated following cellular breakdown (Kadam et al., 2013, 2015a). UAE is a cost-effective and efficient method of extraction when compared to other extraction protocols based on the limited equipment needed and the vast array of solvents that can be used (Kadam et al., 2013). Kadam et al. (2015b, c) optimized the extraction procedure for the isolation of numerous bioactive compounds, including laminarin from both A. nodosum and Laminaria hyperborea.
Enzyme-Assisted Extraction
Enzyme-assisted extraction (E-AE) is an eco-friendly and efficient extraction method as there are no solvents required by the process (Kadam et al., 2013). The efficiency of the extraction lies in the enzymatic degradation of the complex molecules present in the seaweed cell walls (Wijesinghe and Jeon, 2012; Kadam et al., 2013). Enzymes are chosen strategically based on specific molecules digested from seaweed biomass in order to release the bioactive compounds (Wijesinghe and Jeon, 2012). Various carbohydrate-degrading enzymes and proteases such as Viscozyme, Cellucast, Termamyl, Ultraflo, carrageenanase, agarase, xylanase, Kojizyme, Neutrase, Alcalase, and Umamizyme are commonly used for the extraction of bioactive compounds from seaweeds (Ahn et al., 2004; Heo et al., 2005; Wijesinghe and Jeon, 2012; Kadam et al., 2013). The application of hydrolytic enzymes converts the water-insoluble chemical components of selected seaweed biomass to water-soluble products, thus eliminating the problem of water solubility of bioactive compounds (Wijesinghe and Jeon, 2012; Kadam et al., 2013). To date, there are no publications regarding the extraction of bioactive compounds from A. nodosum using E-AE. It has been reported that E-AE extracts of other seaweeds (i.e., Ecklonia cava, Ishige okamurae, Sargassum fulvellum, S. horneri, S. coreanum, S. thunbergii, and Scytosiphon lomentaria) showed higher antioxidative activity, as compared to commercial antioxidants (Heo et al., 2005). In the future, this method might be applied for the extraction of bioactive compounds from A. nodosum.
Super-Critical Fluid Extraction
The super-critical fluid extraction (SFE) method is yet another eco-friendly method of bioactive extraction from seaweeds, based on the lack of toxic solvents required for extraction (Herrero et al., 2010; Michalak et al., 2016b). This method protects the parent seaweed material against thermal or biochemical degradation of the bioactive compounds (Herrero et al., 2010; Michalak et al., 2015a; da Silva et al., 2016). Bioactive compounds are extracted in the presence of super-critical organic solvents (often CO2, based on its critical conditions, availability, and high diffusivity when mixed with ethanol; Herrero et al., 2010; Kadam et al., 2013). The higher penetration of the solvent into the seaweed material during SFE results in better mass transfer between solvent phases (Michalak et al., 2015a; Messyasz et al., 2018). Michalak et al. (2016a) showed that super-critical extracts of A. nodosum enhanced the growth and development of winter wheat.
Pressurized Liquid Extraction
Pressurized liquid extraction (PLE) was first reported by Richter et al. (1996). In this method, extraction was carried out under high pressure (3.5–20 MPa) and temperature (50–200°C) (Kadam et al., 2013). The high pressure elevated the temperature of solvents above their boiling point, facilitating bioactive compound extraction by increasing the solubility of complex algal molecules and increasing mass transfer rate (Kadam et al., 2013; Michalak and Chojnacka, 2015). PLE is a faster extraction method compared to other methods; however, Tierney et al. (2013) showed that the application of high temperatures (50–200°C) and pressures (500–3,000 psi) during extraction did not enhance the antioxidant activities of extracts from A. nodosum, Pelvetia canaliculata, Fucus spiralis, and Ulva intestinalis, as compared to extracts from the traditional solid liquid extraction method.
In addition to the aforementioned techniques, different extraction methods were also used in combination for extracting protein from A. nodosum extract. Kadam et al. (2017) combined ultrasound pretreatment with acid and alkali hydrolysis and, more simply, combined acid and alkali hydrolysis to extract protein from A. nodosum. The initial treatment of the A. nodosum with acid followed by a treatment with alkali was found to be the most efficient method among all methods investigated (Kadam et al., 2017). Similarly, combining enzymatic hydrolysis with alkaline extraction also increases the efficiency of protein extraction in Palmaria palmata (Maehre et al., 2016). The combination of extraction methods has not yet been employed for the extraction of biostimulants from A. nodosum for plant growth, creating opportunities for the future.
Ascophyllum nodosum
Ascophyllum nodosum is commonly known as rockweed, and is abundantly distributed throughout the northwest coast of Europe and the northeastern coast of North America (Moreira et al., 2017). Craigie (2011) reviewed the unique characteristics of A. nodosum as a prominent source for the production and synthesis of biostimulants. One unique feature of A. nodosum is its mutualistic association with the fungal endophyte Mycosphaerella ascophylli (Fries and Thorén-Tolling, 1978; Fries, 1979; Garbary and Gautam, 1989; Craigie, 2011). M. ascophylli protects A. nodosum from desiccation (Garbary and London, 1995). Further, results published by Prithiviraj et al. (2011) showed that M. ascophylli-derived fungal sterols present in the ethyl acetate extract of A. nodosum mitigated salinity stress in plants.
Based on the review published by Van Oosten et al. (2017), nearly 47 companies worldwide are currently involved in manufacturing extracts from A. nodosum for agricultural and horticultural applications. A. nodosum is a rich source of various bioactive phenolic compounds such as phlorotannins and unique polysaccharides, i.e., alginic acid (28%), fucoidans (11.6%), mannitol (7.5%), and laminarin (4.5%) (Ragan and Jensen, 1977; Holdt and Kraan, 2011; Yuan and Macquarrie, 2015a; Moreira et al., 2017). Commercially dried and milled, A. nodosum meal is reported to contain carbohydrate (44.7 ± 2.1%), ash (18.6 ± 0.9%), protein (5.2 ± 0.2%), lipids (3.0 ± 0.1%), phenolics (1.4 ± 0.2%), and other compounds (13.6%) (Yuan and Macquarrie, 2015b; Moreira et al., 2017). Some of these compounds showed considerable seasonal variation (Parys et al., 2009; Craigie, 2011). The bioactive compounds present in A. nodosum were extracted and utilized to promote plant growth according to Van Oosten et al. (2017).
ANE Improves Fruit Quality, Plant Growth, and Yield
Commercial, hydrolyzed extracts from A. nodosum (ANE) have been repeatedly demonstrated to exhibit growth-stimulating activities in treated plants, when applied repeatedly at very low doses, and are referred to as “biostimulants” (Sharma et al., 2014; Van Oosten et al., 2017). Table 1 lists publications on the growth-promoting activities of commercial extracts of A. nodosum. The applications of different extracts of A. nodosum are repeatedly demonstrated to improve the growth and productivity of crops through various modes of action (Figure 1).
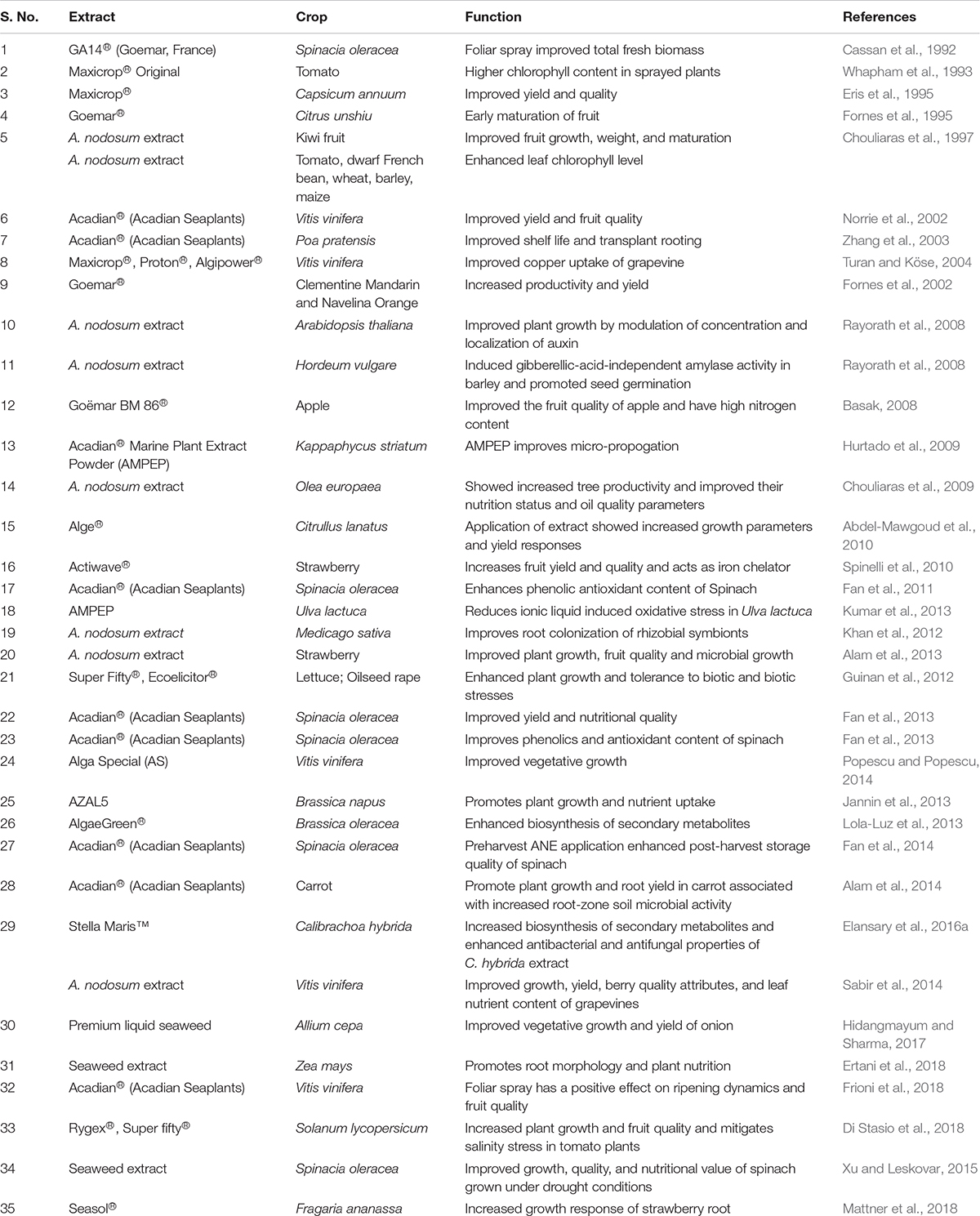
Table 1. List of extracts manufactured from A. nodosum biomass that were reported to improve plant growth.
Fruit Quality
A foliar spray of A. nodosum improved fruit quality of watermelons, apples, olives, and grapes (Basak, 2008; Chouliaras et al., 2009; Abdel-Mawgoud et al., 2010; Frioni et al., 2018). Foliar application of ANE also increased the ripening rate of grapes (Norrie et al., 2002; Sabir et al., 2014; Frioni et al., 2018) and increased oil content and consistency of fruit maturation in olive (Chouliaras et al., 2009).
Nutrient Acquisition, Accumulation, and Biosynthesis
Ascophyllum nodosum was reported to improve both the growth and productivity of agricultural crops by increasing nutrient availability and uptake (Crouch and van Staden, 1993; Khan et al., 2009; Craigie, 2011; Sharma et al., 2014; Van Oosten et al., 2017). Several publications indicated that a foliar application of ANE to the leaves of Vitis vinifera, after full bloom, increased the nutrient content of grapevines, specifically the accumulation of anthocyanins and phenolics (Norrie et al., 2002; Sabir et al., 2014; Frioni et al., 2018). Two commercial extracts of A. nodosum, Rygex® and Super Fifty®, enhanced the macronutrient (N, P, K, Ca, S) and micronutrient (Mg, Zn, Mn, Fe) contents of tomato fruits (Di Stasio et al., 2018). Similarly, olive plants (Olea europaea) treated with ANE showed a higher uptake of K, Fe, and Cu (Chouliaras et al., 2009). When applied at a rate of 0.1% (v/v), AZAL5®, a commercial seaweed extract, improved root and shoot growth of rapeseed (Brassica napus) by stimulating nitrogen and sulfate accumulation (Jannin et al., 2013). Microarray analysis revealed that B. napus plants treated with AZAL5® showed differential regulation of 724 and 298 genes in shoots and roots, respectively, after 3 days of treatment, while 612 and 439 genes were differentially regulated in the shoots and roots, respectively, after 30 days of treatment (Jannin et al., 2013). Treatment with AZAL5® increased the nitrate uptake of B. napus by inducing the expression of BnNRT1.1 and BnNRT1.2 genes, known to be involved in nitrate assimilation and amino acid metabolism. Similarly, plants treated with AZAL5® showed higher sulfate accumulation by the induction of BnSultr1.1 and BnSultr1.2 genes (Jannin et al., 2013). Commercial extracts Maxicrop®, Proton®, and Algipower® were also reported to improve the nutrient uptake of grapevines (V. vinifera) (Turan and Köse, 2004).
Ascophyllum nodosum extracts enhanced the growth of leafy vegetables such as spinach (Spinacia oleracea) and lettuce (Lactuca sativa) (Cassan et al., 1992; Moller and Smith, 1998; Fan et al., 2013; Chrysargyris et al., 2018). A root-drench application of ANE induced the expression of glutamine synthetase in spinach (Fan et al., 2013), which is responsible for the conversion of inorganic ammonium to organic glutamine, and also plays an important role in nitrogen metabolism and assimilation (Oliveira et al., 2002). In addition to this, root application of ANE induced the expression of nitrate reductase, an important enzyme involved in nitrogen assimilation, which catalyzes the conversion of nitrate to nitrite (Fan et al., 2013). Taken together, these results suggest that ANE plays an important role in plant growth by enhancing nutrient uptake through the regulation of genes involved in nutritional acquisition. Pre-harvest treatment with 1.0 g/L of ANE through a root-drench improved the quality and nutrient content of spinach during post-harvest storage (Fan et al., 2014). The foliar application of 1% Biopost AG200® liquid seaweed extract (Cofuna, France) biweekly for 5 weeks enhanced the relative growth and quality (post-harvest) of lettuce grown under K-deficient conditions by increasing antioxidant activity (Chrysargyris et al., 2018).
Pre-harvest root treatment by ANE (Acadian®) was reported to have a profound effect in reducing post-harvest losses by reducing lipid peroxidation (Fan et al., 2014). The results presented by Fan et al. (2011) reported that an application of ANE increased antioxidants and stimulated phenolic compound biosynthesis in spinach. Furthermore, the ANE-induced biosynthesis of phenolic antioxidants in spinach, when provided as a feed intake, protected Caenorhabditis elegans against oxidative and thermal stress (Fan et al., 2011). Similarly, the provision of Tasco®-Forage, a feed supplement (air dried and milled A. nodosum), improved non-enzymatic antioxidant compounds such as α-tocopherol, ascorbic acid, and β-carotene in turf and forage grasses (Allen et al., 2001). Tasco®-Forage also induced the activity of the enzymes superoxide dismutase (SOD), glutathione reductase, and ascorbate peroxidase (APX) in forage grasses (Allen et al., 2001). AlgaeGreen®, a commercial A. nodosum extract, increased the yield and secondary metabolite content of cabbage (Brassica oleraceae) (Lola-Luz et al., 2013). Treatment with ANE significantly enhanced vegetative growth as well as the biosynthesis of bioactive molecules such as phenolics and flavonoids of Calibrachoa hybrid, a medicinal plant (Elansary et al., 2016a). ANE-induced biosynthesis of secondary metabolites further enhanced the antifungal and antibacterial activity of the extract of Calibrachoa (Elansary et al., 2016a).
A root application of 1.0 g/L ANE was reported to induce the accumulation of transcripts of betaine aldehyde dehydrogenase (BADH) and choline mono-oxygenase (CMO) in spinach grown in vitro (Fan et al., 2013). These enzymes are known to catalyze a two-step pathway involved in the biosynthesis of glycine betaine in plants. Glycine betaine, an amphoteric quaternary ammonium compound, is an efficient, compatible solute that protects plants against environmental stresses (Sakamoto and Murata, 2002). The A. nodosum extract used was shown to contain quaternary ammonium compounds such as glycine betaine, δ-aminovaleric acid betaine, γ-aminobutyric acid betaine, and laminine (Blunden et al., 1985, 1996; Whapham et al., 1993; MacKinnon et al., 2010).
Hurtado and Critchley (2018) reviewed the biostimulant effect of Ascophyllum (Acadian®) Marine Plant Extract Powder (AMPEP) in increasing the cultivation and micro-propagation of the commercially important seaweed, Kappaphycus alvarezii. The application of AMPEP, a product derived from A. nodosum, improved the biomass cultivation of K. alvarezii (Marroig et al., 2016). The results of Tibubos et al. (2017) showed that AMPEP induced the direct formation of axes in new plantlets of K. alvarezii. These results provide clear evidence that the Ascophyllum-derived extract can potentiate growth of commercially important seaweed crops.
ANE Improves Plant Growth by Regulating Phytohormone Biosynthesis in Plants
Phytohormones are low-molecular-weight compounds produced in very small quantities that regulate several physiological and developmental processes in plants (Wally et al., 2013; Wani et al., 2016). The most common phytohormones include auxins (IAA), cytokinins (CK), abscisic acid (ABA), gibberellic acid (GA), ethylene, jasmonic acid (JA), and salicylic acid (SA) (Wani et al., 2016). One reported growth-promoting effect of ANE was ascribed to the presence of a variety of “phytohormone-like substances” (Stirk and Van Staden, 1997; Khan et al., 2009; Craigie, 2011; Sharma et al., 2014; Battacharyya et al., 2015).
There is a wide variation in auxin content in A. nodosum extracts reported in the literature. A. nodosum was reported to have a high concentration of indole acetic acid (IAA), approximately 50 mg/g of dry extract (Kingman and Moore, 1982; Khan et al., 2009), whereas Maxicrop®, a different commercial product also prepared from A. nodosum, contained 6.63 mg of IAA per gram of dried powder (Sanderson et al., 1987). By using ultra-performance liquid chromatography–electrospray tandem mass spectrometry, Wally et al. (2013) confirmed the presence of 25–35 ng of IAA per dry gram of the extract they tested. This variation in auxin content is likely to be a function of the method of extraction and processing, as well as the geographical location of the raw material harvested including any possible seasonal variation (Stirk and Van Staden, 1996; Wally et al., 2013).
SAURs (small auxin-up RNAs) are a group of small auxin-induced RNAs that reportedly play an important role in cellular, physiological, and developmental processes (Ren and Gray, 2015). The expression of SAUR33, SAUR59, and SAUR71 were up-regulated by the foliar application (0.2%) of commercially available neutral and alkaline extracts from A. nodosum, while SAUR1 and SAUR50 were down-regulated by both extracts (Goñi et al., 2016). Buggeln and Craigie (1971) reported biologically active homologs of auxin-like compounds in alkaline hydrolysates of A. nodosum. Bioactive compounds present in a methanolic fraction of this commercial ANE elicited plant growth by enhancing root tip growth and showed higher GUS expression in the DR5: GUS transgenic line of Arabidopsis thaliana (Rayorath et al., 2008). These findings strongly suggested that the organic fraction of ANE regulated auxin activity in ANE-treated plants through the regulation of auxin-responsive promoter elements (AuxRE) (Rayorath et al., 2008).
Cytokinins are derivatives of adenines that possess either an isoprenoid or aromatic side chain at the N6 position (Frébort et al., 2011). Previously published reports demonstrated that various cytokinins and “cytokinin-like compounds” were the most abundant plant growth regulators present in commercial extracts of A. nodosum (Stirk and Van Staden, 1997; Khan et al., 2011; Wally et al., 2013). Maxicrop® was reported to contain a complex of cytokinins including zeatin, di-hydrozeatin, iso-petenyladenine, and iso-petenyladenosine (Sanderson et al., 1987). Stirk and Van Staden (1996) investigated the cytokinin activity of the commercial seaweed extract Seamac® by evaluating its effect on soybean callus, where Seamac® induced maximum soybean callus formation. Khan et al. (2011) and Wally et al. (2013) showed that a root application of an alkaline extract of A. nodosum resulted in the activation of the cytokinin-responsive promoter ARR5. The application of this commercial seaweed extract to A. thaliana showed a higher concentration of CK and ABA, coupled with a reduction in IAA levels. This observation helps to explain the varied mechanisms of actions behind higher vegetative plant growth and the reduction in the length of primary roots (Wally et al., 2013).
Wally et al. (2013) showed that the application of an ANE increased cytokinin concentrations in the tissues of A. thaliana, particularly trans-zeatin-type and cis-zeatin-type cytokinins. The first step of cytokinin biosynthesis involves the transfer of an isoprenoid molecule to adenine by isopentenyl transferases (IPTs). ANE applications induced the expression of IPT3, IPT4, and IPT5 in A. thaliana, while the expressions of IPT2 and IPT9 were unchanged (Wally et al., 2013). In this study, ANE also regulated the transcript levels of cytosolic and mitochondrial IPTs (IPT3, IPT4, and IPT5) and induced the production of isopentenyl-type cytokinins via the mevalonate (MVA) pathway (Frébort et al., 2011; Wally et al., 2013). Similar to the expression pattern of IPT3, IPT4, and IPT5, the expression of CK hydroxylases (CYP735A2), which catalyze the biosynthesis of trans-zeatin, was higher in ANE-treated A. thaliana plants (Takei et al., 2004; Wally et al., 2013). Furthermore, the ANE treatment suppressed the expression of genes involved in cytokinin degradation (Wally et al., 2013). The accumulation of cytokinin oxidase 4 (CKX4), involved in cytokinin catabolism, was reduced in ANE-supplemented Arabidopsis plants. This suggests that ANE applications induced a higher metabolic production of cytokinins within treated plant tissues by differentially regulating cytokinin metabolism. High cytokinin content in plants was found to delay senescence (Gan and Amasino, 1995; Lim et al., 2003). Wally et al. (2013) showed that the ANE application retarded senescence in treated Arabidopsis by increasing the endogenous cytokinin content. This result was further supported by the strong inhibition of expression of Senescence Associate Gene 13 (SAG13) in plants treated with ANE (Wally et al., 2013).
The root application of ANE modulated the expression of genes involved in GA biosynthesis and thus resulted in a higher accumulation of GA24 (Wally et al., 2013). Similarly, a foliar application of 0.2% ANE on Arabidopsis leaves also regulated the expression of the GA-responsive genes GASA1 and GASA4, after 1 week of treatment (Goñi et al., 2016). This published evidence concluded that ANE treatment regulated endogenous phytohormone levels and possibly their ratios to one another within treated plant tissues by modulating the expression of genes involved in their biosynthesis. Subsequently, the modulation of gene expression improved plant growth and development.
ANE Mitigates Abiotic Stresses in Plants
Plants, being sessile, are relentlessly challenged by a variety of environmental stresses that limit their growth and productivity (Agarwal et al., 2013; Shukla et al., 2016). Due to the complex metabolic pathways involved in stress tolerance, limited success has been achieved in generating stress-tolerant crops through genetic engineering (Agarwal et al., 2013; Mickelbart et al., 2015). Another sustainable approach to improve stress tolerance in plants is the use of extracts from A. nodosum. Table 2 summarizes studies published on the effect of ANE on plants under abiotic stress.
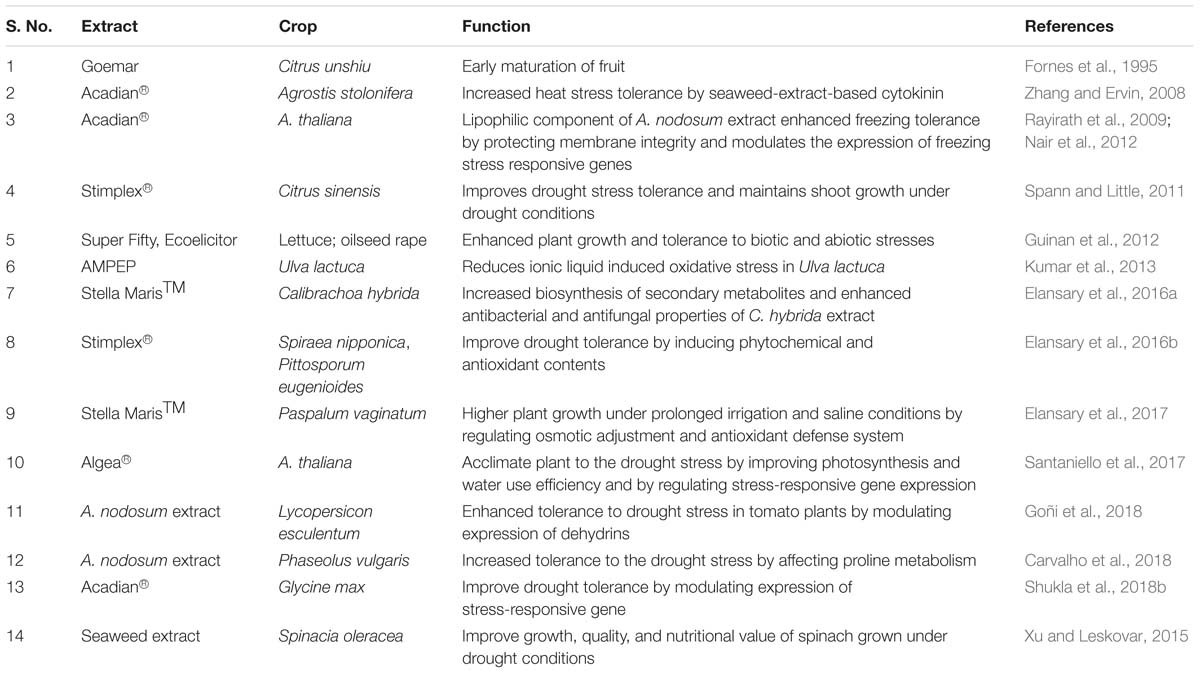
Table 2. List of the different extracts from A. nodosum conferring salinity stress tolerance in various crops.
ANE Improves Salinity Tolerance in Plants
Soil salinity is a global problem, affecting over 800 million hectares of land, resulting in massive impacts on agricultural productivity (Shrivastava and Kumar, 2015; Ferchichi et al., 2018). Mild salinity stress causes physiological drought in plants, impairing cell–water relations, inhibiting cell expansion, and, consequently, reducing growth rate (Hasegawa et al., 2000a). Long-term exposure to high salinity causes ionic stress by disturbing the homeostasis of intracellular ions, which results in membrane dysfunction and attenuation of metabolic activity and secondary effects, inhibiting growth, and inducing cell death (Hasegawa et al., 2000b; Yadav et al., 2012; Hasegawa, 2013; Shukla et al., 2011, 2015). Salinity induces both ionic and osmotic stresses, thus reducing plant growth and productivity (Agarwal et al., 2013). Plants have developed strategies to adapt to salinity stress at molecular, biochemical, and physiological levels (Agarwal et al., 2013; Hasegawa, 2013; Ferchichi et al., 2018).
Studies revealed that the application of various forms of ANE improved salinity stress tolerance in Arabidopsis, tomato (Solanum lycopersicum), passion fruit (Passiflora edulis), and avocado (Persea americana) (Jithesh et al., 2012, 2018; Bonomelli et al., 2018; Di Stasio et al., 2018; Jolinda et al., 2018; Shukla et al., 2018a). Rygex® and Super Fifty®, both commercial extracts of A. nodosum, boosted the accumulation of minerals, antioxidants, and essential amino acids in tomato fruits grown under salinity stress (Di Stasio et al., 2018). Salinity stress reduced both the growth and yield of avocado by almost 50% (Alvarez-Acosta et al., 2018; Bonomelli et al., 2018). The application of A. nodosum-based extracts reportedly alleviated the effects of salinity stress on the growth and productivity of avocado by improving nutrient uptake. A. nodosum extract-treated avocado plants showed higher content of Ca2+ and K+ (Bonomelli et al., 2018). Further, ANE also improved the growth of turf grass grown under salinity stress by maintaining a higher K+/Na+ content (Elansary et al., 2017).
An ethyl acetate fraction of an A. nodosum extract (EAA) reportedly induced salinity tolerance in Arabidopsis. To further investigate the mode of action of ANE in mitigating stress, Jithesh et al. (2018) carried out a study of the global transcriptomics of EAA-treated Arabidopsis grown under salinity stress. This study showed that EAA induced the expression of 184 genes on Day 1 of treatment, further increasing to 257 genes expressed on Day 5, while 91 and 262 genes were down-regulated on Days 1 and 5 post-treatment, respectively. Similarly, Goñi et al. (2016) also compared the transcriptome of Arabidopsis treated with two different extracts of A. nodosum: both prepared at a temperature greater than 100°C, differing in pH and preparation method (one extract had a neutral pH while the other extract was alkaline). The application of these two different extracts induced the expression of a plethora of genes involved in stress tolerance mechanisms (Goñi et al., 2016). Both studies showed the induction of different late embryogenesis abundant (LEA) proteins and dehydrins in Arabidopsis treated with ANE. Thus, it is evident that various bioactive components of an A. nodosum extract were able to mitigate salinity stress through various mechanisms: by protecting cellular structures from water loss, via acting as a hydration buffer, sequestering ions, directly protecting other proteins or by re-naturing unfolded proteins through increased expression of LEAs (Wise and Tunnacliffe, 2004; Goyal et al., 2005; Jithesh et al., 2018).
The molecular and cellular responses of plants to salinity stress include perception, signal transduction to the cytoplasm and nucleus, gene expression, and, finally, metabolic alterations leading to stress tolerance (Agarwal et al., 2006). Salinity stress signals are first perceived by signaling molecules such as ABA and Ca2+, and these molecules start a cascade of events eventually leading to stress tolerance in plants (Chinnusamy et al., 2004; Bhatnagar-Mathur et al., 2008; Agarwal et al., 2013). Arabidopsis treated with an ethyl acetate fraction of ANE (EAA) and grown under salinity stress showed a higher transcript accumulation of SnRK2, a gene involved in the activation of the ABA-signaling network (Coello et al., 2011; Jithesh et al., 2018). Further, EAA treatments induced genes involved in ABA-dependent signaling pathways (Jithesh et al., 2018). Transcription factors (TFs) regulate the expression of various downstream target genes by interacting with the cis-acting element in promoters of respective target genes (Yamaguchi-Shinozaki and Shinozaki, 2006; Agarwal and Jha, 2010; Agarwal et al., 2013). The bioactive component present in A. nodosum extracts has been shown to regulate the convergence and interaction of various TFs such as DREB/CBF, COR47, NF-YA, COR15A, AGF2, CCA1, and LHY1, which confer stress tolerance on plants (Agarwal and Jha, 2010; Todaka et al., 2012; Goñi et al., 2018; Jithesh et al., 2018). ANE regulated both post-transcriptional as well as post-translational regulation of stress-responsive TFs (Shukla et al., 2018a). The application of ANE down-regulated the expression of miR396a-5p, which resulted in a reduction in the expression of its target gene AtGRF7 (Yang et al., 2009; Shukla et al., 2018a). In Arabidopsis, AtGRF7 down-regulated the expression of AtDREB2a by binding to its promoter element, which, in turn, acted as a down-regulator for salinity tolerance (Shukla et al., 2018a). Lower levels of AtGRF7 in the ANE-treated plants under salinity stress led to a higher expression of AtDREB2a and AtRD29 (Shukla et al., 2018a). Similarly, ANE reduced the expression of miR169 in plants grown under salinity stress conditions. The miR169 plays an important role in stress-induced flowering in plants, targeting TF NFYA (Xu et al., 2014). The application of ANE to plants grown under salinity stress delayed the induction of ath-miR169g-5p and showed a higher expression of AtNFYA1. This suggests that benefits of the application of ANE, as a salinity stress mitigation strategy, were due to the partial control of miR169 over NFYA1 expression (Zhao et al., 2009; Li et al., 2013; Jithesh et al., 2018; Shukla et al., 2018a).
Salinity stress leads to the generation of reactive oxygen species (ROS) in plants, which is a well-known cause of damage to proteins, lipids, carbohydrates, and DNA, resulting in oxidative stress, which ultimately results in negative effects on plant development and growth (Mittler et al., 2004; Gill and Tuteja, 2010; Karuppanapandian et al., 2011). Salinity stress-induced production of ROS damages cell membranes by changing the saturation pattern through increased lipid peroxidation (Gossett et al., 1994; Jain et al., 2001; Miller et al., 2010). ANE application has been reported to reduce the effects of ROS generated by salinity stress in turf grass by reducing lipid peroxidation through higher activity of antioxidative enzymes. The various bioactive components of A. nodosum extracts reduced salinity-induced oxidative damage by eliciting the expression of glutathione S transferase in Arabidopsis (Jithesh et al., 2018). ANE application also alleviated oxidative damage by modulating the expression of ath-miR398, regulating the expression of its target gene, copper/zinc SOD (AtCSD1) (Shukla et al., 2018a).
The application of ANE had a significant influence on the expression of genes involved in the biosynthesis and transportation of flavonoids, which protect plants from ROS-induced oxidative damage during salinity stress (Jithesh et al., 2018). In addition to the regulation of regulatory genes, it was reported that ANE applications also regulated the expression of genes involved in the biosynthesis of carbohydrates (starch, sucrose, raffinose), amino acids (proline, isoleucine), and sugar alcohols (inositol, trehalose) (Jithesh et al., 2018). ANE-treated plants accumulated higher proline tissue levels under saline conditions (Elansary et al., 2017). Higher proline levels can mitigate salinity stress by stabilizing sub-cellular structures and scavenging free radicals while also buffering cellular redox potentials (Ashraf and Harris, 2004; Ashraf and Foolad, 2007; Shukla et al., 2015). Salinity stress reduces osmotic potential and affects water availability, causing physiological drought in plants. Sugar accumulation maintains total osmotic potential in plant cells during salt stress (Shukla et al., 2011). In addition to their role in osmotic adjustments, the availability and inter-organ transport of sugars play an important regulatory role in salt-stressed plants (Hare et al., 1998). The data reported by Elansary et al. (2017) indicated that ANE treatments enhanced the total non-structural carbohydrates in turf grass exposed to prolonged salinity, by increasing photochemical efficiency. ANE regulated the expression of genes involved in the metabolism and transport of carbohydrates; thus, unspecified bioactive compounds present in ANE must supply enough carbon and energy to the plant during stressful conditions.
Ascophyllum nodosum treatments were also reported to improve nutrient uptake in plants grown under salinity stress. Supplementation of ANE in growth media deprived of phosphorus (P) improved its uptake and homeostasis in salt-stressed Arabidopsis by modulating the expression of miRNA399, altering the expression of its target gene AtUBC24. In addition to miR399, the ANE treatment also modulated the expression of miR827 and miR2111b, indicating that some components of ANE and their utilization by plant tissues have the ability to improve P-uptake in salt-stressed plants (Shukla et al., 2018a). Similarly, ANE treatments improved the architecture of Arabidopsis root systems when grown under conditions combining phosphorus deprivation and salinity stress. ANE therefore played an important role in sulfur (S) homeostasis in salt-exposed Arabidopsis by modulating the expression of miR395 (Shukla et al., 2018a). In addition to the regulation of sulfur homeostasis, ANE treatments also regulated the expression of SULTR1;2 and SULTR3;1 in plants grown under both normal and saline conditions (Goñi et al., 2016; Shukla et al., 2018a). Thus, ANE prevented the root tip and its meristematic cell from the injurious consequence of both stresses by regulating the expression of regulatory RNAs and genes involved in the efficient relocation of P and S resources (Shukla et al., 2018a). A clear, beneficial role for ANE has been observed in mitigating salinity stress due to its ability to improve a plant’s response to stress, both at the molecular and at the physiological level, as represented in Figure 2.
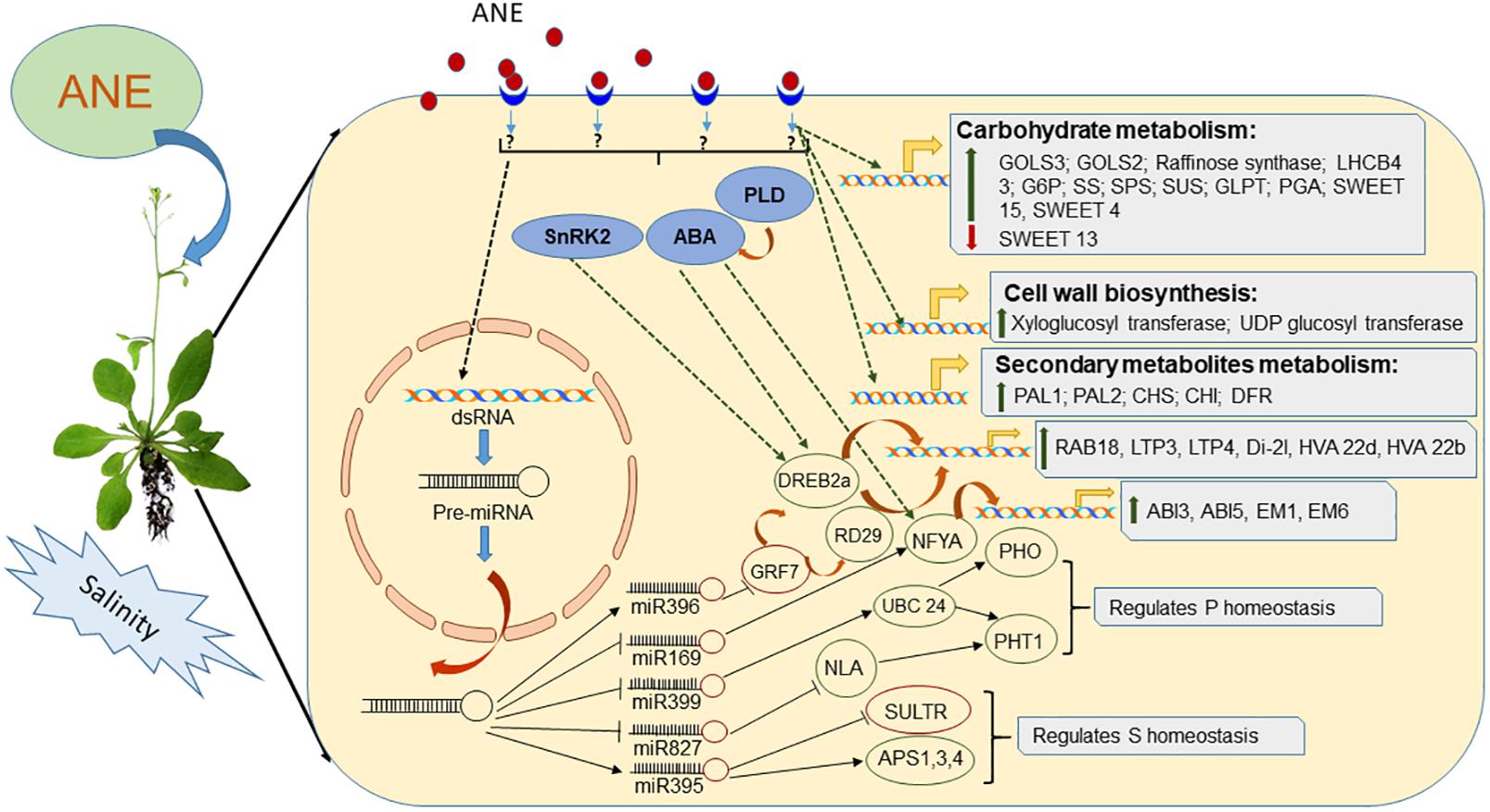
Figure 2. Depiction of mode of action of Ascophyllum nodosum extract (ANE) in mitigating salinity stress.
ANE Mitigates Drought Stress in Plants
Both physical and physiological drought negatively impact plant physiology and thereby crop productivity by impeding nutrient and water relations, photosynthesis, and assimilate partitioning (Fahad et al., 2017; Shukla et al., 2018b). It is estimated that nearly 50% of agricultural crops are affected by drought stress worldwide (Farooq et al., 2009; Bodner et al., 2015; Joshi et al., 2016). Notable progress has been made to mitigate drought stress by using bioactive substances from A. nodosum (Figure 3). Several studies clearly demonstrated that the application of different ANEs alleviated drought stress in soybean (Glycine max), bean (Phaseolus vulgaris), A. thaliana, tomato (Lycopersicon esculentum), sweet orange (Citrus sinensis), spinach (Spinacea oleracea), Spiraea nipponica, and lemon wood (Pittosporum eugenioides) (Spann and Little, 2011; Xu and Leskovar, 2015; Elansary et al., 2016b; Santaniello et al., 2017; Carvalho et al., 2018; Goñi et al., 2018; Shukla et al., 2018b). The bioactive compounds (not yet fully elucidated) present in A. nodosum extracts when applied to stressed plants have reduced the deleterious effects of drought stress by regulating a series of sequential molecular, cellular, and physiological responses including the modulation of several genes, resulting in an accumulation of various osmolytes, an improved antioxidant system, and enhanced gaseous exchange through stomatal regulation.
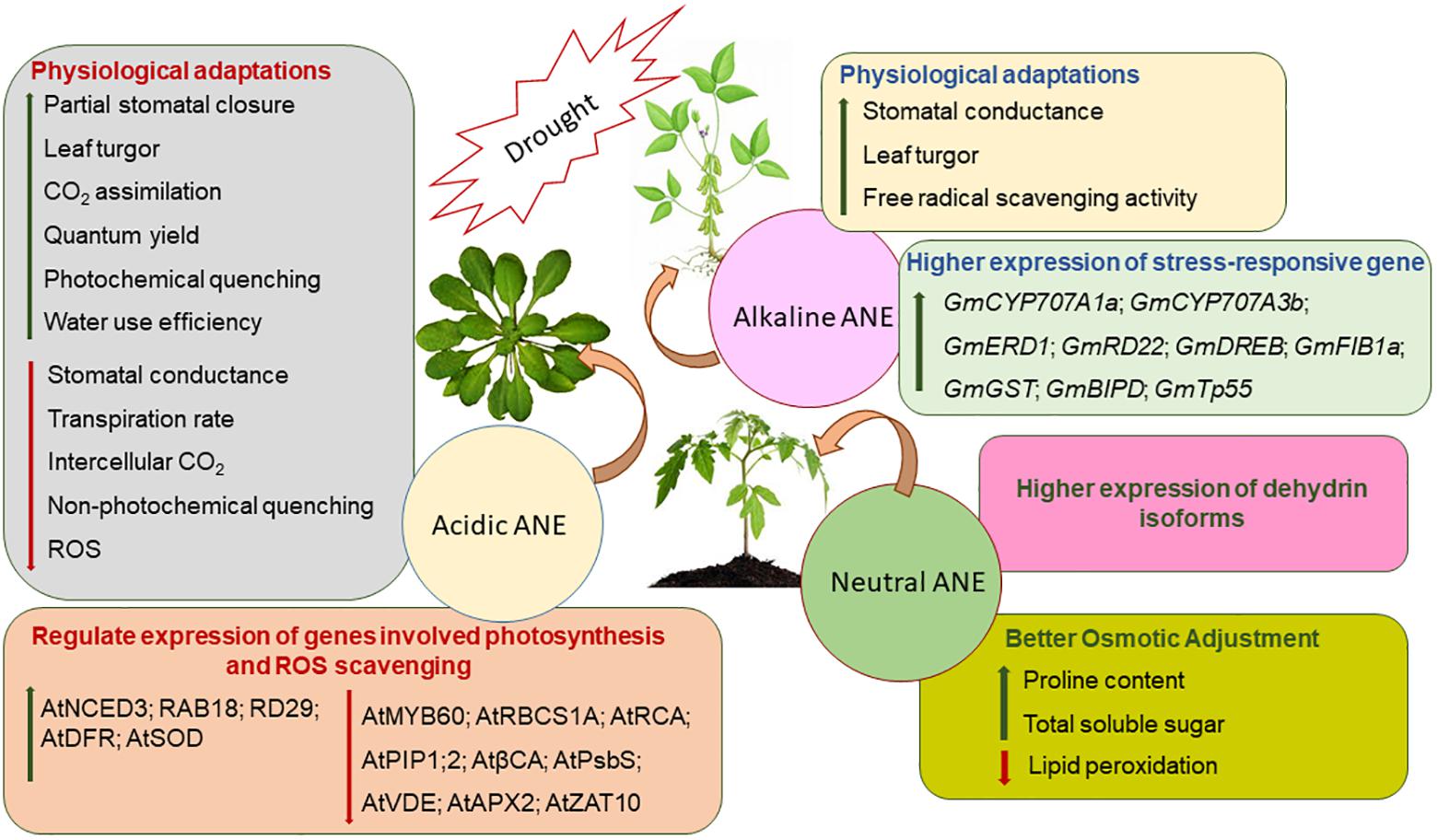
Figure 3. The proposed modes of action of three fractions of Ascophyllum nodosum extract (ANE): acidic, neutral, and alkaline ANE when applied to plants exposed to drought stress.
Drought stress reduces transpirational cooling, therefore increasing leaf temperature (Yordanov et al., 2000). Acadian®, an alkaline commercial extract of A. nodosum, was shown to help soybean plants withstand severe drought conditions by regulating leaf temperature, turgor, and several stress-responsive genes (Martynenko et al., 2016; Shukla et al., 2018b). Stomatal conductance is a key variable of a plant’s physiological process that is influenced during drought stress (Manavalan et al., 2009). Acadian® extract-treated plants showed higher stomatal conductance under drought stress (Shukla et al., 2018b), while in another study, an acidic extract of Ascophyllum also resulted in a reduction of stomatal conductance by down-regulating the expression of AtPIP1;2 and βCA1, key genes involved in the regulation of CO2 diffusion within the mesophyll (Santaniello et al., 2017). Stomatal conductance and ABA concentrations are co-related during drought stress (Manavalan et al., 2009). In drought-stressed soybean, the application of an alkaline ANE extract (Acadian®) modulated the expression of genes involved in the catabolism of ABA by regulating the expression of GmCYP707A1a and GmCYP707A3b (Shukla et al., 2018b). In addition, priming the plants with acid-extracted ANE induced a partial stomatal closure by down-regulating the expression of AtMYB60, which is known to be involved in the regulation of stomatal movement (Santaniello et al., 2017). The presence of ABA negatively regulated the expression of AtMYB60 during drought stress. Thus, ANE-treated plants, under drought stress, induced ABA biosynthesis by boosting the expression of AtNCED3, which resulted in partial stomatal closure for greater water-use efficiency (Santaniello et al., 2017). In addition, ANE treatment also induced the expression of ABA-responsive genes such as AtRAB18 and AtRD29 in response to drought stress (Santaniello et al., 2017). Taken together, these findings suggested that alkaline-extracted ANE has different modes of action in mitigating drought stress, as compared to acid-extracted ANE. Alkaline ANE regulated stomatal conductance in an ABA-independent manner while acid-extracted ANE promotes an ABA-dependent stomatal closure during drought stress.
Drought-induced stomatal closure leads to a reduction in CO2 availability, directly reducing the rate of photosynthesis (Chaves et al., 2003, 2009). Treatment with the various A. nodosum extracts modulated photochemical efficiencies, water-use potential, and stomatal conductance of Arabidopsis, spinach, S. nipponica, and P. eugenioides (Xu and Leskovar, 2015; Elansary et al., 2016b; Santaniello et al., 2017). Acidic-extracted ANE protected Arabidopsis from drought stress by inducing partial stomatal closure, thereby preventing water loss due to transpiration. Furthermore, acid-extracted ANE protected the photosynthetic apparatus by reducing the expression of AtRBCS1A and AtRCA, which catalyze Rubisco activation during photosynthesis (Demirevska et al., 2008; Santaniello et al., 2017). The alkali process extract of A. nodosum regulated the expression of GmFIB1a and protected photosystem II (PSII) from drought-induced damage (Shukla et al., 2018b). GmFIB1a functioned in an ABA-dependent manner and was involved in photo-protection during stress (Yang et al., 2006). Thus, in soybean, alkali-processed ANE extract regulated both ABA-dependent and ABA-independent pathways for conferring drought tolerance (Shukla et al., 2018b).
Plants under drought conditions tend to produce ROS that include superoxide, hydroxyl, perhydroxy, and alkoxy radicals (Mittler, 2002; Farooq et al., 2009). These ROS entities are known to damage cellular constituents such as DNA, proteins, membranes, and lipids (Fu and Huang, 2001). Drought-induced ROS production results in the peroxidation of the PUFAs found in biological membranes (Fu and Huang, 2001; Jiang and Huang, 2001). The MDA (malondialdehyde) content of tissues can be used as an indicator of the extent of drought-induced peroxidative damage (Shukla et al., 2011). As an adaptive mechanism in response to drought, plants detoxify ROS by enzymatic and non-enzymatic pathways (Apel and Hirt, 2004; Baxter et al., 2014). Enzymatic ROS scavenging mechanisms in plants include SOD, APX, glutathione peroxidase (GPX), and catalase (CAT) (Miller et al., 2010). ANE applications were reported to improve drought tolerance by reducing ROS-induced MDA production in the bean (P. vulgaris) by improving CAT activity (Carvalho et al., 2018). Similarly, a foliar spray of ANE reduced lipid peroxidation in Paspalum vaginatum that was grown under prolonged irrigation (Elansary et al., 2017). Reduced ROS in ANE-treated P. vaginatum grown under drought stress was ascribed to increased activity of antioxidative enzymes such as SOD, CAT, and APX, and the higher production of non-enzymatic antioxidants, such as ascorbates (Elansary et al., 2017).
Proline is an important osmolyte and a signaling molecule in plants, and is credited for its role in ROS scavenging as well as in osmotic adjustment (Valliyodan and Nguyen, 2006). However, it is not clear whether proline accumulation is a symptom of stress, a response to stress, or an adaptive strategy (Carillo, 2018). Regardless, proline plays an integral role in drought adaptation by buffering cellular redox potential, stabilizing membranes and proteins, and inducing the expression of stress-responsive genes (Singh et al., 2015; Carillo, 2018). ANE was found to improve proline biosynthesis in P. vulgaris grown under drought stress (Carvalho et al., 2018). Similarly, a soil treatment of ANE on S. nipponica and P. eugenioides reportedly ameliorated drought stress by increasing the accumulation of antioxidants and lipid peroxidation, thus reducing the ROS content and inherent stresses (Elansary et al., 2016b). Goñi et al. (2018) showed that extracts of the same Ascophyllum raw material, prepared by different extraction methods, regulated drought stress in treated tomato in different ways. ANE manufactured using a proprietary process at temperatures greater than 100°C and an alkaline pH was more efficient in mitigating drought stress in L. esculentum (by increased antioxidants, proline, and soluble sugar accumulation) as compared to ANE manufactured at the same temperature (T > 100°C) but at a neutral pH (Goñi et al., 2018). Dehydrins are produced by plants in response to drought, acting as intracellular stabilizers, upon targets in both the nucleus and cytoplasm (Tommasini et al., 2008). Besides the accumulation of proline and soluble sugars in ANE-treated tomato plants, ANE treatments also induced the expression of different dehydrin-like proteins under drought stress. Together, these findings verified that various extracts from A. nodosum mitigated the severity of drought stress by regulating intrinsic molecular and biochemical processes in plants.
ANE Mitigates Freezing Stress in Plants
Nearly 42% of all global land experiences temperatures below −20°C, and plants growing in these regions experience freezing stresses during periodic exposure to temperatures below 0°C (Chinnusamy et al., 2007; Miura and Furumoto, 2013). Freezing stress adversely affects plant growth and development, limiting agricultural productivity (Miura and Furumoto, 2013). During freezing stress, intracellular and extracellular ice are formed, which disrupts the integrity of cells, causing death (Burke et al., 1976; Weiser et al., 1976). Most temperate crops have an inherent tendency to acquire tolerance to low temperatures by a process known as cold acclimation, while tropical and sub-tropical plants are sensitive to low-temperature stress (Chinnusamy et al., 2003). Several studies reported that the bioactive compounds present in various types of extracts from A. nodosum can mitigate low-temperature stress in plants. The application of ANE on winter barley improved winter hardiness and increased frost resistance (Burchett et al., 1998). Rayirath et al. (2009) showed that the lipophilic fraction of an A. nodosum extract improved tolerance of A. thaliana grown under freezing conditions. Under control conditions, the A. thaliana plants grown at −5.5°C showed significant chlorosis and tissue damage, whereas plants treated with the lipophilic fraction of ANE recovered from freezing-induced damage (Rayirath et al., 2009). This study also revealed that ANE application reduced freezing-induced electrolyte leakage by maintaining membrane integrity during freezing stress. ANE also induced the expression of cold-responsive genes such as COR15A, RD29A, and CBF3 (Rayirath et al., 2009). In order to further understand the mode of action of ANE in mediating freezing tolerance in plants, Nair et al. (2012) carried out global transcriptome and metabolome analysis of the lipophilic fraction (LPC) of ANE-treated plants exposed to −2°C. Global transcriptome analysis revealed that the LPC of ANE altered the expression of 1,113 genes in response to freezing stress. Most of these genes were found to be involved in responses to stress, sugar accumulation, and lipid metabolism. In response to freezing stress, plants tend to accumulate proline by simultaneous up-regulation of genes involved in proline biosynthesis (P5CS1, P5CS2) and down-regulation of genes involved in proline catabolism (ProDH). Application of the LPC fraction of ANE increased the proline content in response to freezing stress by modulating the expression of P5CS1, P5CS2, and ProdH (Nair et al., 2012). Therefore, ANE improved freezing tolerance in plants by inducing proline biosynthesis.
Metabolite profiling of the LPC fraction of ANE-treated Arabidopsis plants revealed that protection was achieved by regulating pools of soluble sugars, sugar alcohols, organic acids, and lipophilic components such as fatty acids (Nair et al., 2012). Sugar accumulation helps plants overcome freezing stress by playing an important role in stabilizing various biological components such as the cellular membrane and membrane-bound organelles (Tarkowski and Van den Ende, 2015). The LPC of ANE failed to improve freezing tolerance in the SFR4 mutant of Arabidopsis, which is known to be defective in the accumulation of free sugars (Nair et al., 2012). These results suggested that an ANE treatment, prior to freezing stress exposure, induced the accumulation of soluble sugars. These results provided evidence to support the claim that ANE plays an important role in improving freezing tolerance in plants through molecular, biochemical, and physiological changes.
ANE Improves Plant Defenses Against Various Pathogens
Changing climatic conditions and intensive agricultural practices increase the emergence of infectious plant diseases, causing a reduction in agricultural productivity (Anderson et al., 2004; Ayliffe and Lagudah, 2004). Plant diseases are caused by pathogens such as bacteria, fungi, and viruses (Pieterse and Dicke, 2007; Stadnik and Freitas, 2014) that disrupt plant health as well as their productivity. Plants have evolved several inducible defense mechanisms in order to deter these pathogens following infection (Conrath et al., 2002; Wiesel et al., 2014). Two types of disease resistance mechanisms in plants have been reported: systemic acquired resistance (SAR) and induced systemic resistance (ISR). In SAR, SA plays a crucial role of mediating pathogenesis-related (PR) gene activation, while in ISR, JA, and ethylene (ET) pathways are important for the induction of broad-spectrum disease resistance (Gaffney et al., 1993; van Loon et al., 1998). Elicitors are defined as compounds of biological origin capable of inducing defense responses in plants (Conrath et al., 2002; Wiesel et al., 2014). Elicitors are molecules such as lipo-polysaccharides, chitin, and bacterial flagella. Furthermore, some synthetic chemicals, e.g., chitosan, 2,6-dichloro-isonicotinic acid, β-aminobutyric acid, methyl jasmonate, and benzothiadiazole, have also been reported for their ability to induce SAR and ISR against various plant pathogens (Dixon, 2001; Mercier et al., 2001; Bektas and Eulgem, 2015; Iriti and Varoni, 2015).
Over the course of evolution, various seaweeds have developed efficient defense mechanisms in order to fight their own natural pathogens (Potin et al., 1999; Shukla et al., 2016). Less incidence of pathogen infection is seemingly observed in seaweeds because they are rich sources of unique bioactive compounds such as fucans, carrageenans (e.g., i, k, and λ), ulvans, and laminarins (or fucose containing polymers) (Klarzynski et al., 2003; Sangha et al., 2010; Vera et al., 2011). These seaweed-based bioactive compounds are known to induce defense responses against pathogens by acting as priming or elicitor molecules (Khan et al., 2009; Sharma et al., 2014; Shukla et al., 2016). These elicitors act as pathogen-associated molecular patterns (PAMPs) (Sharma et al., 2014). PAMPs bind to host trans-membrane pattern recognition receptors (PRRs) and prime the plants by inducing ISR and SAR responses (Eckardt, 2008; Zipfel, 2009). Primed plants induced a greater preventative response against the progression of the pathogen infection as compared to unprimed plants.
It was reported that bioactive compounds present in ANE elicited defense responses against various pathogens (Patier et al., 1995; Sharma et al., 2014). Marmarine (IFTCTM, Amman, Jordan), a commercial extract of A. nodosum, improved plant defense against Phytopthora melonis in cucumber (Abkhoo and Sabbagh, 2016). The application of the extract [30 ml per plant, 0.5 or 1% Marmarine, alternating with 2 g L−1 of fungicide (metalaxyl), applied to 21-day-old seedlings through root drench and/or foliar spray at 5-day intervals for a total of three applications] led to enhanced activation of disease resistance enzymes including peroxidase, polyphenol oxidase, lipoxygenase, phenylalanine ammonia lyase, and β-1,3-glucanase. This work highlighted the role of certain seaweed extracts on different plant enzymes and genes that could result in the induction of defense mechanisms (or disease resistance) in cucumber (Abkhoo and Sabbagh, 2016). Similarly, Panjehkeh and Abkhoo (2016) revealed that the same initial application of A. nodosum extract Dalgin [Sustainable Agro Solutions (SAS), Spain] alternating with 2 g L−1 of fungicide (metalaxyl), as in the Abkhoo and Sabbagh (2016) study (30 ml per plant, 0.5 or 1% Dalgin, applied to 21-day-old seedlings), was able to induce resistance (ISR) against Phytophthora capsica, a fungal disease in tomato. Similarly, the alternating application of Stimplex®, a liquid-based extract of A. nodosum with fungicide (chlorothalonil, 2 g L−1), reduced the progression of fungal disease in cucumber through the induction of defense genes and enzymes (Jayaraman et al., 2011).
The mechanism of A. nodosum extract-induced resistance in A. thaliana against Pseudomonas syringae pv. tomato DC3000 was carried out by Subramanian et al. (2011). Different extracts from A. nodosum induced resistance in SA-deficient plants, while extracts did not elicit an effect on JAR1 (jasmonic acid resistance 1) mutant. In addition to this, the application of ANE induced the expression of JA-related genes such as PDF1.2, while expressions of PR1 and ICS1 were not greatly affected by ANE (Subramanian et al., 2011). These results suggested that ANE induced resistance in challenged Arabidopsis by activating the JA-dependent signaling pathway. Different solvent fractions exhibited reduced development of disease symptoms on the leaves, which is correlated with the increased expression of jasmonic-acid-related gene transcripts (Subramanian et al., 2011). Kappaphycus and Eucheuma spp., economically important red algae, were reported to be susceptible to epiphyte infestations (Loureiro et al., 2012). It was reported that a dip application of ANE (as a soluble seaweed extract powder, given the acronym AMPEP—Ascophyllum Marine Plant Extract Powder) elicited a natural defense mechanism in cultivated Kappaphycus against the epiphytes Neosiphonia apiculata, Cladophora, and Ulva, by inducing the phenolic content, free-radical scavenging, and iron chelation (Loureiro et al., 2010, 2012; Hurtado et al., 2012; Ali et al., 2018).
Another A. nodosum-derived extract (Stella Maris®) was reported to boost plant immunity by elevating the production of hydrogen peroxide, which ultimately led to an increase in the concentration of ROS. It was further shown that the expression of plant immune response genes WRKY30, CYP71A12, and PR-1 (genes that activate in early, mid, and late phases of immunity in the plant, respectively) was up-regulated (Cook et al., 2018). The priming of 3-week-old A. thaliana plants with 1 g/L of ANE (25 ml per plant through root drench) 2 days prior to inoculation protected against the necrotic pathogen, Sclerotinia sclerotiorum (Subramanian et al., 2011). Similarly, Jayaraj et al. (2008) showed that a foliar spray of ANE to carrot plants significantly reduced the progression of disease caused by Alternaria radicina and Botrytis cinerea. It was found that the priming of carrot plants with ANE induced the activity of defense-related enzymes including peroxidase (PO), polyphenoloxidase (PPO), phenylalanine ammonia lyase (PAL), chitinase, and β-1,3-glucanase, as well as increasing the transcript accumulation of PR-1, PR-5, NPR-1, LTP, chalcone synthase, and PAL. Based on the available literature, Figure 4 was prepared to depict elicitors present in ANE, which are known to improve plant defense responses against different pathogens. The published evidence suggested that judicious applications of extracts from ANE could be an effective tool in disease management (Table 3). This strategy minimizes the use of chemical-based fungicides and provides an environmentally safe and sustainable method for the management of plant diseases.
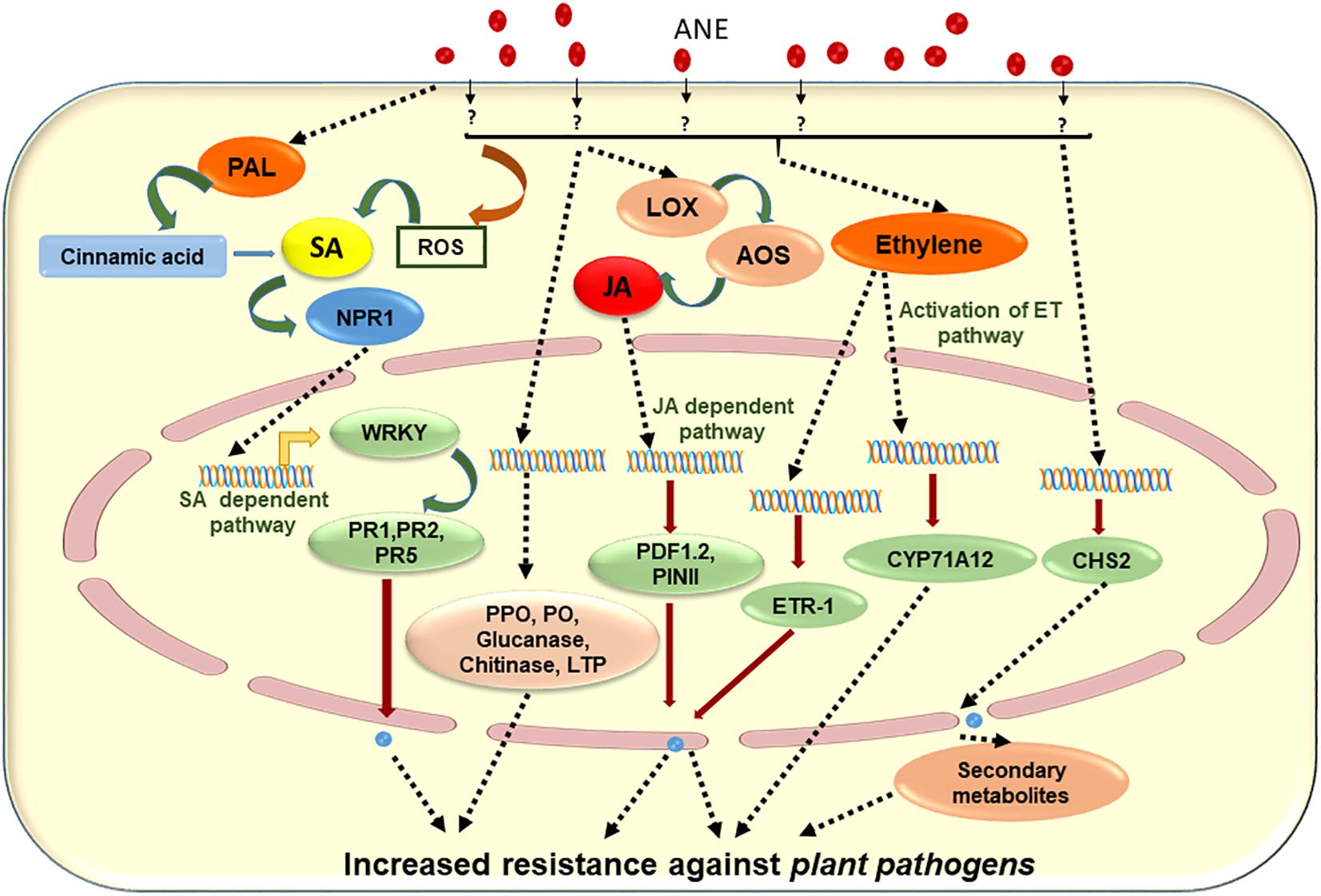
Figure 4. Schematic representation of proposed mode of action of Ascophyllum nodosum extract (ANE) in eliciting plant defense against different plant pathogens.
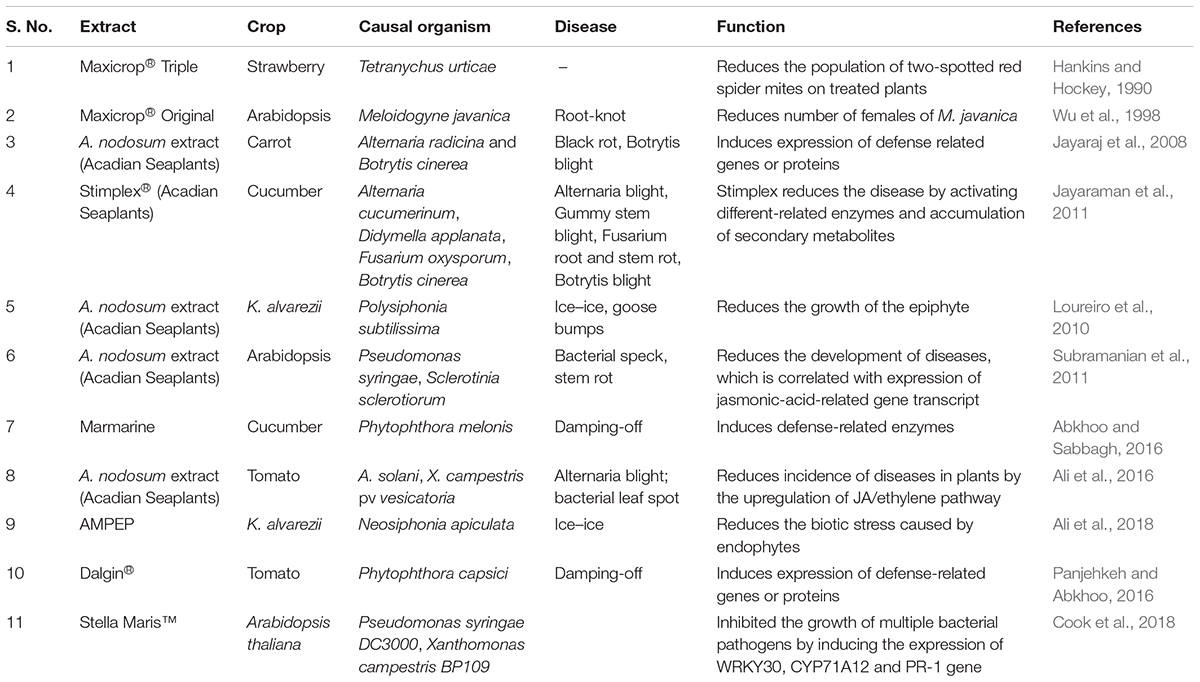
Table 3. List of the different extracts from A. nodosum inducing disease resistance in different plants against different pathogens.
ANE Improves Soil Health
Soil health, alternatively known as soil quality, is simply defined as: “the continued capacity of soil to function as a vital living ecosystem that sustains plants, animals and humans” (U.S. EPA, 2012). A healthy soil contributes to environmental management within the biosphere (air, water, and soil) and the productivity of plants and animals under both natural and managed systems (Karlen et al., 1997; Doran and Zeiss, 2000). Soils need improvement in order to enhance their ability to sustain their environmental and biological purposes. Select seaweed extracts have been studied sufficiently to suggest that their use as agricultural inputs have two modes of action: (1) they are biostimulants, as discussed above, that enhance growth and productivity of crop plants, and (2) they are chelators, directly contributing to the health of the soil (Khan et al., 2009). ANE provides natural chelation in the soil due to the presence of residual alginates present in the hydrolyzed extract, which allows for an increase in plant-available minerals and increased soil aeration and water-holding capacity (Spinelli et al., 2010; Craigie, 2011; du Jardin, 2015; Illera-Vives et al., 2015). Actiwave®, a metabolic enhancer prepared from A. nodosum, was used as a natural iron chelator for improved productivity of strawberry (Spinelli et al., 2010).
Alginic acid is a polysaccharide made up of mannuronic and guluronic acid units derived from brown seaweeds (Yabur et al., 2007; Craigie, 2011; Battacharyya et al., 2015). Alginic acids are a major constituent of the algal cell wall, comprising between 15 and 30% by dry weight (Yabur et al., 2007; Khan et al., 2009; Craigie, 2011; Battacharyya et al., 2015). Once commercially extracted, alginates are able to form natural gums or gels based on their composition (i.e., ratio of M:G, mannuronic acid:guluronic acid) and through their ability to bind water (Glicksman, 1987; Yabur et al., 2007). Alginates have been found to improve the physical conditions of soil (Khan et al., 2009; Illera-Vives et al., 2015). Through natural chelation, alginates bind to metal ions in the soil forming complex polymers (i.e., high molecular weight), and these molecules absorb moisture and swell as a result (Khan et al., 2009; Battacharyya et al., 2015). It is these swollen molecules that increase soil aeration and water-holding capacity (Khan et al., 2009; Spinelli et al., 2010). Further, through the aforementioned process, the presence of alginate in the rhizosphere alters the soil structure to become a more conducive environment for plant and microbial growth activity (Battacharyya et al., 2015).
Change in the Host Plant Induces Change in the Rhizospheric Microbial Population
The interaction between soil microbes and plants is cyclic in nature, known loosely as soil community feedback (Bever et al., 2012). The composition of the soil microbial population is based on the presence of the plant roots in the soil and compounds in the soil. Plants will grow with the help of molecules in the soil provided, in part, by the soil microbial population (Bever et al., 2012). There are interactions between plant roots (inter- and intra-species), between plant roots and insects, and between roots and rhizospheric microbes (Bais and Kaushik, 2010). Furthermore, there are also complex interactions between the aforementioned microbes, insects, and roots with root exudates (Bais and Kaushik, 2010).
The pretreatment of 10-day-old Medicago sativa (alfalfa) plants with 1 g/L of Acadian® (Acadian Seaplants Limited, 100 ml total) 2 days prior to inoculation with Sinorhizobium meliloti more than doubled the number of bacteria present in the rhizosphere, 12 h post-inoculation as compared to the untreated control (Khan et al., 2012). The seaweed extract induced the plant to produce root exudates (i.e., flavonoids) that would attract the bacteria to the root surface (Khan et al., 2012). Similarly, it is also reported that the application of ANE and its organic fractions induced rhizobium nodulation by regulating the legume-rhizobia signaling process (Khan et al., 2013).
Changes in Modes of Action and/or Function of Rhizospheric Microbial Population
The composition of the rhizospheric microbial population is dependent on a plethora of factors, including soil temperature, water-holding capacity, oxygen supply, and soil cultivation practices (i.e., history of fertilizer and pesticide applications and tillage) (Kilian et al., 2000). A change in any one of these factors could significantly impact the composition of the various microbial populations as well as the microbial functionality in the soil (Kilian et al., 2000). The application of select seaweed extracts directly to the soil or indirectly to the plant has also been reported to alter the rhizospheric microbial population.
A soil drench application of Actiwave® (10 ml of extract in 20 ml of water per plant) on strawberry plants increased the rhizospheric microbial population (Spinelli et al., 2010) and subsequent metabolic activity when applied in lower concentrations, compared to untreated soils as a result of stimulation from the bioactive components in the extract (Alam et al., 2013). The root-drench application of an Ascophyllum extract improved the growth of strawberries and carrots by acting as a prebiotic and increasing soil microbial activity (Alam et al., 2013, 2014).
Conversely, constituents in various seaweed extracts have shown effectiveness as biocontrol agents against bacteria, viruses, fungi, and nematodes (Nabti et al., 2017). A soil drench application of an alkaline seaweed extract (Maxicrop Original®, Maxicrop International Limited) to the soil of A. thaliana plants significantly reduced the number of deleterious female nematodes (Meloidogyne javanica) and number of eggs, compared to untreated soils (Wu et al., 1998). This study and others (Wu et al., 1998) suggested that the betaine constituent of the extract was responsible for inducing a defense reaction in A. thaliana and L. esculentum (tomato) against the root-knot nematode (Wu et al., 1998).
Conclusion and Future Challenges
In the current agricultural landscape, cultivation practices are reliant on synthetic chemicals [approximately 200 teragrams (1 Tg = 1012 g) per year worldwide] (Wu et al., 2018) to combat abiotic and biotic stresses (pesticides) and to promote plant growth (fertilizers). The short- and long-term negative impacts of synthetic chemicals on the environment and associated plant and animal health are becoming more prevalent every day. However, to sustain the growing human population, agriculture must be more productive than ever, with less viable resources and variable growing conditions (i.e., cultivatable soils, access to water and nutrients, consistent temperatures, etc.). To reduce reliance on synthetic chemicals, the solution must include multiple sources of natural compounds that are proven to promote crop growth under seemingly inadequate growing conditions and inherently refuel the surrounding ecosystems with more beneficial compounds, i.e., perform the roles of the pesticides and fertilizers without the harmful side effects. The utility of various extracts of A. nodosum-based products as biostimulants is multi-faceted: this complex alga and its extracts have shown efficacy in promoting plant growth and improving crop plant resilience to environmental perturbations, while being a natural, marine species, and therefore, when applied correctly (i.e., defined rates and timings of applications), they pose no harmful effects. Furthermore, ANE has been reported to act as both a biocontrol agent and a soil-microbial supplement.
Although the existing evidence for A. nodosum extracts as biostimulants in agriculture is promising, moving forward, it is important to focus the research in order to fully saturate agricultural practices with these extracts. Now that we are beginning to accumulate evidence on the modes of action of the extracts, we need to evaluate other aspects of extract application to optimize the desired mode of action. This push for more information creates a plethora of research questions: What is the optimal application rate of ANE, and in what application method (i.e., drench or spray)? When is the optimal time of application, and is there need for re-application during the growing season? If so, at what time intervals? How do these answers vary between crops and between climatic locations? Additionally, there are differences between extracts of A. nodosum based on extraction method and the resultant composition of the extract. How can current extraction methods be optimized to reap the most benefits from each extract? Can extraction methods not previously used with A. nodosum be adopted industrially (i.e., E-AE)? How do the resultant extracts compare to currently available (and reasonably well studied) extracts, and how can we exploit their positive modes of action? Furthermore, it is important to investigate whether different modes of application inherently alter the mode of action of the extracts in improving plant growth through the integration of modern interdisciplinary science. The application of the research to real-world producers will be of great benefit to understand any changes in behavior of the extracts under environmental conditions, while further identifying the modes of action will increase the extension of applications of the extracts into other fields.
Author Contributions
PS, EM, AC, and BP conceived the layout. PS, EM, MA, SB, AC, and BP wrote the review. All authors reviewed and agreed with the final version of the submitted manuscript.
Funding
This project was supported partly by Collaborative Research and Development (CRD) grant (459260-13) from the Natural Sciences and Engineering Research Council of Canada and Accelerate Cluster grant (IT08347) from Mitacs (Canada), awarded to BP. The funder provided support in the form of salary for authors (PS, MA, and SB).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
References
Abdel-Mawgoud, A. M. R., Tantaway, A. S., Hafez, M. M., and Habib, H. A. (2010). Seaweed extract improves growth, yield and quality of different watermelon hybrids. Res. J. Agric. Biol. Sci. 6, 161–168.
Abkhoo, J., and Sabbagh, S. K. (2016). Control of Phytophthora melonis damping-off, induction of defense responses, and gene expression of cucumber treated with commercial extract from Ascophyllum nodosum. J. Appl. Phycol. 28, 1333–1342. doi: 10.1007/s10811-015-0693-3
Agarwal, P. K., Agarwal, P., Reddy, M. K., and Sopory, S. K. (2006). Role of DREB transcription factors in abiotic and biotic stress tolerance in plants. Plant Cell Rep. 25, 1263–1274. doi: 10.1007/s00299-006-0204-8
Agarwal, P. K., and Jha, B. (2010). Transcription factors in plants and ABA dependent and independent abiotic stress signalling. Biol. Plant. 54, 201–212. doi: 10.1007/s10535-010-0038-7
Agarwal, P. K., Shukla, P. S., Gupta, K., and Jha, B. (2013). Bioengineering for salinity tolerance in plants: state of the art. Mol. Biotechnol. 54, 102–123. doi: 10.1007/s12033-012-9538-3
Ahmadi, A., Moghadamtousi, S. Z., Abubakar, S., and Zandi, K. (2015). Antiviral potential of algae polysaccharides isolated from marine sources: a review. Biomed. Res. Int. 2015:825203. doi: 10.1155/2015/825203
Ahn, C. B., Jeon, Y. J., Kang, D. S., Shin, T. S., and Jung, B. M. (2004). Free radical scavenging activity of enzymatic extracts from a brown seaweed Scytosiphon lomentaria by electron spin resonance spectrometry. Food Res Int. 37, 253–258. doi: 10.1016/j.foodres.2003.12.002
Alam, M. Z., Braun, G., Norrie, J., and Hodges, D. M. (2013). Effect of Ascophyllum extract application on plant growth, fruit yield and soil microbial communities of strawberry. Can. J. Plant Sci. 93, 23–36. doi: 10.4141/cjps2011-260
Alam, M. Z., Braun, G., Norrie, J., and Hodges, D. M. (2014). Ascophyllum extract application can promote plant growth and root yield in carrot associated with increased root-zone soil microbial activity. Can. J. Plant Sci. 94, 337–348. doi: 10.4141/cjps2013-135
Ale, M. T., Mikkelsen, J. D., and Meyer, A. S. (2012). Designed optimization of a single-step extraction of fucose-containing sulfated polysaccharides from Sargassum sp. J. Appl. Phycol. 24, 715–723. doi: 10.1007/s10811-011-9690-3
Ali, M. K. M., Yasir, S. M., Critchley, A. T., and Hurtado, A. Q. (2018). Impacts of Ascophyllum marine plant extract powder (AMPEP) on the growth, incidence of the endophyte Neosiphonia apiculata and associated carrageenan quality of three commercial cultivars of Kappaphycus. J. Appl. Phycol. 30, 1185–1195. doi: 10.1007/s10811-017-1312-2
Ali, N., Farrell, A., Ramsubhag, A., and Jayaraman, J. (2016). The effect of Ascophyllum nodosum extract on the growth, yield and fruit quality of tomato grown under tropical conditions. J. Appl. Phycol. 28, 1353–1362. doi: 10.1007/s10811-015-0608-3
Allen, V. G., Pond, K. R., Saker, K. E., Fontenot, J. P., Bagley, C. P., Ivy, R. L., et al. (2001). Tasco: influence of a brown seaweed on antioxidants in forages and livestock—A review. J. Anim. Sci. 79:E21. doi: 10.2527/jas2001.79E-SupplE21x
Alvarez-Acosta, C., Marrero-Dominguez, A., Gallo-Llobet, L., and Gonzalez-Rodriguez, A. M. (2018). Physiological response of selected avocados (Persea americana) subjected to NaCl and NaHCO3 stress. Sci. Hortic. 237, 81–88. doi: 10.1016/j.scienta.2018.04.010
Anderson, J. T., Willis, J. H., and Mitchell-Olds, T. (2011). Evolutionary genetics of plant adaptation. Trends Genet. 27, 258–266. doi: 10.1016/j.tig.2011.04.001
Anderson, P. K., Cunningham, A. A., Patel, N. G., Morales, F. J., Epstein, P. R., and Daszak, P. (2004). Emerging infectious diseases of plants: pathogen pollution, climate change and agrotechnology drivers. Trends Ecol Evol. 19, 535–544. doi: 10.1016/j.tree.2004.07.021
Apel, K., and Hirt, H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399. doi: 10.1146/annurev.arplant.55.031903.141701
Ashraf, M., and Foolad, M. R. (2007). Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 59, 206–216. doi: 10.1016/j.envexpbot.2005.12.006
Ashraf, M., and Harris, P. J. C. (2004). Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 166, 3–16. doi: 10.1016/j.plantsci.2003.10.024
Ayliffe, M. A., and Lagudah, E. S. (2004). Molecular genetics of disease resistance in cereals. Ann. Bot. 94, 765–773. doi: 10.1093/aob/mch207
Bais, H. P., and Kaushik, S. (2010). Catechin secretion & phytotoxicity: fact not fiction. Commun. Integr. Biol. 3, 468–470. doi: 10.4161/cib.3.5.12559
Basak, A. (2008). Effect of preharvest treatment with seaweed products, Kelpak® and Goëmar BM 86®, on fruit quality in apple. Int. J. Fruit Sci. 8, 1–14. doi: 10.1080/15538360802365251
Battacharyya, D., Babgohari, M. Z., Rathor, P., and Prithiviraj, B. (2015). Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 196, 39–48. doi: 10.1016/j.scienta.2015.09.012
Baxter, A., Mittler, R., and Suzuki, N. (2014). ROS as key players in plant stress signalling. J. Exp. Bot. 65, 1229–1240. doi: 10.1093/jxb/ert375
Bektas, Y., and Eulgem, T. (2015). Synthetic plant defense elicitors. Front. Plant Sci. 5:804. doi: 10.3389/fpls.2014.00804
Bever, J. D., Platt, T. G., and Morton, E. R. (2012). Microbial population and community dynamics on plant roots and their feedbacks on plant communities. Annu. Rev. Microbiol. 66, 265–283. doi: 10.1146/annurev-micro-092611-150107
Bhatnagar-Mathur, P., Vadez, V., and Sharma, K. K. (2008). Transgenic approaches for abiotic stress tolerance in plants: retrospect and prospects. Plant Cell Rep. 27, 411–424. doi: 10.1007/s00299-007-0474-9
Bleakley, S., and Hayes, M. (2017). Algal proteins: extraction, application, and challenges concerning production. Foods 6:E33. doi: 10.3390/foods6050033
Blunden, G., Gordon, S. M., Smith, B. E., and Fletcher, R. L. (1985). Quaternary ammonium compounds in species of the fucaceae (Phaeophyceae) from Britain. Br. Phycol. J. 20, 105–108. doi: 10.1080/00071618500650121
Blunden, G., Jenkins, T., and Liu, Y. W. (1996). Enhanced leaf chlorophyll levels in plants treated with seaweed extract. J. Appl. Phycol. 8, 535–543. doi: 10.1007/BF02186333
Blunden, G., and Wildgoose, P. B. (1977). The effects of aqueous seaweed extract and kinetin on potato yields. J. Sci. Food Agric. 28, 121–125. doi: 10.1002/jsfa.2740280203
Bodner, G., Nakhforoosh, A., and Kaul, H. P. (2015). Management of crop water under drought: a review. Agron. Sustain. Dev. 32, 1–13. doi: 10.1007/s13593-015-0283-4
Bonomelli, C., Celis, V., Lombardi, G., and Mártiz, J. (2018). Salt stress effects on Avocado (Persea americana Mill). plants with and without seaweed extract (Ascophyllum nodosum) application. Agronomy 8:64. doi: 10.3390/agronomy8050064
Boyer, J. S. (1982). Plant productivity and environment. Science 218, 443–448. doi: 10.1126/science.218.4571.443
Buggeln, R. G., and Craigie, J. S. (1971). Evaluation of evidence for the presence of indole-3-acetic acid in marine algae. Planta 97, 173–178. doi: 10.1007/BF00386764
Burchett, S., Fuller, M. P., and Jellings, A. J. (1998). Application of seaweed extract improves winter hardiness of winter barley cv Igri. J. Exp. Bot. 49:97.
Burke, M. J., Gusta, L. V., Quamme, H. A., Weiser, C. J., and Li, P. (1976). Freezing and injury in plants. Annu. Rev. Plant Physiol. 27, 507–528. doi: 10.1146/annurev.pp.27.060176.002451
Calvo, P., Nelson, L., and Kloepper, J. W. (2014). Agricultural uses of plant biostimulants. Plant Soil 383, 3–41. doi: 10.1007/s11104-014-2131-8
Carillo, P. (2018). GABA shunt in durum wheat. Front. Plant Sci. 9:100. doi: 10.3389/fpls.2018.00100
Carvalho, M. E. A., De Camargo, E., Castro, P. R., Gaziola, S. A., and Azevedo, R. A. (2018). Is seaweed extract an elicitor compound? Changing proline content in drought-stressed bean plants. Comun. Sci. 9, 292–297. doi: 10.14295/CS.v9i2.2134
Cassan, L., Jeannin, I., Lamaze, T., and Morot-Gaudry, J. F. (1992). The effect of the Ascophyllum nodosum extract Goëmar GA 14 on the growth of spinach. Bot. Mar. 35, 437–440. doi: 10.1515/botm.1992.35.5.437
Chaves, M. M., Flexas, J., and Pinheiro, C. (2009). Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann. Bot. 103, 551–560. doi: 10.1093/aob/mcn125
Chaves, M. M., Maroco, J. P., and Pereira, J. S. (2003). Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 30, 239–264. doi: 10.1071/FP02076
Chinnusamy, V., Ohta, M., Kanrar, S., Lee, B., Hong, X., Agarwal, M., et al. (2003). ICE1: a regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev. 17, 1043–1054. doi: 10.1101/gad.1077503
Chinnusamy, V., Schumaker, K., and Zhu, J. K. (2004). Molecular genetic perspectives on cross-talk and specificity in abiotic stress signalling in plants. J. Exp. Bot. 55, 225–236. doi: 10.1093/jxb/erh005
Chinnusamy, V., Zhu, J., and Zhu, J. K. (2007). Cold stress regulation of gene expression in plants. Trends Plant Sci. 12, 444–451. doi: 10.1016/j.tplants.2007.07.002
Chojnacka, K., Michalak, I., Dmytryk, A., Gramza, M., Słowiński, A., and Górecki, H. (2015). “Algal extracts as plant growth biostimulants,” in Marine Algae Extracts: Processes, Products, and Applications, eds S. K. Kim, and K. Chojnacka (Hoboken, NJ: Wiley), 189–212. doi: 10.1002/9783527679577.ch11
Chouliaras, V., Gerascapoulos, D., and Lionakis, S. (1997). Effects of seaweed extract on fruit growth, weight and maturation of ‘Hayward’ kiwifruit. Acta. Hortic. 444, 485–492. doi: 10.17660/ActaHortic.1997.444.74
Chouliaras, V., Tasioula, M., Chatzissavvidis, C., Therios, I., and Tsabolatidou, E. (2009). The effects of a seaweed extract in addition to nitrogen and boron fertilization on productivity, fruit maturation, leaf nutritional status and oil quality of the olive (Olea europaea L). cultivar Koroneiki. J. Sci. Food Agric. 89, 984–988. doi: 10.1002/jsfa.3543
Chrysargyris, A., Xylia, P., Anastasiou, M., Pantelides, I., and Tzortzakis, N. (2018). Effects of Ascophyllum nodosum seaweed extracts on lettuce growth, physiology and fresh-cut salad storage under potassium deficiency. J. Sci. Food Agric. 98, 5861–5872. doi: 10.1002/jsfa.9139
Coello, P., Hey, S. J., and Halford, N. G. (2011). The sucrose non-fermenting-1-related (SnRK) family of protein kinases: potential for manipulation to improve stress tolerance and increase yield. J. Exp. Bot. 62, 883–893. doi: 10.1093/jxb/erq331
Conrath, U., Pieterse, C. M. J., and Mauch-Mani, B. (2002). Priming in plant-pathogen interactions. Trends Plant Sci. 7, 210–216. doi: 10.1016/S1360-1385(02)02244-6
Cook, J., Zhang, J., Norrie, J., Blal, B., and Cheng, Z. (2018). Seaweed extract (Stella Maris®) activates innate immune responses in Arabidopsis thaliana and protects host against bacterial pathogens. Mar. Drugs 16:E221. doi: 10.3390/md16070221
Courtois, J. (2009). Oligosaccharides from land plants and algae: production and applications in therapeutics and biotechnology. Curr. Opin. Microbiol. 12, 261–273. doi: 10.1016/j.mib.2009.04.007
Craigie, J. S. (2011). Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 23, 371–393. doi: 10.1007/s10811-010-9560-4
Crouch, I. J., and van Staden, J. (1993). Evidence for the presence of plant growth regulators in commercial seaweed products. Plant Growth Regul. 13, 21–29. doi: 10.1007/BF00207588
da Silva, R. P. F. F., Rocha-Santos, T. A. P., and Duarte, A. C. (2016). Supercritical fluid extraction of bioactive compounds. TrAC Trends Anal. Chem. 76, 40–51. doi: 10.1016/j.trac.2015.11.013
Damalas, C., and Koutroubas, S. (2016). Farmers’ exposure to pesticides: toxicity types and ways of prevention. Toxics 4:1. doi: 10.3390/toxics4010001
De Jesus Raposo, M. F., De Morais, R. M. S. C., and De Morais, A. M. M. B. (2013). Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar. Drugs 11, 233–252. doi: 10.3390/md11010233
Demirevska, K., Simova-Stoilova, L., Vassileva, V., and Feller, U. (2008). Rubisco and some chaperone protein responses to water stress and rewatering at early seedling growth of drought sensitive and tolerant wheat varieties. Plant Growth Regul. 56, 97–106. doi: 10.1007/s10725-008-9288-1
Di Stasio, E., Van Oosten, M. J., Silletti, S., Raimondi, G., dell’Aversana, E., Carillo, P., et al. (2018). Ascophyllum nodosum-based algal extracts act as enhancers of growth, fruit quality, and adaptation to stress in salinized tomato plants. J. Appl. Phycol. 30, 2675–2686. doi: 10.1007/s10811-018-1439-9
Dixon, R. A. (2001). Natural products and plant disease resistance. Nature 411, 843–847. doi: 10.1038/35081178
Doran, J. W., and Zeiss, M. R. (2000). Soil health and sustainability: managing the biotic component of soil quality. Appl. Soil Ecol. 15, 3–11. doi: 10.1016/s0929-1393(00)00067-6
dos Reis, S. P., Lima, A. M., and de Souza, C. R. B. (2012). Recent molecular advances on downstream plant responses to abiotic stress. Int. J. Mol. Sci. 13, 8628–8647. doi: 10.3390/ijms13078628
du Jardin, P. (2015). Plant biostimulants: definition, concept, main categories and regulation. Sci. Hortic. 196, 3–14. doi: 10.1016/j.scienta.2015.09.021
Eckardt, N. A. (2008). Chitin signaling in plants: insights into the perception of fungal pathogens and rhizobacterial symbionts. Plant Cell 20, 241–243. doi: 10.1105/tpc.108.058784
Elad, Y., and Pertot, I. (2014). Climate change impacts on plant pathogens and plant diseases. J. Crop Improv. 28, 99–139. doi: 10.1080/15427528.2014.865412
Elansary, H. O., Norrie, J., Ali, H. M., Salem, M. Z. M., Mahmoud, E. A., and Yessoufou, K. (2016a). Enhancement of Calibrachoa growth, secondary metabolites and bioactivity using seaweed extracts. BMC Complement. Altern. Med. 16:341. doi: 10.1186/s12906-016-1332-5
Elansary, H. O., Skalicka-Woźniak, K., and King, I. W. (2016b). Enhancing stress growth traits as well as phytochemical and antioxidant contents of Spiraea and Pittosporum under seaweed extract treatments. Plant Physiol. Biochem. 105, 310–320. doi: 10.1016/j.plaphy.2016.05.024
Elansary, H. O., Yessoufou, K., Abdel-Hamid, A. M. E., El-Esawi, M. A., Ali, H. M., and Elshikh, M. S. (2017). Seaweed extracts enhance Salam turfgrass performance during prolonged irrigation intervals and saline shock. Front. Plant Sci. 8:830. doi: 10.3389/fpls.2017.00830
Eris, A., Sivritepe, H. Ö., and Sivritepe, N. (1995). “The effect of seaweed (Ascophyllum nodosum) extract on yield and quality criteria in peppers,” in I International Symposium on Solanacea for Fresh Market, Vol. 412, eds R. Fernández-Muñoz, J. Cuartero, and M. L. Gómez-Guillamón (Malaga: ISHS), 185–192. doi: 10.17660/actahortic.1995.412.21
Ertani, A., Francioso, O., Tinti, A., Schiavon, M., Pizzeghello, D., and Nardi, S. (2018). Evaluation of seaweed extracts from Laminaria and Ascophyllum nodosum spp. as biostimulants in Zea mays L. using a combination of chemical, biochemical and morphological approaches. Front. Plant Sci. 9:428. doi: 10.3389/fpls.2018.00428
Eskilsson, C. S., and Björklund, E. (2000). Analytical-scale microwave-assisted extraction. J. Chromatogr. 902, 227–250. doi: 10.1016/S0021-9673(00)00921-3
Fahad, S., Bajwa, A. A., Nazir, U., Anjum, S. A., Farooq, A., Zohaib, A., et al. (2017). Crop production under drought and heat stress: plant responses and management options. Front. Plant Sci. 8:1147. doi: 10.3389/fpls.2017.01147
Fan, D., Hodges, D. M., Critchley, A. T., and Prithiviraj, B. (2013). A commercial extract of Brown Macroalga (Ascophyllum nodosum) affects yield and the nutritional quality of Spinach in vitro. Commun. Soil Sci. Plant Anal. 44, 1873–1884. doi: 10.1080/00103624.2013.790404
Fan, D., Hodges, D. M., Zhang, J., Kirby, C. W., Ji, X., Locke, S. J., et al. (2011). Commercial extract of the brown seaweed Ascophyllum nodosum enhances phenolic antioxidant content of spinach (Spinacia oleracea L) which protects Caenorhabditis elegans against oxidative and thermal stress. Food Chem. 124, 195–202. doi: 10.1016/j.foodchem.2010.06.008
Fan, D., Kandasamy, S., Hodges, D. M., Critchley, A. T., and Prithiviraj, B. (2014). Pre-harvest treatment of spinach with Ascophyllum nodosum extract improves post-harvest storage and quality. Sci. Hortic. 170, 70–74. doi: 10.1016/j.scienta.2014.02.038
Farooq, M., Wahid, A., Kobayashi, N., Fujita, D., and Basra, S. M. A. (2009). Plant drought stress: effects, mechanisms and management. Agron. Sustain. Dev. 29, 185–212. doi: 10.1051/agro:2008021
Ferchichi, S., Hessini, K., Dell’Aversana, E., D’Amelia, L., Woodrow, P., Ciarmiello, L. F., et al. (2018). Hordeum vulgare and Hordeum maritimum respond to extended salinity stress displaying different temporal accumulation pattern of metabolites. Funct. Plant Biol. 45, 1096–1109.
Flórez, N., Conde, E., and Domínguez, H. (2015). Microwave assisted water extraction of plant compounds. J. Chem. Technol. Biotechnol. 90, 590–607. doi: 10.1002/jctb.4519
Flórez-Fernández, N., Torres, M. D., González-Muñoz, M. J., and Domínguez, H. (2018). Potential of intensification techniques for the extraction and depolymerization of fucoidan. Algal Res. 30, 128–148. doi: 10.1016/j.algal.2018.01.002
Fornes, F., Sánchez-Perales, M., and Guardiola, J. L. (1995). Effect of a seaweed extract on citrus fruit maturation. Acta Hortic. 379, 75–82. doi: 10.17660/actahortic.1995.379.6
Fornes, F., Sánchez-Perales, M., and Guardiola, J. L. (2002). Effect of a seaweed extract on the productivity of ‘de Nules’ clementine mandarin and navelina orange. Bot. Mari. 45, 486–489. doi: 10.1515/BOT.2002.051
Frébort, I., Kowalska, M., Hluska, T., Frébortová, J., and Galuszka, P. (2011). Evolution of cytokinin biosynthesis and degradation. J. Exp. Bot. 62, 2431–2452. doi: 10.1093/jxb/err004
Fries, N. (1979). Physiological characteristics of Mycosphaerella ascophylli, a fungal endophyte of the marine brown alga Ascophyllum nodosum. Physiol. Plant. 45, 117–121. doi: 10.1111/j.1399-3054.1979.tb01674.x
Fries, N., and Thorén-Tolling, K. (1978). Identity of the fungal endophyte of Ascophyllum with Mycosphaerella ascophylli established by means of fluorescent antibody technique. Bot. Mar. 21, 409–412.
Frioni, T., Sabbatini, P., Tombesi, S., Norrie, J., Poni, S., Gatti, M., et al. (2018). Effects of a biostimulant derived from the brown seaweed Ascophyllum nodosum on ripening dynamics and fruit quality of grapevines. Sci. Hortic. 232, 97–106. doi: 10.1016/j.scienta.2017.12.054
Fry, S. C., Aldington, S., Hetherington, P. R., and Aitken, J. (1993). Oligosaccharides as signals and substrates in the plant cell wall. Plant Physiol. 103, 1–5. doi: 10.1104/pp.103.1.1
Fu, J., and Huang, B. (2001). Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ. Exp. Bot. 45, 105–114. doi: 10.1016/S0098-8472(00)00084-8
Gaffney, T., Friedrich, L., Vernooij, B., Negrotto, D., Nye, G., Uknes, S., et al. (1993). Requirement of salicylic acid for the induction of systemic acquired resistance. Science 261, 754–756. doi: 10.1126/science.261.5122.754
Gan, S., and Amasino, R. M. (1995). Inhibition of leaf senescence by autoregulated production of cytokinin. Science 270, 1986–1988. doi: 10.1126/science.270.5244.1986
Garbary, D., and Gautam, A. (1989). The Ascophyllum, Polysiphonia, Mycosphaerella symbiosis. I. Population ecology of Mycosphaerella from Nova Scotia. Bot. Mar. 32, 181–186.
Garbary, D., and London, J. (1995). The Ascophyllum Polysiphonial Mycosphaerella symbiosis V. Fungal infection protects A. nosodum from desiccation. Bot Mar. 38, 529–534.
Gill, S. S., and Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48, 909–930. doi: 10.1016/j.plaphy.2010.08.016
Glicksman, M. (1987). “Utilization of seaweed hydrocolloids in the food industry,” in Proceedings of the Twelfth International Seaweed Symposium, (Dordrecht: Springer), 31–47. doi: 10.1007/978-94-009-4057-4_3
Goñi, O., Fort, A., Quille, P., McKeown, P. C., Spillane, C., and O’Connell, S. (2016). Comparative transcriptome analysis of two Ascophyllum nodosum extract biostimulants: same seaweed but different. J. Agric. Food Chem. 64, 2980–2989. doi: 10.1021/acs.jafc.6b00621
Goñi, O., Quille, P., and O’Connell, S. (2018). Ascophyllum nodosum extract biostimulants and their role in enhancing tolerance to drought stress in tomato plants. Plant Physiol. Biochem. 126, 63–73. doi: 10.1016/j.plaphy.2018.02.024
Gossett, D., Millhollon, E., Lucas, M. C., Banks, S., and Marney, M.-M. (1994). The effects of NaCl on antioxidant enzyme activities in callus tissue of salt-tolerant and salt-sensitive cotton cultivars (Gossypium hirsutum L). Plant Cell Rep. 13, 498–503. doi: 10.1007/BF00232944
Goyal, K., Walton, L. J., and Tunnacliffe, A. (2005). LEA proteins prevent protein aggregation due to water stress. Biochem. J. 388, 151–157. doi: 10.1042/BJ20041931
Guinan, K. J., Sujeeth, N., Copeland, R. B., Jones, P. W., O’brien, N. M., Sharma, H. S. S., et al. (2012). Discrete roles for extracts of Ascophyllum nodosum in enhancing plant growth and tolerance to abiotic and biotic stresses. Acta Hortic. 1009, 127–135. doi: 10.17660/actahortic.2013.1009.15
Hankins, S. D., and Hockey, H. P. (1990). “The effect of a liquid seaweed extract from Ascophyllum nodosum (Fucales, Phaeophyta) on the two-spotted red spider mite Tetranychus urticae,” in Proceedings of the Thirteenth International Seaweed Symposium, (Dordrecht: Springer), 555–559. doi: 10.1007/978-94-009-2049-1_80
Hare, P. D., Cress, W. A., and Van Staden, J. (1998). Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ. 21, 535–553. doi: 10.3390/md17010070
Hasegawa, P. M. (2013). Sodium (Na+) homeostasis and salt tolerance of plants. Environ. Exp. Bot. 92, 19–31. doi: 10.1016/j.envexpbot.2013.03.001
Hasegawa, P. M., Bressan, R. A., Zhu, J. K., and Bohnert, H. J. (2000a). Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 51, 463–499. doi: 10.1146/annurev.arplant.51.1.463
Hasegawa, M., Bressan, R., and Pardo, J. M. (2000b). The dawn of plant salt tolerance genetics. Trends Plant Sci. 5, 317–319. doi: 10.1016/S1360-1385(00)01692-7
Heo, S. J., Park, E. J., Lee, K. W., and Jeon, Y. J. (2005). Antioxidant activities of enzymatic extracts from brown seaweeds. . Bioresour. Technol. 96, 1613–1623. doi: 10.1016/j.biortech.2004.07.013
Herrero, M., Mendiola, J. A., Cifuentes, A., and Ibáñez, E. (2010). Supercritical fluid extraction: recent advances and applications. J. Chromatogr. A 1217, 2495–2511. doi: 10.1016/j.chroma.2009.12.019
Hidangmayum, A., and Sharma, R. (2017). Effect of different concentrations of commercial seaweed liquid extract of Ascophyllum nodosum as a plant bio stimulant on growth, yield and biochemical constituents of onion (Allium cepa L). J. Pharmacogn. Phytochem. 6, 658–663.
Holdt, S. L., and Kraan, S. (2011). Bioactive compounds in seaweed: functional food applications and legislation. J. Appl. Phycol. 23, 543–597. doi: 10.1007/s10811-010-9632-5
Hurtado, A. Q., and Critchley, A. T. (2018). A review of multiple biostimulant and bioeffector benefits of AMPEP, an extract of the brown alga Ascophyllum nodosum, as applied to the enhanced cultivation and micropropagation of the commercially important red algal carrageenophyte Kappaphycus alvarezii and its selected cultivars. J. Appl. Phycol. 30, 2859–2873. doi: 10.1007/s10811-018-1407-4
Hurtado, A. Q., Joe, M., Sanares, R. C., Fan, D., Prithiviraj, B., and Critchley, A. T. (2012). Investigation of the application of Acadian Marine Plant Extract Powder (AMPEP) to enhance the growth, phenolic content, free radical scavenging, and iron chelating activities of Kappaphycus Doty (Solieriaceae, Gigartinales, Rhodophyta). J. Appl. Phycol. 24, 601–611. doi: 10.1007/s10811-011-9785-x
Hurtado, A. Q., Yunque, D. A., Tibubos, K., and Critchley, A. T. (2009). Use of Acadian marine plant extract powder from Ascophyllum nodosum in tissue culture of Kappaphycus varieties. J. Appl. Phycol. 21, 633–639. doi: 10.1007/s10811-008-9395-4
Illera-Vives, M., López-Fabal, A., López-Mosquera, M. E., and Ribeiro, H. M. (2015). Mineralization dynamics in soil fertilized with seaweed–fish waste compost. J. Sci. Food Agric. 95, 3047–3054. doi: 10.1002/jsfa.7207
Iriti, M., and Varoni, E. M. (2015). Chitosan-induced antiviral activity and innate immunity in plants. Environ. Sci. Pollut. Res. 22, 2935–2944. doi: 10.1007/s11356-014-3571-7
Jain, M., Mathur, G., Koul, S., and Sarin, N. B. (2001). Ameliorative effects of proline on salt stress-induced lipid peroxidation in cell lines of groundnut (Arachis hypogaea L). Plant Cell Rep. 20, 463–468. doi: 10.1007/s002990100353
Jannin, L., Arkoun, M., Etienne, P., Laîné, P., Goux, D., Garnica, M., et al. (2013). Brassica napus growth is promoted by Ascophyllum nodosum (L). Le Jol. seaweed extract: microarray analysis and physiological characterization of N, C, and S metabolisms. J. Plant Growth Regul. 32, 31–52. doi: 10.1007/s00344-012-9273-9
Jayaraj, J., Wan, A., Rahman, M., and Punja, Z. K. (2008). Seaweed extract reduces foliar fungal diseases on carrot. Crop Prot. 27, 1360–1366. doi: 10.1016/j.cropro.2008.05.005
Jayaraman, J., Norrie, J., and Punja, Z. K. (2011). Commercial extract from the brown seaweed Ascophyllum nodosum reduces fungal diseases in greenhouse cucumber. J. Appl. Phycol. 23, 353–361. doi: 10.1007/s10811-010-9547-1
Jiang, Y., and Huang, B. (2001). Drought and heat stress injury to two cool-season turfgrasses in relation to antioxidant metabolism and lipid peroxidation. Crop Sci. 41, 436–442. doi: 10.2135/cropsci2001.412436x
Jithesh, M. N., Shukla,P., Joshi, J., Critchley, A. T., and Prithiviraj, B. (2018). Physiological and transcriptomics analyses reveal that Ascophyllum nodosum extracts induce salinity tolerance in Arabidopsis by regulating the expression of stress responsive genes. J. Plant Growth Regul. 1–16. doi: 10.1007/s00344-018-9861-4
Jithesh, M. N., Wally, O. S. D., Manfield, I., Critchley, A. T., Hiltz, D., and Prithiviraj, B. (2012). Analysis of seaweed extract-induced transcriptome leads to identification of a negative regulator of salt tolerance in Arabidopsis. Hort. Sci. 47, 704–709. doi: 10.21273/hortsci.47.6.704
Jolinda, M., Leitão, E. T., Gomes, C. D., Rodrigues, M. H., Valéria, F. D. O., dos Santos, G. L., et al. (2018). The initial growth of passion fruit plant irrigated with saline water and the application of biostimulants. J. Agric. Sci. 10, 357–363.
Joshi, R., Wani, S. H., Singh, B., Bohra, A., Dar, Z. A., Lone, A. A., et al. (2016). Transcription factors and plants response to drought stress: current understanding and future directions. Front. Plant Sci. 7:1029. doi: 10.3389/fpls.2016.01029
Kadam, S. U., Álvarez, C., Tiwari, B. K., and O’Donnell, C. P. (2017). Extraction and characterization of protein from Irish brown seaweed Ascophyllum nodosum. Food Res. Int. 99, 1021–1027. doi: 10.1016/j.foodres.2016.07.018
Kadam, S. U., O’Donnell, C. P., Rai, D. K., Hossain, M. B., Burgess, C. M., Walsh, D., et al. (2015a). Laminarin from Irish brown seaweeds Ascophyllum nodosum and Laminaria hyperborea: ultrasound assisted extraction, characterization and bioactivity. Mar. Drugs 13, 4270–4280. doi: 10.3390/md13074270
Kadam, S. U., Tiwari, B. K., O’Connell, S., and O’Donnell, C. P. (2015b). Effect of ultrasound pretreatment on the extraction kinetics of bioactives from brown seaweed (Ascophyllum nodosum). Sep. Sci. Technol. 50, 670–675. doi: 10.1080/01496395.2014.960050
Kadam, S. U., Tiwari, B. K., and O’Donnell, C. P. (2013). Application of novel extraction technologies for bioactives from marine algae. J. Agric. Food Chem. 61, 4667–4675. doi: 10.1021/jf400819p
Kadam, S. U., Tiwari, B. K., Smyth, T. J., and O’Donnell, C. P. (2015c). Optimization of ultrasound assisted extraction of bioactive components from brown seaweed Ascophyllum nodosum using response surface methodology. Ultrason. Sonochem. 23, 308–316. doi: 10.1016/j.ultsonch.2014.10.007
Karlen, D. L., Mausbach, M. J., Doran, J. W., Cline, R. G., Harris, R. F., and Schuman, G. E. (1997). Soil quality: a concept, definition, and framework for evaluation (a guest editorial). Soil Sci. Soc. Am. J. 61, 4–10.
Karuppanapandian, T., Moon, J. C., Kim, C., Manoharan, K., and Kim, W. (2011). Reactive oxygen species in plants: their generation, signal transduction, and scavenging mechanisms. Aust. J. Crop Sci. 5, 709–725.
Khan, W., Hiltz, D., Critchley, A. T., and Prithiviraj, B. (2011). Bioassay to detect Ascophyllum nodosum extract-induced cytokinin-like activity in Arabidopsis thaliana. J. Appl. Phycol. 23, 409–414. doi: 10.1007/s10811-010-9583-x
Khan, W., Palanisamy, R., Critchley, A. T., Smith, D. L., Papadopoulos, Y., and Prithiviraj, B. (2013). Ascophyllum nodosum extract and its organic fractions stimulate rhizobium root nodulation and growth of Medicago sativa (Alfalfa). Commun. Soil Sci. Plant Anal. 44, 900–908. doi: 10.1080/00103624.2012.744032
Khan, W., Rayirath, U. P., Subramanian, S., Jithesh, M. N., Rayorath, P., Hodges, D. M., et al. (2009). Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 28, 386–399. doi: 10.1007/s00344-009-9103-x
Khan, W., Zhai, R., Souleimanov, A., Critchley, A. T., Smith, D. L., and Prithiviraj, B. (2012). Commercial extract of Ascophyllum nodosum improves root colonization of alfalfa by its bacterial symbiont Sinorhizobium meliloti. Commun. Soil Sci. Plant Anal. 43, 2425–2436. doi: 10.1080/00103624.2012.708079
Kilian, M., Steiner, U., Krebs, B., Junge, H., Schmiedeknecht, G., and Hain, R. (2000). FZB24® Bacillus subtilis–mode of action of a microbial agent enhancing plant vitality. Pflanzensch. Nachr. Bayer 53, 72–93.
Kingman, A. R., and Moore, J. (1982). Isolation, purification and quantitation of deveral growth regulating substances in Ascophyllum nodosum (Phaeophyta). Bot. Mar. 25, 149–154. doi: 10.1515/botm.1982.25.4.149
Klarzynski, O., Descamps, V., Plesse, B., Yvin, J.-C., Kloareg, B., and Fritig, B. (2003). Sulfated fucan oligosaccharides elicit defense responses in tobacco and local and systemic resistance against tobacco mosaic virus. Mol. Plant Microbe Interact. 16, 115–122. doi: 10.1094/MPMI.2003.16.2.115
Kumar, M., Reddy, C. R. K., and Jha, B. (2013). The ameliorating effect of Acadian marine plant extract against ionic liquids-induced oxidative stress and DNA damage in marine macroalga Ulva lactuca. J. Appl. Phycol. 25, 369–378. doi: 10.1007/s10811-012-9871-8
Li, Y. J., Fang, Y., Fu, Y. R., Huang, J. G., Wu, C. A., and Zheng, C. C. (2013). NFYA1 is involved in regulation of postgermination growth arrest under salt stress in Arabidopsis. PLoS One 8:e61289. doi: 10.1371/journal.pone.0061289
Lim, P. O., Woo, H. R., and Nam, H. G. (2003). Molecular genetics of leaf senescence in Arabidopsis. Trends Plant Sci. 8, 272–278. doi: 10.1016/s1360-1385(03)00103-1
Lola-Luz, T., Hennequart, F., and Gaffney, M. (2013). Enhancement of phenolic and flavonoid compounds in cabbage (Brassica oleraceae) following application of commercial seaweed extracts of the brown seaweed, (Ascophyllum nodosum). Agric. Food Sci. 22, 288–295. doi: 10.23986/afsci.7676
Loureiro, R. R., Reis, R. P., Berrogain, F. D., and Critchley, A. T. (2012). Extract powder from the brown alga Ascophyllum nodosum (Linnaeus) Le Jolis (AMPEP): a “vaccine-like” effect on Kappaphycus alvarezii (Doty) Doty ex P.C, Silva. J. Appl. Phycol. 24, 427–432. doi: 10.1007/s10811-011-9735-7
Loureiro, R. R., Reis, R. P., and Critchley, A. T. (2010). In vitro cultivation of three Kappaphycus alvarezii (Rhodophyta, Areschougiaceae) variants (green, red and brown) exposed to a commercial extract of the brown alga Ascophyllum nodosum (Fucaceae, Ochrophyta). J. Appl. Phycol. 22, 101–104. doi: 10.1007/s10811-009-9412-2
Lucchesi, M. E., Chemat, F., and Smadja, J. (2004). Solvent-free microwave extraction of essential oil from aromatic herbs: comparison with conventional hydro-distillation. J. Chromatogr. A 1043, 323–327. doi: 10.1016/j.chroma.2004.05.083
MacKinnon, S. L., Hiltz, D., Ugarte, R., and Craft, C. A. (2010). Improved methods of analysis for betaines in Ascophyllum nodosum and its commercial seaweed extracts. J. Appl. Phycol. 22, 489–494. doi: 10.1007/s10811-009-9483-0
Maehre, H., Jensen, I. J., and Eilertsen, K. E. (2016). Enzymatic pre-treatment increases the protein bioaccessibility and extractability in Dulse (Palmaria palmata). Mar. Drugs 14:E196. doi: 10.3390/md14110196
Magnusson, M., Yuen, A. K. L., Zhang, R., Wright, J. T., Taylor, R. B., Maschmeyer, T., et al. (2017). A comparative assessment of microwave assisted (MAE) and conventional solid-liquid (SLE) techniques for the extraction of phloroglucinol from brown seaweed. Algal Res. 23, 28–36. doi: 10.1016/j.algal.2017.01.002
Manavalan, L. P., Guttikonda, S. K., Tran, L.-S., and Nguyen, H. T. (2009). Physiological and molecular approaches to improve drought resistance in soybean. Plant Cell Physiol. 50, 1260–1276. doi: 10.1093/pcp/pcp082
Marais, M. F., and Joseleau, J. P. (2001). A fucoidan fraction from Ascophyllum nodosum. Carbohydr. Res. 336, 155–159. doi: 10.1016/S0008-6215(01)00257-9
Marroig, R. G., Loureiro, R. R., and Reis, R. P. (2016). The effect of Ascophyllum nodosum (Ochrophyta) extract powder on the epibiosis of Kappaphycus alvarezii (Rhodophyta) commercially cultivated on floating rafts. J. Appl. Phycol. 28, 2471–2477. doi: 10.1007/s10811-015-0770-7
Martynenko, A., Shotton, K., Astatkie, T., Petrash, G., Fowler, C., Neily, W., et al. (2016). Thermal imaging of soybean response to drought stress: the effect of Ascophyllum nodosum seaweed extract. Springerplus 5:1393. doi: 10.1186/s40064-016-3019-2
Matesanz, S., Gianoli, E., and Valladares, F. (2010). Global change and the evolution of phenotypic plasticity in plants. Ann. N. Y. Acad. Sci. 1206, 35–55. doi: 10.1111/j.1749-6632.2010.05704.x
Mattner, S. W., Milinkovic, M., and Arioli, T. (2018). Increased growth response of strawberry roots to a commercial extract from Durvillaea potatorum and Ascophyllum nodosum. J. Appl. Phycol. 30, 2943–2951. doi: 10.1007/s10811-017-1387-9
Mercier, L., Lafitte, C., Borderies, G., Briand, X., Esquerré-Tugayé, M. T., and Fournier, J. (2001). The algal polysaccharide carrageenans can act as an elicitor of plant defence. New Phytol. 149, 43–51. doi: 10.1046/j.1469-8137.2001.00011.x
Messyasz, B., Michalak, I., Łȩska, B., Schroeder, G., Górka, B., Korzeniowska, K., et al. (2018). Valuable natural products from marine and freshwater macroalgae obtained from supercritical fluid extracts. J. Appl. Phycol. 30:591. doi: 10.1007/s10811-017-1257-5
Michalak, I., and Chojnacka, K. (2015). Algae as production systems of bioactive compounds. Eng. Life Sci. 15, 160–176. doi: 10.1002/elsc.201400191
Michalak, I., Chojnacka, K., Dmytryk, A., Wilk, R., Gramza, M., and Rój, E. (2016a). Evaluation of supercritical extracts of algae as biostimulants of plant growth in field trials. Front. Plant Sci. 7:1591. doi: 10.3389/fpls.2016.01591
Michalak, I., Dmytryk, A., Wieczorek, P. P., Rój, E., Łȩska, B., Górka, B., et al. (2015a). Supercritical algal extracts: a source of biologically active compounds from nature. J. Chem. 2015:597140. doi: 10.1155/2015/597140
Michalak, I., Górka, B., Wieczorek, P. P., Rój, E., Lipok, J., Łȩska, B., et al. (2016b). Supercritical fluid extraction of algae enhances levels of biologically active compounds promoting plant growth. Eur. J. Phycol. 51, 1–10. doi: 10.1080/09670262.2015.1134813
Michalak, I., Tuhy, Ł, and Chojnacka, K. (2015b). Seaweed extract by microwave assisted extraction as plant growth biostimulant. Open Chem. 13, 1183–1195. doi: 10.1515/chem-2015-0132
Mickelbart, M. V., Hasegawa, P. M., and Bailey-Serres, J. (2015). Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability. Nat. Rev. Genet. 16, 237–251. doi: 10.1038/nrg3901
Miller, G., Suzuki, N., Ciftci-Yilmaz, S., and Mittler, R. (2010). Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 33, 453–467. doi: 10.1111/j.1365-3040.2009.02041.x
Mittler, R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7, 405–410. doi: 10.1016/S1360-1385(02)02312-9
Mittler, R., Vanderauwera, S., Gollery, M., and Van Breusegem, F. (2004). Reactive oxygen gene network of plants. Trends Plant Sci. 9, 490–498. doi: 10.1016/j.tplants.2004.08.009
Miura, K., and Furumoto, T. (2013). Cold signaling and cold response in plants. Int. J. Mol. Sci. 14, 5312–5337. doi: 10.3390/ijms14035312
Moller, M., and Smith, M. L. (1998). The significance of the mineral component of seaweed suspensions on lettuce (Lactuca sativa L). seedling growth. J. Plant Physiol. 153, 658–663. doi: 10.1016/S0176-1617(98)80217-4
Moreira, R., Sineiro, J., Chenlo, F., Arufe, S., and Díaz-Varela, D. (2017). Aqueous extracts of Ascophyllum nodosum obtained by ultrasound-assisted extraction: effects of drying temperature of seaweed on the properties of extracts. J. Appl. Phycol. 29, 3191–3200. doi: 10.1007/s10811-017-1159-6
Nabti, E., Jha, B., and Hartmann, A. (2017). Impact of seaweeds on agricultural crop production as biofertilizer. Int. J. Environ. Sci. Technol. 14, 1119–1134. doi: 10.1007/s13762-016-1202-1
Nair, P., Kandasamy, S., Zhang, J., Ji, X., Kirby, C., Benkel, B., et al. (2012). Transcriptional and metabolomic analysis of Ascophyllum nodosum mediated freezing tolerance in Arabidopsis thaliana. BMC Genomics 13:643. doi: 10.1186/1471-2164-13-643
Norrie, J., Branson, T., and Keathley, P. E. (2002). Marine plant extracts impact on grape yield and quality. Acta Hortic. 594, 315–319. doi: 10.17660/ActaHortic.2002.594.38
Okolie, C. L., Mason, B., and Critchley, A. T. (2018). “Seaweeds as a source of proteins for use in pharmaceuticals and high-value applications,” in Novel Proteins for Food, Pharmaceuticals, and Agriculture: Sources, Applications, and Advances, ed. M. Hayes (Hoboken, NJ: Wiley), 217.
Oliveira, A. L. M., Urquiaga, S., Döbereiner, J., and Baldani, J. I. (2002). The effect of inoculating endophytic N2-fixing bacteria on micropropagated sugarcane plants. Plant Soil 242, 205–215. doi: 10.1023/A:1016249704336
Panjehkeh, N., and Abkhoo, J. (2016). Influence of marine brown alga extract (Dalgin) on damping-off tolerance of tomato. JMES 7, 2369–2374.
Parys, S., Kehraus, S., Pete, R., Küpper, F. C., Glombitza, K. W., and König, G. M. (2009). Seasonal variation of polyphenolics in Ascophyllum nodosum (Phaeophyceae). Eur. J. Phycol. 44, 331–338. doi: 10.1080/09670260802578542
Patier, P., Potin, P., Rochas, C., Kloareg, B., Yvin, J., and Licnart, Y. (1995). Free or silica-bound oligokappa-carrageenans elicit laminarinase activity in Rubus cells and protoplasts. Plant Sci. 9452, 27–35. doi: 10.1016/0168-9452(95)04182-T
Paulert, R., Talamini, V., Cassolato, J. E. F., Duarte, M. E. R., Noseda, M. D., Smania, A., et al. (2009). Effects of sulfated polysaccharide and alcoholic extracts from green seaweed Ulva fasciata on anthracnose severity and growth of common bean (Phaseolus vulgaris L). J. Plant Dis. Prot. 116:263. doi: 10.1007/BF03356321
Peng, S., Huang, J., Sheehy, J. E., Laza, R. C., Visperas, R. M., Zhong, X., et al. (2004). Rice yields decline with higher night temperature from global warming. Proc. Natl. Acad. Sci. U.S.A. 101, 9971–9975. doi: 10.1073/pnas.0403720101
Pieterse, C. M. J., and Dicke, M. (2007). Plant interactions with microbes and insects: From molecular mechanisms to ecology. Trends Plant Sci. 12, 564–569. doi: 10.1016/j.tplants.2007.09.004
Popescu, G. C., and Popescu, M. (2014). Effect of the brown alga Ascophyllum nodosum as biofertilizer on vegetative growth in grapevine (Vitis vinifera L). Curr. Trends Nat. Sci. 3, 61–67.
Potin, P., Bouarab, K., Küpper, F., and Kloareg, B. (1999). Oligosaccharide recognition signals and defence reactions in marine plant-microbe interactions. Curr. Opin. Microbiol. 2, 276–283. doi: 10.1016/s1369-5274(99)80048-4
Prithiviraj, B., Kant, P., Narayanan, J. M., Khan, W., Hankins, S., Neily, W., et al. (2011). Bioactive compounds of Ascophyllum nodosum and their use for alleviating salt induced stress in plants. U.S. Patent No. 12/936,074. Washington, DC: U.S. Patent and Trademark Office. doi: 10.1016/s1369-5274(99)80048-4
Qin, F., Shinozaki, K., and Yamaguchi-Shinozaki, K. (2011). Achievements and challenges in understanding plant abiotic stress responses and tolerance. Plant Cell Physiol. 52, 1569–1582. doi: 10.1093/pcp/pcr106
Ragan, M. A., and Jensen, A. (1977). Quantitative studies on brown algal phenols. I. Estimation of absolute polyphenol content of Ascophyllum nodosum (L). Le Jol. and Fucus vesiculosus (L). J. Exp. Mar. Bio Ecol. 30, 209–221. doi: 10.1016/0022-0981(77)90013-2
Rayirath, P., Benkel, B., Mark Hodges, D., Allan-Wojtas, P., MacKinnon, S., Critchley, A. T., et al. (2009). Lipophilic components of the brown seaweed, Ascophyllum nodosum, enhance freezing tolerance in Arabidopsis thaliana. Planta 230, 135–147. doi: 10.1007/s00425-009-0920-8
Rayorath, P., Jithesh, M. N., Farid, A., Khan, W., Palanisamy, R., Hankins, S. D., et al. (2008). Rapid bioassays to evaluate the plant growth promoting activity of Ascophyllum nodosum (L). Le Jol. using a model plant, Arabidopsis thaliana (L). Heynh. J. Appl. Phycol. 20, 423–429. doi: 10.1007/s10811-007-9280-6
Ren, H., and Gray, W. M. (2015). SAUR proteins as effectors of hormonal and environmental signals in plant growth. Mol. Plant 8, 1153–1164. doi: 10.1016/j.molp.2015.05.003
Richter, B. E., Jones, B., Ezzell, J. L., and Porter, N. L. (1996). Accelerated solvent extraction&xxx202F;: a technique for sample preparation. Anal. Chem. 68, 1033–1039. doi: 10.1021/ac9508199
Routray, W., and Orsat, V. (2012). Microwave-assisted extraction of flavonoids: a review. Food Bioprocess Technol. 5, 409–424. doi: 10.1007/s11947-011-0573-z
Sabir, A., Yazar, K., Sabir, F., Kara, Z., Yazici, M. A., and Goksu, N. (2014). Vine growth, yield, berry quality attributes and leaf nutrient content of grapevines as influenced by seaweed extract (Ascophyllum nodosum) and nanosize fertilizer pulverizations. Sci. Hortic. 175, 1–8. doi: 10.1016/j.scienta.2014.05.021
Sakamoto, A., and Murata, N. (2002). The role of glycine betaine in the protection of plants from stress: clues from transgenic plants. Plant Cell Environ. 25, 163–171. doi: 10.1046/j.0016-8025.2001.00790.x
Sanderson, K. J., Jameson, P. E., and Zabkiewicz, J. A. (1987). Auxin in a seaweed extract: Identification and quantitation of indole-3-acetic acid by gas chromatography-mass spectrometry. J. Plant Physiol. 129, 363–367. doi: 10.1016/S0176-1617(87)80093-7
Sangha, J. S., Ravichandran, S., Prithiviraj, K., Critchley, A. T., and Prithiviraj, B. (2010). Sulfated macroalgal polysaccharides λ-carrageenan and ι-carrageenan differentially alter Arabidopsis thaliana resistance to Sclerotinia sclerotiorum. Physiol. Mol. Plant Pathol. 75, 38–45. doi: 10.1016/j.pmpp.2010.08.003
Santaniello, A., Scartazza, A., Gresta, F., Loreti, E., Biasone, A., Di Tommaso, D., et al. (2017). Ascophyllum nodosum seaweed extract alleviates drought stress in Arabidopsis by affecting photosynthetic performance and related gene expression. Front. Plant Sci. 8:1362. doi: 10.3389/fpls.2017.01362
Sharma, H. S. S., Fleming, C., Selby, C., Rao, J. R., and Martin, T. (2014). Plant biostimulants: a review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J. Appl. Phycol. 26, 465–490. doi: 10.1007/s10811-013-0101-9
Shrivastava, P., and Kumar, R. (2015). Soil salinity: a serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 22, 123–131. doi: 10.1016/j.sjbs.2014.12.001
Shukla, P. S., Agarwal, P. K., and Jha, B. (2011). Improved salinity tolerance of Arachis hypogaea (L). by the interaction of halotolerant plant-growth-promoting rhizobacteria. J. Plant Growth Regul. 31, 195–206. doi: 10.1007/s00344-011-9231-y
Shukla, P. S., Borza, T., Critchley, A. T., Hiltz, D., Norrie, J., and Prithiviraj, B. (2018a). Ascophyllum nodosum extract mitigates salinity stress in Arabidopsis thaliana by modulating the expression of miRNA involved in stress tolerance and nutrient acquisition. PLoS One 13:e0206221. doi: 10.1371/journal.pone.0206221
Shukla, P. S., Borza, T., Critchley, A. T., and Prithiviraj, B. (2016). Carrageenans from red seaweeds as promoters of growth and elicitors of defense response in plants. Front. Mar. Sci. 3:81. doi: 10.3389/fmars.2016.00081
Shukla, P. S., Gupta, K., Agarwal, P., Jha, B., and Agarwal, P. K. (2015). Overexpression of a novel SbMYB15 from Salicornia brachiata confers salinity and dehydration tolerance by reduced oxidative damage and improved photosynthesis in transgenic tobacco. Planta 242, 1291–1308. doi: 10.1007/s00425-015-2366-5
Shukla, P. S., Shotton, K., Norman, E., Neily, W., Critchley, A. T., and Prithiviraj, B. (2018b). Seaweed extract improve drought tolerance of soybean by regulating stress-response genes. AoB Plants 10:plx051. doi: 10.1093/aobpla/plx051
Singh, M., Kumar, J., Singh, S., Singh, V. P., and Prasad, S. M. (2015). Roles of osmoprotectants in improving salinity and drought tolerance in plants: a review. Rev. Environ. Sci. Biotechnol. 14, 407–426. doi: 10.1007/s11157-015-9372-8
Spann, T. M., and Little, H. A. (2011). Applications of a commercial extract of the brown seaweed Ascophyllum nodosum increases drought tolerance in container-grown “hamlin” sweet orange nursery trees. Hortic. Sci. 46, 577–582. doi: 10.21273/hortsci.46.4.577
Spinelli, F., Fiori, G., Noferini, M., Sprocatti, M., and Costa, G. (2010). A novel type of seaweed extract as a natural alternative to the use of iron chelates in strawberry production. Sci. Hortic. 125, 263–269. doi: 10.1016/j.scienta.2010.03.011
Stadnik, M. J., and Freitas, M. B. (2014). Algal polysaccharides as source of plant resistance inducers. Trop. Plant Pathol. 39, 111–118. doi: 10.1590/s1982-56762014000200001
Stirk, W. A., and Van Staden, J. (1996). Comparison of cytokinin- and auxin-like activity in some commercially used seaweed extracts. J. Appl. Phycol. 8, 503–508. doi: 10.1007/BF02186328
Stirk, W. A., and Van Staden, J. (1997). Isolation and identification of cytokinins in a new commercial seaweed product made from Fucus serratus L. J. Appl. Phycol. 9, 327–330. doi: 10.1023/A:1007910110045
Subramanian, S., Sangha, J. S., Gray, B. A., Singh, R. P., Hiltz, D., Critchley, A. T., et al. (2011). Extracts of the marine brown macroalga, Ascophyllum nodosum, induce jasmonic acid dependent systemic resistance in Arabidopsis thaliana against Pseudomonas syringae pv. tomato DC3000 and Sclerotinia sclerotiorum. Eur. J. Plant Pathol. 131, 237–248. doi: 10.1007/s10658-011-9802-6
Takei, K., Ueda, N., Aoki, K., Kuromori, T., Hirayama, T., Shinozaki, K., et al. (2004). AtIPT3 is a key determinant of nitrate-dependent cytokinin biosynthesis in Arabidopsis. Plant Cell Physiol. 45, 1053–1062. doi: 10.1093/pcp/pch119
Tarkowski, Ł. P., and Van den Ende, W. (2015). Cold tolerance triggered by soluble sugars: a multifaceted countermeasure. Front. Plant Sci. 6:203. doi: 10.3389/fpls.2015.00203
Tibubos, K. R., Hurtado, A. Q., and Critchley, A. T. (2017). Direct formation of axes in new plantlets of Kappaphycus alvarezii (Doty) Doty, as influenced by the use of AMPEP K+, spindle inhibitors, and plant growth hormones. J. Appl. Phycol. 29, 2345–2349. doi: 10.1007/s10811-016-0988-z
Tierney, M. S., Smyth, T. J., Rai, D. K., Soler-Vila, A., Croft, A. K., and Brunton, N. (2013). Enrichment of polyphenol contents and antioxidant activities of Irish brown macroalgae using food-friendly techniques based on polarity and molecular size. Food Chem. 139, 753–761. doi: 10.1016/j.foodchem.2013.01.019
Todaka, D., Nakashima, K., Shinozaki, K., and Yamaguchi-Shinozaki, K. (2012). Toward understanding transcriptional regulatory networks in abiotic stress responses and tolerance in rice. Rice 5, 1–9. doi: 10.1186/1939-8433-5-6
Tommasini, L., Svensson, J. T., Rodriguez, E. M., Wahid, A., Malatrasi, M., Kato, K., et al. (2008). Dehydrin gene expression provides an indicator of low temperature and drought stress: transcriptome-based analysis of barley (Hordeum vulgare L). Funct. Integr. Genomics 8, 387–405. doi: 10.1007/s10142-008-0081-z
Turan, M., and Köse, C. (2004). Seaweed extracts improve copper uptake of grapevine. Acta Agric. Scand. Sect. B Soil Plant Sci. 54, 213–220. doi: 10.1080/09064710410030311
U.S. EPA (2012). Summary Report: Global Anthropogenic Non-CO2 Greenhouse Gas Emissions: 1990-2030. Washington, DC: EPA.
Valliyodan, B., and Nguyen, H. T. (2006). Understanding regulatory networks and engineering for enhanced drought tolerance in plants. Curr. Opin. Plant Biol. 9, 189–195. doi: 10.1016/j.pbi.2006.01.019
van Loon, L. C., Bakker, P., and Pieterse, C. M. (1998). Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 36, 453–483. doi: 10.1146/annurev.phyto.36.1.453
Van Oosten, M. J., Pepe, O., De Pascale, S., Silletti, S., and Maggio, A. (2017). The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 4:5. doi: 10.1186/s40538-017-0089-5
Vera, J., Castro, J., Gonzalez, A., and Moenne, A. (2011). Seaweed polysaccharides and derived oligosaccharides stimulate defense responses and protection against pathogens in plants. Mar. Drugs 9, 2514–2525. doi: 10.3390/md9122514
Vinocur, B., and Altman, A. (2005). Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr. Opin. Biotechnol. 16, 123–132. doi: 10.1016/j.copbio.2005.02.001
Voss-Fels, K., and Snowdon, R. J. (2016). Understanding and utilizing crop genome diversity via high-resolution genotyping. Plant Biotechnol. J. 14, 1086–1094. doi: 10.1111/pbi.12456
Wally, O. S. D., Critchley, A. T., Hiltz, D., Craigie, J. S., Han, X., Zaharia, L. I., et al. (2013). Regulation of phytohormone biosynthesis and accumulation in Arabidopsis following treatment with commercial extract from the marine macroalga Ascophyllum nodosum. J. Plant Growth Regul. 32, 324–339. doi: 10.1007/s00344-012-9301-9
Wani, S. H., Kumar, V., Shriram, V., and Sah, S. K. (2016). Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J. 4, 162–176. doi: 10.1016/j.cj.2016.01.010
Weiser, C. J., Quamme, H. A., Li, P., Gusta, L. V., and Burke, M. J. (1976). Freezing and injury in plants. Annu. Rev. Plant Physiol. 27, 507–528. doi: 10.1146/annurev.pp.27.060176.002451
West, J. S., Townsend, J. A., Stevens, M., and Fitt, B. D. (2012). Comparative biology of different plant pathogens to estimate effects of climate change on crop diseases in Europe. Eur. J. Plant Pathol. 133, 315–331. doi: 10.1007/s10658-011-9932-x
Whapham, C. A., Blunden, G., Jenkins, T., and Hankins, S. D. (1993). Significance of betaines in the increased chlorophyll content of plants treated with seaweed extract. J. Appl. Phycol. 5, 231–234. doi: 10.1007/BF00004023
Wiesel, L., Newton, A. C., Elliott, I., Booty, D., Gilroy, E. M., Birch, P. R. J., et al. (2014). Molecular effects of resistance elicitors from biological origin and their potential for crop protection. Front. Plant Sci. 5:655. doi: 10.3389/fpls.2014.00655
Wijesinghe, W. A., and Jeon, Y. J. (2012). Enzyme-assistant extraction (EAE) of bioactive components: a useful approach for recovery of industrially important metabolites from seaweeds: a review. Fitoterapia 83, 6–12. doi: 10.1016/j.fitote.2011.10.016
Wise, M. J., and Tunnacliffe, A. (2004). POPP the question: What do LEA proteins do? Trends Plant Sci. 9, 13–17. doi: 10.1016/j.tplants.2003.10.012
Wu, Y., Jenkins, T., Blunden, G., von Mende, N., and Hankins, S. D. (1998). Suppression of fecundity of the root-knot nematode, Meloidogyne javanica, in monoxenic cultures of Arabidopsis thaliana treated with an alkaline extract of Ascophyllum nodosum. J. Appl. Phycol. 10:91. doi: 10.1128/AEM.03905-13
Wu, Y., Xi, X., Tang, X., Luo, D., Gu, B., Lam, S. K., et al. (2018). Policy distortions, farm size, and the overuse of agricultural chemicals in China. Proc. Natl. Acad. Sci. U.S.A. 115, 7010–7015. doi: 10.1073/pnas.1806645115
Xu, C., and Leskovar, D. I. (2015). Effects of A. nodosum seaweed extracts on spinach growth, physiology and nutrition value under drought stress. Sci. Hortic. 183, 39–47. doi: 10.1016/j.scienta.2014.12.004
Xu, M. Y., Zhang, L., Li, W. W., Hu, X. L., Wang, M. B., Fan, Y. L., et al. (2014). Stress-induced early flowering is mediated by miR169 in Arabidopsis thaliana. J. Exp. Bot. 65, 89–101. doi: 10.1093/jxb/ert353
Yabur, R., Bashan, Y., and Hernández-Carmona, G. (2007). Alginate from the macroalgae Sargassum sinicola as a novel source for microbial immobilization material in wastewater treatment and plant growth promotion. J. Appl. Phycol. 19, 43–53. doi: 10.1007/s10811-006-9109-8
Yadav, N. S., Shukla, P. S., Jha, A., Agarwal, P. K., and Jha, B. (2012). The SbSOS1 gene from the extreme halophyte Salicornia brachiata enhances Na+ loading in xylem and confers salt tolerance in transgenic tobacco. BMC Plant Biol. 12:188. doi: 10.1186/1471-2229-12-188
Yakhin, O. I., Lubyanov, A. A., Yakhin, I. A., and Brown, P. H. (2017). Biostimulants in plant science: a global perspective. Front. Plant Sci. 7:671. doi: 10.3389/fpls.2016.02049
Yamaguchi-Shinozaki, K., and Shinozaki, K. (2006). Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 57, 781–803. doi: 10.1146/annurev.arplant.57.032905.105444
Yang, F., Liang, G., Liu, D., and Yu, D. (2009). Arabidopsis MiR396 mediates the development of leaves and flowers in transgenic tobacco. J. Plant Biol. 52, 475–481. doi: 10.1007/s12374-009-9061-7
Yang, Y., Sulpice, R., Himmelbach, A., Meinhard, M., Christmann, A., and Grill, E. (2006). Fibrillin expression is regulated by abscisic acid response regulators and is involved in abscisic acid-mediated photoprotection. Proc. Natl. Acad. Sci. U.S.A. 103, 6061–6066. doi: 10.1073/pnas.0501720103
Yordanov, I., Velikova, V., and Tsonev, T. (2000). Plant responses to drought, acclimation, and stress tolerance. Photosynthetica 38, 171–186. doi: 10.1023/A:1007201411474
Yuan, Y., and Macquarrie, D. (2015a). Microwave assisted extraction of sulfated polysaccharides (fucoidan) from Ascophyllum nodosum and its antioxidant activity. Carbohydr. Polym. 129, 101–107. doi: 10.1016/j.carbpol.2015.04.057
Yuan, Y., and Macquarrie, D. J. (2015b). Microwave assisted step-by-step process for the production of fucoidan, alginate sodium, sugars and biochar from Ascophyllum nodosum through a biorefinery concept. Bioresour. Technol. 198, 819–827. doi: 10.1016/j.biortech.2015.09.090
Yuan, Y., Zhang, J., Fan, J., Clark, J., Shen, P., Li, Y., et al. (2018). Microwave assisted extraction of phenolic compounds from four economic brown macroalgae species and evaluation of their antioxidant activities and inhibitory effects on α-amylase, α-glucosidase, pancreatic lipase and tyrosinase. Food Res. Int. 113, 288–297. doi: 10.1016/j.foodres.2018.07.021
Zhang, X., Ervin, E. H., and Schmidt, R. E. (2003). Plant growth regulators can enhance the recovery of Kentucky bluegrass sod from heat injury. Crop Sci. 43, 952–956.
Zhang, X., and Ervin, E. H. (2008). Impact of seaweed extract-based cytokinins and zeatin riboside on creeping bentgrass heat tolerance. Crop Sci. 48, 364–370. doi: 10.2135/cropsci2007.05.0262
Zhao, B., Ge, L., Liang, R., Li, W., Ruan, K., Lin, H., et al. (2009). Members of miR-169 family are induced by high salinity and transiently inhibit the NF-YA transcription factor. BMC Mol. Biol. 10:29. doi: 10.1186/1471-2199-10-29
Keywords: Ascophyllum nodosum, biostimulants, plant growth, stress tolerance, disease management
Citation: Shukla PS, Mantin EG, Adil M, Bajpai S, Critchley AT and Prithiviraj B (2019) Ascophyllum nodosum-Based Biostimulants: Sustainable Applications in Agriculture for the Stimulation of Plant Growth, Stress Tolerance, and Disease Management. Front. Plant Sci. 10:655. doi: 10.3389/fpls.2019.00655
Received: 28 March 2019; Accepted: 01 May 2019;
Published: 29 May 2019.
Edited by:
Jose M. Garcia-Mina, University of Navarra, SpainReviewed by:
Serenella Nardi, University of Padua, ItalyPetronia Carillo, Università degli Studi della Campania Luigi Vanvitelli, Italy
Copyright © 2019 Shukla, Mantin, Adil, Bajpai, Critchley and Prithiviraj. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Balakrishnan Prithiviraj, bprithiviraj@dal.ca
 Pushp Sheel Shukla
Pushp Sheel Shukla Emily Grace Mantin
Emily Grace Mantin Mohd Adil
Mohd Adil Sruti Bajpai
Sruti Bajpai Alan T. Critchley
Alan T. Critchley Balakrishnan Prithiviraj
Balakrishnan Prithiviraj