Corrigendum: Interactive Brain Activity: Review and Progress on EEG-Based Hyperscanning in Social Interactions
- 1School of Humanities and Social Science, University of Science and Technology of China, Hefei, China
- 2Department of Education, Hefei University, Hefei, China
- 3School of Foreign Languages, Anhui Jianzhu University, Hefei, China
- 4Department of Mechanical and Automation Engineering, The Chinese University of Hong Kong, Hong Kong, China
- 5Department of Social and Behavioural Sciences, City University of Hong Kong, Hong Kong, China
- 6CAS Key Laboratory of Brain Function and Disease, and School of Life Sciences, University of Science and Technology of China, Hefei, China
- 7School of Chemistry and Materials Science, University of Science and Technology of China, Hefei, China
- 8Hefei Medical Research Center on Alcohol Addiction, Anhui Mental Health Center, Hefei, China
- 9Academy of Psychology and Behavior, Tianjin Normal University, Tianjin, China
When individuals interact with others, perceived information is transmitted among their brains. The EEG-based hyperscanning technique, which provides an approach to explore dynamic brain activities between two or more interactive individuals and their underlying neural mechanisms, has been applied to study different aspects of social interactions since 2010. Recently there has been an increase in research on EEG-based hyperscanning of social interactions. This paper summarizes the application of EEG-based hyperscanning on the dynamic brain activities during social interactions according to the experimental designs and contents, discusses the possibility of applying inter-brain synchrony to social communication systems and analyzes the contributions and the limitations of these investigations. Furthermore, this paper sheds light on some new challenges to future EEG-based hyperscanning studies and the emerging field of EEG-based hyperscanning for pursuing the broader research field of social interactions.
Introduction
Social interaction, a fundamental part of our daily life, is at the core of human behaviors (Dumas, 2011; Dumas et al., 2014). There are many social interactions in our daily lives, which involves different kinds of interpersonal synchronies. For example, we synchronize our footsteps with those of our partners unconsciously when we walk together (Reddish et al., 2013). This phenomenon is considered as interpersonal synchrony. Synchronous behaviors, playing a central role in establishing and promoting social ties, are socially important. In addition, the degree of synchrony predicts subsequent affiliation ratings (Reindl et al., 2018). Some researchers found that there was a close relationship between neural dynamics and interpersonal behavioral synchronization (Hove and Risen, 2009). Based on those findings, some researchers are dedicated to exploring the mechanism of interpersonal synchrony and the functional significance of inter-brain synchrony in interpersonal interactions.
Through social interactions with others, human beings know each other and form a family or a state (Decety and Lamm, 2007). Although the social nature of human beings was noticed thousands of years ago, the studying of brain activities during social interactions in neuroscience has only been carried out for about 10 years (Hari and Kujala, 2009). In recent years, social neuroscientists suggest that the dynamic brain activities between two or more interactive individuals should be analyzed in order to provide a window into how their minds (Hari et al., 2015). A technique called hyperscanning or pseudo-hyperscanning is used to assess the level of between-brain coupling, which requires the measurement of brain activities of two or more participants involved in social interactions. Hyperscanning is a measurement of brain activities of participants at the same time, and pseudo-hyperscanning is a similar measurement but measures each participant at a time (Schoot et al., 2016). The first study on dynamic brain activities between individuals by virtue of electroencephalography (EEG) can be traced back to an experiment conducted by Duane and Behrendt (1965). It took the lead in using the EEG to record twins' brain activities simultaneously and calculate the correlation between EEG traces. From then on, several researchers investigated the dynamic brain activities and reported correlated brain signals by using simultaneous electroencephalographic recordings from interactive participants. However, these studies on how two brains interact with each other used offline designs and the subjects were isolated from one another without actually taking part in social interactions due to technological limitations (Kohler, 1969; Perez-Rincon et al., 1981).
The idea of recording multi-subjects' brain activities simultaneously was proposed by Montague et al. (2002) and it was called the “hyperscanning” technique. The term “hyperscanning” refers to simultaneous recording of hemodynamic or neuro-electric activity of the brains from multiple subjects involved in social interactions. By means of cognitive neuroscience equipment (e.g., EEG, fMRI, fNIRS), the “hyperscanning technique” has the potential to explore interpersonal brain mechanisms underlying neuronal correlation between interaction during two or more people taking part in social interactions (Balconi and Molteni, 2015). Combined with fNIRS, fMRI and EEG, the study on the neural mechanism of interpersonal social interactions has currently gained momentum in the young field of social neuroscience since 2002 (Konvalinka and Roepstorff, 2012; Babiloni and Astolfi, 2014; Koike et al., 2015; Schoot et al., 2016; Xue et al., 2018). Conventionally, the fMRI- or fNIRS-based hyperscanning has the general drawback of the low temporal resolution due to the inertia of the bold response, whereas EEG has finer temporal resolution and thus becomes the most frequently used technique in hyperscanning studies (Koike et al., 2015). One of the advantages of EEG is that it has finer temporal resolution than fMRI. EEG provides the opportunity to record activation on the millisecond scale (Spiegelhalder et al., 2014). Another advantage of EEG is it allows us to observe the inter-brain neural synchronization in more natural settings despite the argument that EEG is susceptible to head movements (Koike et al., 2015). Therefore, studies of EEG-based hyperscanning on social interactions have been extended increasingly, from simple imitative interactions to complicated affective communications in the last decade.
Thus, this review firstly introduced the social interaction and its importance as well as provided an overview of the application of EEG-based hyperscanning technique on the dynamic brain activities during social interactions. Then, this review introduced four specific domains of inter-brain activities according to the experimental designs and contents. Next, this review analyzed the contributions as well as the limitations of these investigations and shed light on the new challenges to future EEG-based hyperscanning studies. Furthermore, this review discussed the upcoming field of EEG-based hyperscanning to pursue a broader study field of social interactions.
Inter-Brain Activities of Joint Action
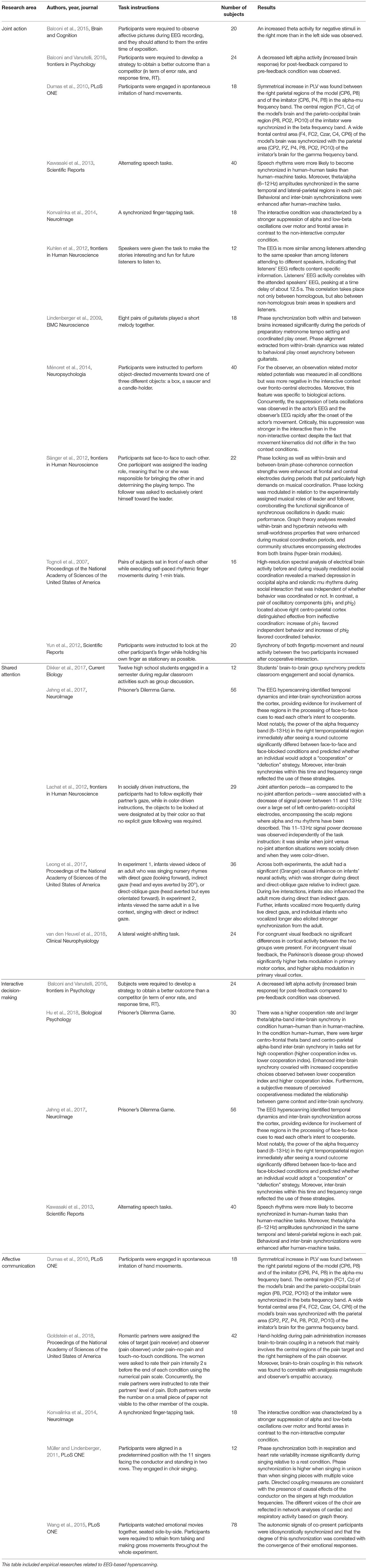
In recent years, with the development of EEG-based hyperscanning technique, several independent research teams have used movement synchronized task (Tognoli et al., 2007; Lindenberger et al., 2009; Dumas et al., 2011; Yun et al., 2012), leader-follow task (Sänger et al., 2012; Konvalinka et al., 2014), speech rhythm synchronization task (Kawasaki et al., 2013), cooperative task (Balconi et al., 2015) and actor-observer interaction paradigm (Ménoret et al., 2014) to explore the neural mechanisms of social coordination. These are all related to joint actions and believed to involve a variety of mechanisms (Della Gatta et al., 2017). In order to know how brain-to-brain interacted with each other during joint actions, some researchers investigated the dynamic brain activities between pairs of subjects while executing spontaneous imitation movements toward the vision of each other's actions (Dumas et al., 2010). The results showed that the alpha–mu band showed the strongest inter-brain synchrony among the right centroparietal regions. Another unconsciously synchronized fingertip movement experiment was conducted to explore the mechanisms of body movement synchrony (Yun et al., 2012).
In order to reduce similarities in movement and perception which may enhance inter-brain synchrony of the experiment, Sänger et al. (2012) used a modified leader-follower task with two guitarists playing in two voices. The enhanced phase locking, within- and between-brain phase coherence was found during musical coordination periods, especially at frontal and central sites. The results extended previous findings and attributed between-brain phase coherence to interpersonal action coordination rather than interpersonal similar action. However, some EEG-based hyperscanning studies showed that asymmetric brain-coupling1 patterns of leader-follower participants in a dyad during coordinated movements (Dumas et al., 2010). The asymmetric phenomena were also emerged from some studies of decision-making in game contexts (Balconi and Vanutelli, 2016). This asymmetric pattern of coupling may be explained by the differential roles of the partners during the interaction, and the participants may have different expectations for the assigned roles. Whether the asymmetric brain-coupling pattern is the mechanism of leader-follower communication remains unresolved, which can be explored in future researches.
In addition, the inter-brain synchrony was also found in other experiments of interpersonal behavioral coordination. For example, in a cooperative and competitive task, the inter-brain synchrony between subjects was significantly higher when they cooperated with each other than that when they were in the competitive condition (Davis et al., 2016). Kuhlen et al. (2012) revealed the coordination of brain activities between the speakers and listeners during verbal communications. Kawasaki et al. (2013) investigated the relationship of brain rhythm synchronization during speech rhythm synchronization between individuals and found the inter-brain synchrony of theta/alpha (6–12 Hz) amplitudes in the temporal and lateral-parietal regions in each pair. Moreover, Ménoret et al. (2014) found that the suppression of beta oscillations was observed in the actor's EEG and the observer's EEG rapidly after the onset of the actor's movement during a face-to-face actor-observer interaction paradigm, and this suppression was stronger for the observer in the interactive than in the non-interactive context independent of the act conducted by a human or a robot.
Based on quantifying functional similarities or temporal synchronization between brains during social interactions, most results attributed inter-brain synchrony or phase coherence to interpersonal action coordination.
Inter-Brain Activities of Shared Attention
Among social signals, the non-verbal signals are deemed to be crucial visual cues for communicative intentions (Jahng et al., 2017). During these processes, people share the same perspective with one another, and this phenomenon is called shared attention (Shteynberg, 2018).
Mutual gaze and shared attention play an essential role in our abilities to detect others' focuses of interest, as well as to infer their intentions, desires and thoughts. The importance of mutual gaze and shared attention on the development of social cognition has been underlined (Koike et al., 2016). To investigate the neural mechanisms of interpersonal shared attention, researchers measured the brain activities of two people who engaged in actual mutual gaze or shared attention experimental task with inter-subjective sharing reciprocal information without words by recording simultaneously dual-EEG. Lachat et al. (2012) set up a live shared attention paradigm to investigate the influence of shared attention on oscillatory activities within the alpha-mu (8–12 Hz) frequency band. Compared with the no-shared attention periods, a decrease of 11–13 Hz signal was found during the shared attention periods over a large set of left centroparietal electrodes extending to occipital electrodes. Another EEG-based hyperscanning study was performed by Leong et al. (2017) to verify whether direct gaze increased neural coupling between adult-infant partners during social interactions. Dikker et al. (2017) found that the highest pairwise alpha coherence emerged in student pairings who sat face-to-face compared to the other two student pairings (adjacent and no face-to-face or no adjacent) and the inter-brain synchrony between students consistently predicted class engagement and social dynamics.
The studies mentioned above supported the view that alpha frequency band was involved in visual processing (van den Heuvel et al., 2018), arousal and attentional mechanisms (Foxe and Snyder, 2011). People exchange reciprocal information via eye-to-eye contact and act according to the interpretation of the information. The results in certain degree showed that eye contact enhanced neural coupling between interactive individuals during social interactions. The conclusion was verified by the experiment about autism spectrum disorders (Yates and Couteur, 2016).
Inter-Brain Activities of Interactive Decision-making
Interactive decision-making is defined as the dynamic process of making choices depending on the antecedent decision behaviors of the partner and other social cues in interactive tasks. It is one of the most omnipresent activities in human beings (Nummenmaa et al., 2018). The decision-making process always requires higher degree of cognitive involvement between interactive individuals in real life. Such an activity involves goal-directed behaviors, social cognition, and theory-of-mind abilities (Gilam and Hendler, 2016). By using EEG-based hyperscanning, a series of studies in game contexts provide abundant evidences for the neural process of interactive decision-making during social interactions.
For example, Balconi and Vanutelli (2016) for the first time explored the neural process during interactive decision-making with EEG-based hyperscanning technique. The experiment was performed in five groups of four subjects during a cooperative card game that involved groups of two subjects against other two. The game was played with two teams of subjects sitting at north and south against those two sitting at east and west. The results showed that causal links emerged from prefrontal areas of the different subjects when they were performing cooperative games in different frequency bands. One of the remarkable things among the Prisoner's Dilemma experiments is the controversial result of the connectivity between the two brains in the defect condition during interactive decision making. The inter-brain synchrony refers to dynamical similarity in brain signals. Even competitive behavior could lead to them if the people need to represent the same information at the same moment. Jahng et al. (2017) found that the pattern of inter-brain connectivity in the cooperation condition was denser than in the defect condition when the individuals engaged in the Prisoner's Dilemma game. Cooperation and defection are different types of social interactions. Some studies also found the inter-brain links with EEG-based hyperscanning during interactive decision-making (Hu et al., 2018). The result also emerged in previous fMRI-based hyperscanning studies in interactive decision-making (King-Casas et al., 2005). For example, Hu et al. (2018) stepped further to compare inter-brain synchrony between H-H (human played the Prisoner's Dilemma game with partner) with H-M (human played the Prisoner's Dilemma game with computer) and found that there was a higher rate of cooperation and larger theta/alpha-band inter-brain synchrony in H-H condition. These findings were in keeping with some neuroimaging studies which suggested cooperation promotes inter-brain synchrony (Pan et al., 2016).
In this part, we discussed how two brains interacted with each other when individuals engage in a more complicated social activity—interactive decision-making. There are two hypotheses proposed to explain the emergence of inter-brain synchrony in interactive decision making: the cooperative interaction hypothesis and the similar task hypothesis (Hu et al., 2018). A line of evidence has demonstrated that neural activities of two individuals are more synchronized when they perform cooperative interactions (Tognoli et al., 2007; Lindenberger et al., 2009; Dumas et al., 2011; Yun et al., 2012).
Inter-Brain Activities of Affective Communication
Affective communication is a complex process during which interactive individuals express and perceive emotional signals and exchange information about internal affective states (Symons et al., 2016). It is a form of emotional support having direct and indirect effects on the stress process (Viswesvaran et al., 1999). Emotions play an important role in regulating and motivating a person's thoughts, feelings, and behavior in almost every aspect of people's life. Interpersonal emotions are evoked when we implicitly or explicitly reflect on ourselves and evaluate ourselves in the context of our surrounding social world (Symons et al., 2016). Müller and Lindenberger (2011) found oscillatory couplings of cardiac and respiratory activities among singers and conductor while they were engaging in choir singing. It seems that favorable affective communication between two individuals is closely related to their physiological states. However, the interactive mechanism of the two dynamic brains is unclear as to affective communication.
Physicians' affective communication has a supportive function particularly effective in situations where individuals lack control (Ommen et al., 2008; Dumas et al., 2012; Abrams et al., 2013; Fowler et al., 2013; Novembre et al., 2017). By means of the EEG-based hyperscanning technique, Müller and Lindenberger (2011) found that theta-alpha hyper-brain networks bound the two brains of kissing partners together with a method of network construction based on the cross-frequency coupling. Thus, it can be inferred that brain-to-brain coupling is a neural marker for interpersonal communication of affection. It is noticeable that there is a relatively weaker inter-brain coupling between the right parietal regions of the female partner and the right parieto-occipito-temporal areas of the male partner in the control (no-touch-no-pain) condition. The inter-brain coupling pattern is in line with the previous findings of interpersonal action coordination (Dumas et al., 2010; Konvalinka et al., 2014). Wang et al. (2015) proposed that co-presence of two speakers could result in their autonomic physiological coupling.
Human emotional experience naturally occurs while interacting in a spontaneous, dynamic and response-contingent fashion with other humans (Gilam and Hendler, 2016). Based on above-mentioned, it can be assumed that the inter-brain coupling pattern in the control condition may constitute a basic interpersonal interaction. However, there are few tasks related to interactive affection communication, which needs future exploration.
Future Challenges and Directions
Many studies with EEG-based hyperscanning have elucidated that the inter-brain synchrony as a result of ongoing social interactions is more directly and precisely, from coordinated behaviors to affective communication, and have focused on describing the specific time and frequency ranges of the neural processing (Dumas et al., 2010; Kawasaki et al., 2013). Based on quantifying functional similarities or temporal synchronization between brains during social interactions, most studies regarded inter-brain synchrony or phase coherence as an important index of interpersonal interaction and attributed inter-brain synchrony or phase coherence to interpersonal action coordination (Konvalinka et al., 2014). With different experimental tasks, the findings showed that the inter-brain synchrony got across different frequencies. With the portability of EEG devices, people were able to interact naturally and the inter-brain effect was recorded in a very natural setting (Astolfi et al., 2011). The design of social interactive experiments mentioned is more realistic, and studies on social interactions have been extended to a wide range of fields. Though neuroscience has made great progress in recent decades, we only have a preliminary understanding of how two brains interact with each other during social interactions. All studies this review mentioned can be seen in Table 1. For the purpose of studying and comprehending the neural process of social communications in greater depth, we need to focus on some challenges as well as future directions.
Firstly, the psychological significance of inter-brain synchrony is unclear, neither is the minimum inter-brain synchrony requirements. The inter-brain synchrony between brains has been found in most of these studies above-mentioned. There are two patterns of inter-brain synchrony, symmetric and asymmetric inter-brain coherence, among these EEG-based hyperscanning studies. Whether the asymmetric pattern of coupling can be interpreted as the differential roles and different psychological process of the partners during social interactions, the issue should be fully investigated in the future. A series of studies have demonstrated that the neural activities of two individuals are more synchronized when they did synchronized action (Lindenberger et al., 2009; Dumas et al., 2011; Yun et al., 2012; Kawasaki et al., 2013). Another series of studies have attributed the inter-brain synchrony to interpersonal mutual cooperation (Jahng et al., 2017; Hu et al., 2018). However, the inter-brain synchrony has been shown in some non-cooperation or non-interaction activities, for example, the inter-brain synchrony emerged among listeners who attended to the same speaker (Kuhlen et al., 2012), between subjects who participated in a task and the subjects who observed (Kawasaki et al., 2013; Ménoret et al., 2014), and between the participants who just sat facing each other (Goldstein et al., 2018). Participants in these studies performed the task in an independent way that interactions scarcely existed (Hu et al., 2018). Whether the inter-brain synchrony reflects the functional similarity in common task or a basic neural constitution of interpersonal interaction, the assumptions are needed to be verified by future researches. In addition, the mental constituent of these tasks involved in the psychological process of the coherence of inter-brain activities were complicated, including empathy, attention and closeness. Maybe it was the reason that the inter-brain synchrony got across different frequencies in different experiments. An interesting question arises about how to decompose the complicated mental constituent into basic psychological processes. Answers to that question will help us understand the synchronization effects better.
Secondly, EEG-based hyperscanning studies told little about how this inter-brain synchrony was generated (Hu et al., 2018). Most experimental paradigms over the past decades were correlated with long-term neural activities and these studies described the inter-brain activities as a result of ongoing social interactions. In most of these experimental paradigms, participant remained unaltered during the process of social interactions. However, social interactions are interrelated and interacted with each other and it is also a process of turn-taking interaction. It is important to set up well-designed experiments investigating neural transient dynamics related to the real reciprocal interactions. EEG-based hyperscanning studies still have a long way to go, with a view to the methodological challenges of studying brains interactive mechanisms of social interaction.
Thirdly, the inter-brain synchrony can be applied to groups with social cognitive impairment. For example, it is useful to compare social interactions between normal individuals and normal one as well as between normal individuals with abnormal one (e.g., individuals with social cognitive impairment). There may be difference in the inter-brain synchrony in these two different circumstances. Based on these research findings, researchers can ensure the corresponding brain regions to social cognitive impairment in order to provide efficient treatment. For example, impairment of reciprocal social interactions is regarded as a key sign of individuals with autism spectrum disorder (ASD; Qualls and Corbett, 2017). However, the etiology of ASD remains largely unknown. With the advantage of the ability to localize the epicenter of brain activation, fMRI-based hyperscanning can precisely detect the regions exhibiting inter-brain activation. In the future, we can combine EEG-based hyperscanning with fMRI-based hyperscanning to study social brain disorders and get a better knowledge of the social interaction mechanisms of patients with autism spectrum disorder or borderline personality disorder. In the end, we can localize the target and put forward the applicable therapies. For example, the behavioral synchrony between psychotherapists and patients can be improved to enhance their inter-brain synchrony in order to promote communications and attain good treatment effect.
Fourthly, future researches should pay more attention to the influencing factors of the inter-brain synchrony. According to Zhang and Liu (2018), four factors including the aim of communication, the object of communication, the form of communication and the content of communication, are influencing interpersonal neural synchronization. For example, Dumas et al. used a biophysical model to quantify the correlation between the anatomical and functional similarity of the two brains and inter-brain synchronizations. Therefore, future studies should employ the EEG-based hyperscanning technique to investigate more factors that may influence the inter-brain synchrony.
Author Contributions
SL and XZ: Conceive and writing frame design; DL, SL and XZ: Wrote the paper; DL, SL, XL, CZ, AL, CJ, YC, HW, and XZ: Revise the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by grants from Humanities and Social Science Project of Hefei University (18RW12ZDA), the National Key Basic Research Program (2016YFA0400900, 2018YFC0831101), the National Natural Science Foundation of China (31471071, 31771221, 61773360, and 71874170), and the Fundamental Research Funds for the Central Universities of China.
Footnotes
1. ^The asymmetric brain-coupling in Dumas et al. (2010). meant that there was a neural coupling from the leader to the follower while there was no neural coupling from the follower to the leader. On the contrary, the symmetric brain-coupling means that mutual neural couplings.
References
Abrams, D. A., Ryali, S., Chen, T., Chordia, P., Khouzam, A., Levitin, D. J., et al. (2013). Inter-subject synchronization of brain responses during natural music listening. Eur. J. Neurosci. 37, 1458–1469. doi: 10.1111/ejn.12173
Astolfi, L., Toppi, J., De Vico Fallani, F., Vecchiato, G., Cincotti, F., Wilke, C. T., et al. (2011). Imaging the social brain by simultaneous hyperscanning during subject interaction. IEEE Intell. Syst. 26, 38–45. doi: 10.1109/MIS.2011.61
Babiloni, F., and Astolfi, L. (2014). Social neuroscience and hyperscanning techniques: past, present and future. Neurosci. Biobehav. Rev. 44, 76–93. doi: 10.1016/j.neubiorev.2012.07.006
Balconi, M., Grippa, E., and Vanutelli, M. E. (2015). What hemodynamic (fNIRS), electrophysiological (EEG) and autonomic integrated measures can tell us about emotional processing? Brain Cogn. 95, 67–76. doi: 10.1016/j.bandc.2015.02.001
Balconi, M., and Molteni, E. (2015). Past and future of near-infrared spectroscopy in studies of emotion and social neuroscience. J. Cogn. Psychol. 28, 129–146. doi: 10.1080/20445911.2015.1102919
Balconi, M., and Vanutelli, M. E. (2016). Competition in the brain. The contribution of EEG and fNIRS modulation and personality effects in social ranking. Front. Psychol. 7:1587. doi: 10.3389/fpsyg.2016.01587
Davis, T. J., Brooks, T. R., and Dixon, J. A. (2016). Multi-scale interactions in interpersonal coordination. J. Sport Health Sci. 5, 25–34. doi: 10.1016/j.jshs.2016.01.015
Decety, J., and Lamm, C. (2007). The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. Neuroscientist 13, 580–593. doi: 10.1177/1073858407304654
Della Gatta, F., Garbarini, F., Rabuffetti, M., Viganò, L., Butterfill, S. A., and Sinigaglia, C. (2017). Drawn together: when motor representations ground joint actions. Cognition 165, 53–60. doi: 10.1016/j.cognition.2017.04.008
Dikker, S., Wan, L., Davidesco, I., Kaggen, L., Oostrik, M., Mcclintock, J., et al. (2017). Brain-to-brain synchrony tracks real-world dynamic group interactions in the classroom. Curr. Biol. 27, 1375–1380. doi: 10.1016/j.cub.2017.04.002
Duane, T. D., and Behrendt, T. (1965). Extrasensory electroencephalographic induction between identical twins. Science 150, 367–367.
Dumas, G. (2011). Towards a two-body neuroscience. Commun. Integr. Biol. 4, 349–352. doi: 10.4161/cib.4.3.15110
Dumas, G., Chavez, M., Nadel, J., and Martinerie, J. (2012). Anatomical connectivity influences both intra- and inter-brain synchronizations. PLoS ONE 7:e36414. doi: 10.1371/journal.pone.0036414
Dumas, G., Lachat, F., Martinerie, J., Nadel, J., and George, N. (2011). From social behavior to brain synchronization: review and perspectives in hyperscanning. IRBM 32, 48–53. doi: 10.1016/j.irbm.2011.01.002
Dumas, G., Laroche, J., and Lehmann, A. (2014). Your body, my body, our coupling moves our bodies. Front. Human Neurosci. 8:1004. doi: 10.3389/fnhum.2014.01004
Dumas, G., Nadel, J., Soussignan, R., Martinerie, J., and Garnero, L. (2010). Inter-brain synchronization during social interaction. PLoS ONE 5:e12166. doi: 10.1371/journal.pone.0012166
Fowler, J. C., Allen, J. G., Oldham, J. M., and Frueh, B. C. (2013). Exposure to interpersonal trauma, attachment insecurity, and depression severity. J. Affect. Disord. 149, 313–318. doi: 10.1016/j.jad.2013.01.045
Foxe, J. J., and Snyder, A. C. (2011). The role of alpha-band brain oscillations as a sensory suppression mechanism during selective attention. Front. Psychol. 2:154. doi: 10.3389/fpsyg.2011.00154
Gilam, G., and Hendler, T. (2016). With love, from me to you: embedding social interactions in affective neuroscience. Neurosci. Biobehav. Rev. 68, 590–601. doi: 10.1016/j.neubiorev.2016.06.027
Goldstein, P., Weissman-fogel, I., Dumas, G., and Shamay-Tsoory, S. G. (2018). Brain-to-brain coupling during handholding is associated with pain reduction. Proc. Natl. Acad. Sci. U.S.A. 115, E2528–E2537. doi: 10.1073/pnas.1703643115
Hari, R., Henriksson, L., Malinen, S., and Parkkonen, L. (2015). Centrality of social interaction in human brain function. Neuron 88, 181–193. doi: 10.1016/j.neuron.2015.09.022
Hari, R., and Kujala, M. V. (2009). Brain basis of human social interaction: from concepts to brain imaging. Physiol. Rev. 89, 453–479. doi: 10.1152/physrev.00041.2007
Hove, M. J., and Risen, J. L. (2009). It's all in the timing: Interpersonal synchrony increases affiliation. Soc. Cogn. 27, 949–961. doi: 10.1521/soco.2009.27.6.949
Hu, Y., Pan, Y., Shi, X., Cai, Q., Li, X., and Cheng, X. (2018). Inter-brain synchrony and cooperation context in interactive decision making. Biol. Psychol. 133, 54–62. doi: 10.1016/j.biopsycho.2017.12.005
Jahng, J., Kralik, J. D., Hwang, D. U., and Jeong, J. (2017). Neural dynamics of two players when using nonverbal cues to gauge intentions to cooperate during the Prisoner's Dilemma game. Neuroimage 157, 263–274. doi: 10.1016/j.neuroimage.2017.06.024
Kawasaki, M., Yamada, Y., Ushiku, Y., Miyauchi, E., and Yamaguchi, Y. (2013). Inter-brain synchronization during coordination of speech rhythm in human-to-human social interaction. Sci. Rep. 3:1692. doi: 10.1038/srep01692
King-Casas, B., Tomlin, D., Anen, C., Camerer, C. F., Quartz, S. R., and Montague, P. R. (2005). Getting to know you: reputation and trust in a two-person economic exchange. Science 308, 78–83. doi: 10.1126/science.1108062
Kohler, W. C. (1969). Sleep EEG patterns in identical twins with developmental discordance. South. Med. J. 62, 17–22. doi: 10.1097/00007611-196901000-00005
Koike, T., Tanabe, H. C., Okazaki, S., Nakagawa, E., Sasaki, A. T., Shimada, K., et al. (2016). Neural substrates of shared attention as social memory: a hyperscanning functional magnetic resonance imaging study. Neuroimage 125, 401–412. doi: 10.1016/j.neuroimage.2015.09.076
Koike, T., Tanabe, H. C., and Sadato, N. (2015). Hyperscanning neuroimaging technique to reveal the “two-in-one” system in social interactions. Neurosci. Res. 90, 25–32. doi: 10.1016/j.neures.2014.11.006
Konvalinka, I., Bauer, M., Stahlhut, C., Hansen, L. K., Roepstorff, A., and Frith, C. D. (2014). Frontal alpha oscillations distinguish leaders from followers: multivariate decoding of mutually interacting brains. Neuroimage 94, 79–88. doi: 10.1016/j.neuroimage.2014.03.003
Konvalinka, I., and Roepstorff, A. (2012). The two-brain approach: how can mutually interacting brains teach us something about social interaction? Front. Human Neurosci. 6:215. doi: 10.3389/fnhum.2012.00215
Kuhlen, A. K., Allefeld, C., and Haynes, J. D. (2012). Content-specific coordination of listeners' to speakers' EEG during communication. Front. Human Neurosci. 6:266. doi: 10.3389/fnhum.2012.00266
Lachat, F., Hugueville, L., Lemaréchal, J. D., Conty, L., and George, N. (2012). Oscillatory brain correlates of live joint attention: a dual-EEG study. Front. Human Neurosci. 6:156. doi: 10.3389/fnhum.2012.00156
Leong, V., Byrne, E., Clackson, K., Georgieva, S., Lam, S., and Wass, S. (2017). Speaker gaze increases information coupling between infant and adult brains. Proc. Natl. Acad. Sci. U.S.A. 114, 13290–13295. doi: 10.1073/pnas.1702493114
Lindenberger, U., Li, S. C., Gruber, W., and Müller, V. (2009). Brains swinging in concert: cortical phase synchronization while playing guitar. BMC Neurosci. 10:22. doi: 10.1186/1471-2202-10-22
Ménoret, M., Varnet, L., Fargier, R., Cheylus, A., Curie, A., des Portes, V., et al. (2014). Neural correlates of non-verbal social interactions: a dual-EEG study. Neuropsychologia 55, 85–97. doi: 10.1016/j.neuropsychologia.2013.10.001
Montague, P. R., Berns, G. S., Cohen, J. D., Mcclure, S. M., Pagnoni, G., Dhamala, M., et al. (2002). Hyperscanning: simultaneous fMRI during linked social interactions. Neuroimage 16, 1159–1164. doi: 10.1006/nimg.2002.1150
Müller, V., and Lindenberger, U. (2011). Cardiac and respiratory patterns synchronize between persons during choir singing. PLoS ONE 6:e24893. doi: 10.1371/journal.pone.0024893
Novembre, G., Knoblich, G., Dunne, L., and Keller, P. E. (2017). Interpersonal synchrony enhanced through 20 Hz phase-coupled dual brain stimulation. Soc. Cogn. Affect. Neurosci. 11:539. doi: 10.1093/scan/nsw172
Nummenmaa, L., Lahnakoski, J. M., and Glerean, E. (2018). Sharing the social world via intersubject neural synchronization. Curr. Opin. Psychol. 24, 7–14. doi: 10.1016/j.copsyc.2018.02.021
Ommen, O., Janssen, C., Neugebauer, E., Bouillon, B., Rehm, K., Rangger, C., et al. (2008). Trust, social support and patient type—associations between patients perceived trust, supportive communication and patients' preferences in regard to paternalism, clarification and participation of severely injured patients. Patient Educ. Couns. 73, 196–204. doi: 10.1016/j.pec.2008.03.016
Pan, Y., Cheng, X., Zhang, Z., Li, X., and Hu, Y. (2016). Cooperation in lovers: an fNIRS based hyperscanning study. Hum. Brain Mapp. 38, 831–841. doi: 10.1002/hbm.23421
Perez-Rincon, H., Pallares, A. M., and Alvarez-Rueda, J. M. (1981). Case of behavioral, psychological and electroencephalographic identity in a pair of identical twins. Acta Psiquiatr. Psicol. Am. Lat. 27, 292–294.
Qualls, L. R., and Corbett, B. A. (2017). Examining the relationship between social communication on the ADOS and real-world reciprocal social communication in children with ASD. Res. Autism Spectr. Disord. 33 1–9. doi: 10.1016/j.rasd.2016.10.003
Reddish, P., Bulbulia, J., and Fischer, R. (2013). Does synchrony promote generalized pro-sociality? Relig. Brain Behav. 4, 3–19. doi: 10.1080/2153599X.2013.764545
Reindl, V., Gerloff, C., Scharke, W., and Konrad, K. (2018). Brain-to-brain synchrony in parent-child dyads and the relationship with emotion regulation revealed by fNIRS-based hyperscanning. Neuroimage 178, 493–502. doi: 10.1016/j.neuroimage.2018.05.060
Sänger, J., Müller, V., and Lindenberger, U. (2012). Intra-and interbrain synchronization and network properties when playing guitar in duets. Front. Human Neurosci. 6:312. doi: 10.3389/fnhum.2012.00312
Schoot, L., Hagoort, P., and Segaert, K. (2016). What can we learn from a two-brain approach to verbal interaction? Neurosci. Biobehav. Rev. 68, 454–459. doi: 10.1016/j.neubiorev.2016.06.009
Shteynberg, G. (2018). A collective perspective: shared attention and the mind. Curr. Opin. Psychol. 23, 93–97. doi: 10.1016/j.copsyc.2017.12.007
Spiegelhalder, K., Ohlendorf, S., Regen, W., Feige, B., Tebartz van Elst, L., Weiller, C., et al. (2014). Interindividual synchronization of brain activity during live verbal communication. Behav. Brain Res. 258, 75–79. doi: 10.1016/j.bbr.2013.10.015
Symons, A. E., El-Deredy, W., Schwartze, M., and Kotz, S. A. (2016). The functional role of neural oscillations in non-verbal emotional communication. Front. Human Neurosci. 10:239. doi: 10.3389/fnhum.2016.00239
Tognoli, E., Lagarde, J., DeGuzman, G. C., and Kelso, J. A. (2007). The phi complex as a neuromarker of human social coordination. Proc. Natl. Acad. Sci. U.S.A. 104, 8190–8195. doi: 10.1073/pnas.0611453104
van den Heuvel, M. R. C., van Wegen, E. E. H., Beek, P. J., Kwakkel, G., and Daffertshofer, A. (2018). Incongruent visual feedback during a postural task enhances cortical alpha and beta modulation in patients with Parkinson's disease. Clin. Neurophysiol. 129, 1357–1365. doi: 10.1016/j.clinph.2018.04.602
Viswesvaran, C., Sanchez, J. I., and Fisher, J. (1999). The role of social support in the process of work stress: a meta-analysis. J. Vocat. Behav. 54, 314–334. doi: 10.1006/jvbe.1998.1661
Wang, X. L., Li, J. T., and Liu, S. Z. (2015). The mere co-presence: synchronization of autonomic signals and emotional responses across co-present individuals not engaged in direct interaction. PLoS ONE 10:e0125804. doi: 10.1371/journal.pone.0125804
Xue, H., Lu, K., and Hao, N. (2018). Cooperation makes two less-creative individuals turn into a highly-creative pair. Neuroimage 172, 527–537. doi: 10.1016/j.neuroimage.2018.02.007
Yates, K., and Couteur, A. L. (2016). Diagnosing autism/autism spectrum disorders. Paediatr. Child Health 26, 513–518. doi: 10.1016/j.paed.2016.08.004
Yun, K., Watanabe, K., and Shimojo, S. (2012). Interpersonal body and neural synchronization as a marker of implicit social interaction. Sci. Rep. 2:959. doi: 10.1038/srep00959
Keywords: social interaction, EEG-based hyperscanning, inter-brain synchrony, phase coherence, inter-brain activities
Citation: Liu D, Liu S, Liu X, Zhang C, Li A, Jin C, Chen Y, Wang H and Zhang X (2018) Interactive Brain Activity: Review and Progress on EEG-Based Hyperscanning in Social Interactions. Front. Psychol. 9:1862. doi: 10.3389/fpsyg.2018.01862
Received: 02 July 2018; Accepted: 12 September 2018;
Published: 08 October 2018.
Edited by:
Jan Antfolk, Åbo Akademi University, FinlandReviewed by:
Guillaume Dumas, Institut Pasteur, FranceGadi Gilam, Stanford University, United States
Copyright © 2018 Liu, Liu, Liu, Zhang, Li, Jin, Chen, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shen Liu, liushenpsy@ustc.edu.cn
Xiaochu Zhang, zxcustc@ustc.edu.cn
†These authors have contributed equally to this work and shared first authorship
 Difei Liu
Difei Liu Shen Liu
Shen Liu Xiaoming Liu
Xiaoming Liu Chong Zhang4
Chong Zhang4 Chenggong Jin
Chenggong Jin Xiaochu Zhang
Xiaochu Zhang