Cytosporone B as a Biological Preservative: Purification, Fungicidal Activity and Mechanism of Action against Geotrichum citri-aurantii
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experiment Procedures
2.2. Fungi and Culture
2.3. Fermentation, Extraction, and Isolation
2.4. Bioassays
2.4.1. In Vitro Assays
2.4.2. In Vivo Assays
2.5. Scanning Electron Microscopy
2.6. Determination of Cytoplasmic Membrane Integrity
2.7. Transcriptional Analysis
2.7.1. RNA Extraction and Illumina Sequencing
2.7.2. Sequence Assembly, Annotation, and Expression Analysis
2.8. Quantitative Real-Time PCR Analysis
2.9. Statistical Analysis
3. Results
3.1. Structure Identification of Compounds 1–12
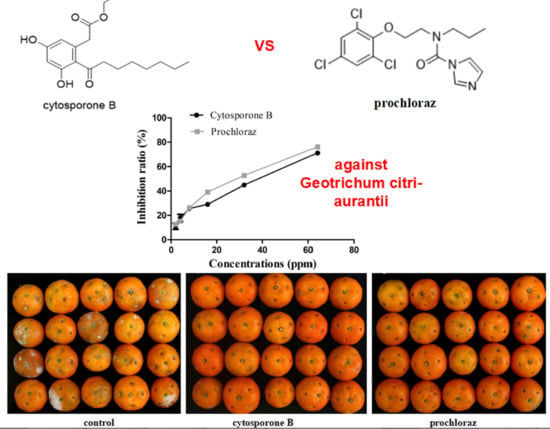
3.2. In Vivo and In Vitro Activity of Cytosporone B
3.3. Membrane Integrity of G. citri-aurantii under the Treatment of Cytosporone B
3.4. Transcriptome Sequencing and DGEs Analysis of G. citri-aurantii
3.5. Identification of Genes Related to Amino Acid Synthesis and Metabolism
3.6. Identification of Genes Related to Signal Transduction Mechanisms
4. Discussion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| EC50 | Median effect concentration |
| MIC | minimum inhibitory concentration |
| SEM | scanning electron microscopy |
| PI | propidium iodide |
| PDA | potato dextrose agar |
| NR | nonredundant protein database |
| GO | gene Ontology |
| COG | Clusters of Orthologous Groups |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| PCC | pearson’s correlation coefficient |
| DEGs | differential expression of genes |
| qRT-PCR | quantitative real-time PCR |
References
- FAOSTAT Database. Available online: http://www.fao.org/faostat/en (accessed on 1 October 2017).
- Schirra, M.; D’Aquino, S.; Cabras, P.; Angioni, A. Control of postharvest diseases of fruit by heat and fungicides: Efficacy, residue levels, and residue persistence. A review. J. Agric. Food Chem. 2011, 59, 8531–8542. [Google Scholar] [CrossRef]
- Butler, E.; Fogle, D.; Miranda, M. Galactomyces citri-aurantii a newly found teleomorph of Geotrichum citri-aurantii—The cause of sour rot of citrus fruit. Mycotaxon 1988, 33, 197–212. [Google Scholar]
- Sparks, T.C.; Hunter, J.E.; Lorsbach, B.A.; Hanger, G.; Gast, R.E.; Kemmitt, G.; Bryant, R.J. Crop Protection Discovery: Is Being the First Best? J. Agric. Food Chem. 2018, 66, 10337–10346. [Google Scholar] [CrossRef]
- Xu, S.X.; Li, Y.C.; Liu, X.; Mao, L.J.; Zhang, H.; Zheng, X.D. In vitro and in vivo antifungal activity of a water-dilutable cassia oil microemulsion against Geotrichum citri-aurantii. J. Sci. Food Agric. 2012, 92, 2668–2671. [Google Scholar] [CrossRef] [PubMed]
- Karim, H.; Boubaker, H.; Askarne, L.; Talibi, I.; Msanda, F.; Boudyach, E.H.; Saadi, B.; Ait Ben Aoumar, A. Antifungal properties of organic extracts of eight Cistus L. species against postharvest citrus sour rot. Lett. Appl. Microbiol. 2016, 62, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, L.P.; Cunha, T.D.; da Silva, A.C.; Kupper, K.C. Biocontrol ability and putative mode of action of yeasts against Geotrichum citri-aurantii in citrus fruit. Microbiol. Res. 2016, 188–189, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Droby, S.; Wisniewski, M. The fruit microbiome: A new frontier for postharvest biocontrol and postharvest biology. Postharvest Biol. Technol. 2018, 140, 107–112. [Google Scholar] [CrossRef]
- Bafort, F.; Parisi, O.; Perraudin, J.P.; Jijakli, M.H. The Lactoperoxidase System: A Natural Biochemical Biocontrol Agent for Pre-and Postharvest Applications. J. Phytopathol. 2017, 165, 22–34. [Google Scholar] [CrossRef]
- Brady, S.F.; Wagenaar, M.M.; Singh, M.P.; Janso, J.E.; Clardy, J. The cytosporones, new octaketide antibiotics isolated from an endophytic fungus. Org. Lett. 2000, 2, 4043–4046. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lv, C.; Sun, W.; Li, Z.; Han, X.; Li, Y.; Shen, Y. Cytosporone B, an inhibitor of the type III secretion system of Salmonella enterica serovar typhimurium. Antimicrob. Agents Chemother. 2013, 57, 2191–2198. [Google Scholar] [CrossRef]
- Egarnes, B.; Blanchet, M.R.; Gosselin, J. Treatment with the NR4A1 agonist cytosporone B controls influenza virus infection and improves pulmonary function in infected mice. PLoS ONE 2017, 12, e0186639. [Google Scholar] [CrossRef] [PubMed]
- Hiwatashi, N.; Mukudai, S.; Bing, R.; Branski, R.C. The effects of cytosporone-B, a novel antifibrotic agent, on vocal fold fibroblasts. Laryngoscope 2018. [Google Scholar] [CrossRef]
- Zhan, Y.; Du, X.; Chen, H.; Liu, J.; Zhao, B.; Huang, D.; Li, G.; Xu, Q.; Zhang, M.; Weimer, B.C.; et al. Cytosporone B is an agonist for nuclear orphan receptor Nur77. Nat. Chem. Biol. 2008, 4, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Cheng, Y.; Yang, M.; Liu, Y.; Chen, K.; Long, C.A.; Deng, X. Mechanisms of action for 2-phenylethanol isolated from Kloeckera apiculata in control of Penicillium molds of citrus fruits. BMC Microbiol. 2014, 14, 242. [Google Scholar] [CrossRef]
- Feng, S.; Eucker, T.P.; Holly, M.K.; Konkel, M.E.; Lu, X.; Wang, S. Investigating the responses of Cronobacter sakazakii to garlic-drived organosulfur compounds: A systematic study of pathogenic-bacterium injury by use of high-throughput whole-transcriptome sequencing and confocal micro-raman spectroscopy. Appl. Environ. Microb. 2014, 80, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Li, T.; Chen, F.; Duan, X.; Yuan, Y.; Zhang, D.; Jiang, Y.M. An inclusion complex of eugenol into β-cyclodextrin: Preparation, and physicochemical and antifungal characterization. Food Chem. 2016, 196, 324–330. [Google Scholar] [CrossRef]
- Karim, H.; Boubaker, H.; Askarne, L.; Cherifi, K.; Lakhtar, H.; Msanda, F.; Boudyach, E.H.; Ait Ben Aoumar, A. Use of Cistus aqueous extracts as botanical fungicides in the control of Citrus sour rot. Microb. Pathog. 2017, 104, 263–267. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full length transcriptome assembly from RNA Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Schulze, S.K.; Kanwar, R.; Gölzenleuchter, M.; Therneau, T.M.; Beutler, A.S. SERE: Single-parameter quality control and sample comparison for RNA-Seq. BMC Genom. 2012, 13, 524. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010, 11, R25. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using Real-Time quantitative PCR and the 2–ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- von Delius, M.; Le, C.M.; Dong, V.M. Rhodium-phosphoramidite catalyzed alkene hydroacylation: Mechanism and octaketide natural product synthesis. J. Am. Chem. Soc. 2012, 134, 15022–15032. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.Y.; Wang, J.F.; Huang, Y.J.; Zheng, Z.H.; Song, S.Y.; Zhang, Y.M.; Su, W.J. Metabolites from a mangrove ednophyitc fungus Dothioreall sp. Acta Oeeanol. Sin. 2004, 23, 541–547. [Google Scholar]
- Shubina, L.K.; Makar’eva, T.N.; Denisenko, V.A.; Stonik, V.A. 4-Hydroxybenzaldehyde from the baikal sponge Lubomirskia baicalensis. Chem. Nat. Comp. 2005, 41, 93–94. [Google Scholar] [CrossRef]
- Singh, D.; Kumar, R.; Chaudhuri, P.K. A New phenolic compound from the flowers of Jasminum multiflorum. Chem. Nat. Comp. 2014, 50, 48–49. [Google Scholar] [CrossRef]
- Weber, D.; Gorzalczany, S.; Martino, V.; Acevedo, C.; Sterner, O.; Anke, T. Metabolites from endophytes of the medicinal plant Erythrina crista-galli. Zeitschrift für Naturforschung C 2005, 60, 467–477. [Google Scholar] [CrossRef]
- Sassa, T.; Kenmoku, H.; Sato, M.; Murayama, T.; Kato, N. (+)-Menthol and its hydroxy derivatives, novel fungal monoterpenols from the fusicoccin-producing Fungi, Phomopsis amygdali F6a and Niigata 2. Biosci. Biotechnol. Biochem. 2003, 67, 475–479. [Google Scholar] [CrossRef]
- El-Beih, A.A.; Kato, H.; Ohta, T.; Tsukamoto, S. (3R,4aR,5S,6R)-6-Hydroxy-5-methylramulosin: A new ramulosin derivative from a marine-derived sterile mycelium. Chem. Pharm. 2007, 55, 953–954. [Google Scholar] [CrossRef]
- Abrell, L.M.; Cheng, X.C.; Crews, P. New nectriapyrones by salt water culture of a fungus separated from an Indo-Pacific sponge. Tetrahedron Lett. 1994, 35, 9159–9160. [Google Scholar] [CrossRef]
- Gong, T.; Zhen, X.; Li, B.J.; Yang, J.L.; Zhu, P. Two new monoterpenoid a-pyrones from a fungus Nectria sp. HLS206 associated with the marine sponge Gelliodes carnosa. J. Asian Nat. Prod. Res. 2015, 17, 633–635. [Google Scholar] [CrossRef]
- Hellin, P.; King, R.; Urban, M.; Hammond-Kosack, K.E.; Legrève, A. The adaptation of Fusarium culmorum to DMI fungicides is mediated by major transcriptome modifications in response to azole fungicide, including the overexpression of a PDR transporter (FcABC1). Front. Microbiol. 2018, 9, 1385. [Google Scholar] [CrossRef]
- Wang, J.; Yu, J.; Liu, J.; Yuan, Y.; Li, N.; He, M.; Qi, T.; Hui, G.; Xiong, L.; Liu, D. Novel mutations in CYP51B from Penicillium digitatum involved in prochloraz resistance. J. Microbiol. 2014, 52, 762–770. [Google Scholar] [CrossRef]
- Udayanga, D.; Liu, X.; McKenzie, E.H.C.; Chukeatirote, E.; Bahkali, A.H.A.; Hyde, K.D. The genus Phomopsis: Biology, applications, species concepts and names of common phytopathogens. Fungal Divers. 2011, 50, 189–225. [Google Scholar] [CrossRef]
- Qin, G.Z.; Zong, Y.Y.; Chen, Q.L.; Hua, D.L.; Tian, S.P. Inhibitory effect of boron against Botrytis cinerea on table grapes and its possible mechanisms of action. Int. J. Food Microbiol. 2010, 138, 145–150. [Google Scholar] [CrossRef]
- Zhang, Z.; Qin, G.; Li, B.; Tian, S. Effect of cinnamic acid for controlling gray mold on table grape and its possible mechanisms of action. Curr. Microbiol. 2015, 71, 396–402. [Google Scholar] [CrossRef]
- Mieszkin, S.; Hymery, N.; Debaets, S.; Coton, E.; Le Blay, G.; Valence, F.; Mounier, J. Action mechanisms involved in the bioprotective effect of Lactobacillus harbinensis K.V9.3.1.Np against Yarrowia lipolytica in fermented milk. Int. J. Food Microbiol. 2017, 248, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Jian, W.; He, D.; Xi, P.; Li, X. Synthesis and biological evaluation of novel fluorine-containing stilbene derivatives as fungicidal agents against phytopathogenic fungi. J. Agric. Food Chem. 2015, 63, 9963–9969. [Google Scholar] [CrossRef]
- Wu, J.; Liu, J.; Jia, R.; Song, H. Nur77 inhibits androgen-induced bladder cancer growth. Cancer Investig. 2013, 31, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Ohnishi, T.; Ishida, H.; Tomida, K.; Sakai, M.; Hara, M.; Watanabe, N. Functional characterization of aromatic amino acid aminotransferase involved in 2-phenylethanol biosynthesis in isolated rose petal protoplasts. J. Plant. Physiol. 2012, 169, 444–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, Y.; Chen, X.; Yang, C.; Chang, J.; Shen, W.; Fan, Y. Engineering Eschericha coli for enhanced tyrosol production. J. Agric. Food Chem. 2017, 65, 4708–4714. [Google Scholar] [CrossRef] [PubMed]
- Hegge, A.; Lønborg, R.; Nielsen, D.M.; Sørensen, J.L. Factors influencing production of fusaristatin A in Fusarium graminearum. Metabolites 2015, 5, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Ding, X.; Liu, X.; Liu, S.; Sun, Y.; Yu, Z.; Hu, S.; Rang, J.; He, H.; He, L.; et al. Differential proteomic profiling reveals regulatory proteins and novel links between primary metabolism and spinosad production in Saccharopolyspora spinosa. Microb. Cell Fact. 2014, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hou, Y.; Yue, L.; Liu, S.; Du, J.; Sun, S. Potential targets for antifungal drug discovery based on growth and virulence in Candida albicans. Antimicrob. Agents Chemother. 2015, 59, 5885–5891. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Jamal, S.B.; Hassan, S.S.; Carvalho, P.V.S.D.; Almeida, S.; Barh, D.; Ghosh, P.; Silva, A.; Castro, T.L.P.; Azevedo, V. Two-component signal transduction systems of pathogenic bacteria as targets for antimicrobial therapy: An overview. Front. Microbiol. 2017, 8, 1878. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, C.; Liu, H.; Shan, Y.; Gupta, V.K.; Jiang, Y.; Zhang, W.; Tan, H.; Gong, L. Cytosporone B as a Biological Preservative: Purification, Fungicidal Activity and Mechanism of Action against Geotrichum citri-aurantii. Biomolecules 2019, 9, 125. https://0-doi-org.brum.beds.ac.uk/10.3390/biom9040125
Yin C, Liu H, Shan Y, Gupta VK, Jiang Y, Zhang W, Tan H, Gong L. Cytosporone B as a Biological Preservative: Purification, Fungicidal Activity and Mechanism of Action against Geotrichum citri-aurantii. Biomolecules. 2019; 9(4):125. https://0-doi-org.brum.beds.ac.uk/10.3390/biom9040125
Chicago/Turabian StyleYin, Chunxiao, Hongxin Liu, Yang Shan, Vijai Kumar Gupta, Yueming Jiang, Weimin Zhang, Haibo Tan, and Liang Gong. 2019. "Cytosporone B as a Biological Preservative: Purification, Fungicidal Activity and Mechanism of Action against Geotrichum citri-aurantii" Biomolecules 9, no. 4: 125. https://0-doi-org.brum.beds.ac.uk/10.3390/biom9040125