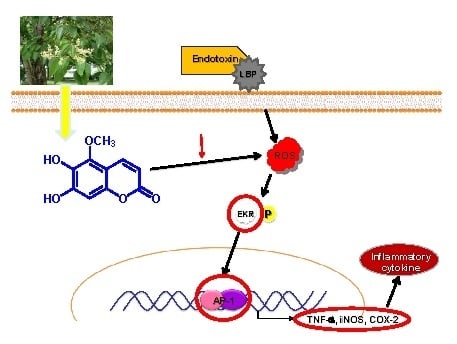
5-Methoxyl Aesculetin Abrogates Lipopolysaccharide-Induced Inflammation by Suppressing MAPK and AP-1 Pathways in RAW 264.7 Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect Of 5-Methoxyl Aesculetin (MOA) on the Viability of RAW 264.7 Cells
2.2. Effect of MOA on Nitric Oxide (NO) Production and Pro-Inflammatory Cytokine Production
2.3. Effects of MOA on the Expression Levels of Inducible NO Synthase (iNOS), Cyclooxygenase-2 (COX-2), and TNF-α mRNA in RAW 264.7 Cells
2.4. Effects of MOA on Free Radicals and Intracellular Reactive Oxygen Species (ROS) Levels
2.5. Effects of MOA on Lipopolysaccharide (LPS)-Induced Mitogen-Activated Protein Kinases (MAPK)/Activator Protein-1 (AP-1) Activation in RAW 264.7 Cells
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Cell Line and Cell Culture
3.3. Cell Viability Assay
3.4. Determination of NO, Prostaglandin E2 (PGE2), Tumor Necrosis Factor-α (TNF-α), Interleukin-1β (IL-1β), and IL-6 Production
3.5. RNA Extraction and Reverse Transcription-Polymerase Chain Reaction
3.6. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Radical-Scavenging Activity
3.7. Superoxide-Radical Scavenging Assay
3.8. Intracellular ROS Inhibition Activity
3.9. SDS-PAGE and Western Blot Analysis
3.10. Molecular Docking Study of MOA
3.11. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| LPS | Lipopolysaccharide |
| NO | Nitric oxide |
| TNF-α | Tumor necrosis factor-α |
| NF-κB | Nuclear factor-kappa B |
| AP-1 | Activator protein-1 |
| MOA | 5-Methoxyl aesculetin |
| ECL | Enhanced chemiluminescence |
| PBS | Phosphate-buffered saline |
| PGE2 | Prostaglandin E2 |
| ERK1/2 | Extracellular signal-regulated kinase |
| MAPKs | Mitogen-activated protein kinases |
| PI3K | Phosphoinositide 3-kinase |
| iNOS | Inducible nitric oxide synthase |
| DPPH | 1,1-Diphenyl-2-picrylhydrazyl |
| NBT | Nitro blue tetrazolium |
| DCFH-DA | 2,7-Dichlorofluorescin diacetate |
| PMS | Phenazine methosulphate |
| MTT | 1-(4,5-Dimethylthiazol-2-yl)-3,5-diphenylformazan |
| l-NMA | NG-monomethyl-l-arginine |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
References
- Takeda, K.; Akira, S. Toll-like receptors in innate immunity. Int. Immunol. 2005, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Lee, C.H.; Chang, Y.W.; Wang, H.M.; Chen, C.Y.; Chen, Y.H. Pheophytin a inhibits inflammation via suppression of LPS-induced nitric oxide synthase-2, prostaglandin E2, and interleukin-1β of macrophages. Int. J. Mol. Sci. 2014, 15, 22819–22834. [Google Scholar] [CrossRef] [PubMed]
- Cheon, S.Y.; Chung, K.S.; Jeon, E.; Nugroho, A.; Park, H.J.; An, H.J. Anti-inflammatory activity of saxifragin via inhibition of NF-κB involves caspase-1 activation. J. Nat. Prod. 2015, 78, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Zamora, R.; Vodovotz, Y.; Billiar, T.R. Inducible nitric oxide synthase and inflammatory diseases. Mol. Med. 2000, 6, 347–373. [Google Scholar] [PubMed]
- Sergent, T.; Piront, N.; Meurice, J.; Toussaint, O.; Schneider, Y.J. Anti-inflammatory effects of dietary phenolic compounds in an in vitro model of inflamed human intestinal epithelium. Chem. Biol. Interact. 2010, 188, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Gökhan, S.H. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar]
- Kathryn, E.W.; Gökhan, S.H. Inflammation, stress, and diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar]
- Yamamoto, M.; Sato, S.; Hemmi, H.; Hoshino, K.; Kaisho, T.; Sanjo, H.; Takeuchi, O.; Sugiyama, M.; Okabe, M.; Takeda, K.; et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 2003, 301, 640–643. [Google Scholar] [CrossRef] [PubMed]
- De, N. Toll-like receptors: Activation, signalling and transcriptional modulation. Cytokine 2015, 74, 181–189. [Google Scholar]
- Jeong, J.W.; Lee, H.H.; Han, M.H.; Kim, G.Y.; Kim, W.J.; Choi, Y.H. Anti-inflammatory effects of genistein via suppression of the toll-like receptor 4-mediated signaling pathway in lipopolysaccharide-stimulated BV2 microglia. Chem. Biol. Interact. 2014, 212, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.H.; Chen, T.H.; Chou, T.C. Magnolol inhibits RANKL-induced osteoclast differentiation of RAW 264.7 macrophages through heme oxygenase-1-dependent inhibition of NFATc1 expression. J. Nat. Prod. 2015, 78, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.L.; Tong, Q.; Wang, W.Q.; Shi, C.Y.; Xiong, W.; Chen, J.; Liu, X.; Fang, J.G. Suppression of inflammatory responses by dihydromyricetin, a flavonoid from Ampelopsis grossedentata, via inhibiting the activation of NF-κB and MAPK signaling pathways. J. Nat. Prod. 2015, 78, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.K.; Lin, C.K.; Chang, H.W.; Wu, Y.H.; Yen, F.L.; Chang, F.R.; Chen, W.C.; Yeh, C.C.; Lee, J.C. Aqueous extract of Gracilaria tenuistipitata suppresses LPS-induced NF-κB and MAPK activation in RAW 264.7 and rat peritoneal macrophages and exerts hepatoprotective effects on carbon tetrachloride-treated rat. PLoS ONE 2014, 9, e86557. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.H.; Park, M.J.; Ra, J.E.; Han, S.I.; Nam, M.H.; Kim, J.H.; Lee, J.H.; Seo, W.D. Saponarin from barley sprouts inhibits NF-κB and MAPK on LPS-induced RAW 264.7 cells. Food Funct. 2014, 5, 3005–3013. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.E.; Park, H.S.; Kim, J.A.; Nagappan, A.; Zhang, J.; Kang, S.R.; Won, C.K.; Cho, J.H.; Kim, E.H.; Kim, G.S. Anti-oxidant and anti-inflammatory effects of Fraxinus rhynchophylla on lipopolysaccharide (LPS)-induced murine Raw 264.7 cells. J. Biomed. Res. 2012, 13, 331–338. [Google Scholar] [CrossRef]
- Wu, Z.B.; Liu, Y.; Tian, S.S.; Wen, C. Chemical constituents of the stem bark of Fraxinus rhynchophylla. Chem. Nat. Compd. 2014, 49, 1162–1163. [Google Scholar] [CrossRef]
- Si, C.L.; Liu, Z.; Su, Y.F.; Kim, J.K.; Bae, Y.S. Coumarins and secoiridoid glucosides from bark of Fraxinus rhynchophylla Hance. Holzforschung 2008, 62, 553–555. [Google Scholar] [CrossRef]
- Pan, Y.M.; Zhu, J.C.; Wang, H.S.; Zhang, X.P.; Zhang, Y.; He, C.H.; Ji, X.W.; Li, H.Y. Antioxidant activity of ethanolic extract of Cortex fraxini and use in peanut oil. Food Chem. 2007, 103, 913–918. [Google Scholar] [CrossRef]
- Zhou, L.; Kang, J.; Fan, L.; Ma, X.C.; Zhao, H.Y.; Han, J.; Wang, B.R.; Guo, D.A. Simultaneous analysis of coumarins and secoiridoids in Cortex Fraxini by high-performance liquid chromatography–diode array detection–electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. Anal. 2008, 47, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, Y.; Ekunwe, S.I.N.; Yi, X.H.; Liu, X.X.; Wang, H.S.; Pan, Y.M. Antioxidant activity and inhibition effect on the growth of human colon carcinoma (HT-29) cells of esculetin from Cortex Fraxini. Med. Chem. Res. 2011, 20, 968–974. [Google Scholar] [CrossRef]
- Lee, B.C.; Lee, S.Y.; Lee, H.J.; Sim, G.S.; Kim, J.H.; Kim, J.H.; Cho, Y.H.; Lee, D.H.; Pyo, H.B.; Choe, T.B.; et al. Anti-oxidative and photo-protective effects of coumarins isolated from Fraxinus chinensis. Arch. Pharm. Res. 2007, 30, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Xing, W.; Li, W.; Fan, T.; Hu, H.; Li, Y. Isofraxidin exhibited anti-inflammatory effects in vivo and inhibited TNF-α production in LPS-induced mouse peritoneal macrophages in vitro via the MAPK pathway. Int. Immunopharmacol. 2012, 14, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Song, X.Y.; Hu, J.F.; Sun, M.N.; Li, Z.P.; Zhu, Z.X.; Song, L.K.; Yuan, Y.H.; Liu, G.; Chen, N.H. IMM-H004 attenuated the production of inflammatory mediatory mediators in LPS-stimulated BV2 microglia. Brain Res. Bull. 2014, 106, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Chiou, W.F.; Don, M.J.; Liao, J.F.; Wei, B.L. Psoralidin inhibits LPS-induced iNOS expression via repressing Syk-mediated activation of PI3K-IKK-IκB signaling pathways. Eur. J. Pharmacol. 2011, 650, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Si, C.L.; Zhang, Y.; Zhu, Z.Y.; Xu, J.; Kim, J.K.; Bae, Y.S. Isolation and structure elucidation of secoiridoid glucosides from Fraxinus rhynchophylla leaves. Chem. Nat. Compd. 2009, 45, 814–816. [Google Scholar] [CrossRef]
- Xiao, K.; Song, Q.H.; Zhang, S.W.; Xuan, L.J. Water-soluble constituents of the root barks of Fraxinus rhynchophylla (Chinese drug Qinpi). J. Asian Nat. Prod. Res. 2008, 10, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Pae, H.O.; Ko, Y.S.; Yoo, J.C.; Choi, B.M.; Jun, C.D.; Chung, H.T.; Inagaki, M.; Higuchi, R.; Kim, Y.C. In vitro inducible nitric oxide synthesis inhibitory active constituents from Fraxinus rhynchophylla. Planta Med. 1999, 65, 656–658. [Google Scholar] [CrossRef] [PubMed]
- Abdur, R.; Rehan, K.; Haroon, K.; Samreen, P.; Saboor, P.A. In vivo antinociceptive and anti-inflammatory activities of umbelliferone isolated from Potentilla evestita. Nat. Prod. Res. 2014, 28, 1371–1374. [Google Scholar]
- Witaicenis, A.; Seito, L.N.; Di Stasi, L.C. Intestinal anti-inflammatory activity of esculetin and 4-methylesculetin in the trinitrobenzenesulphonic acid model of rat colitis. Chem. Biol. Interact. 2010, 186, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Sun, X.R.; Du, Z.Z. Studies on the growth inhiibitory effect of Esculin on human pulmonary adenocarcinoma cell line A-549 and its mechanism of anti-inflammation and anti-asthma. Chin. Pharmacol. Bull. 1995, 11, 192–195. [Google Scholar]
- Goode, E.C.; Warburton, R.C.; Gelson, W.T.; Watson, A.J. Activated intestinal macrophages in patients with cirrhosis release NO and IL-6 that may disrupt intestinal barrier function. Gastroenterology 2013, 145, 1481–1484. [Google Scholar] [CrossRef] [PubMed]
- Siragusa, M.; Fröhlich, F.; Park, E.J.; Schleicher, M.; Walther, T.C.; Sessa, W.C. Stromal cell–derived factor 2 is critical for Hsp90-dependent eNOS activation. Sci. Signal. 2015, 8, ra81. [Google Scholar] [CrossRef] [PubMed]
- Kawano, A.; Tsukimoto, M.; Mori, D.; Noguchi, T.; Harada, H.; Takenouchi, T.; Kitani, H.; Kojima, S. Regulation of P2X7-dependent inflammatory functions by P2X4 receptor in mouse macrophages. Biochem. Biophys. Res. Commun. 2012, 420, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Font-Nieves, M.; Sans-Fons, M.G.; Gorina, R.; Bonfill-Teixidor, E.; Salas-Pérdomo, A.; Márquez-Kisinousky, L.; Santalucia, T.; Planas, A.M. Induction of COX-2 enzyme and down-regulation of COX-1 expression by lipopolysaccharide (LPS) control prostaglandin E2 production in astrocytes. J. Biol. Chem. 2012, 287, 6454–6468. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.J.; Tang, Y.M. Cytokine release syndrome in cancer immunotherapy with chimeric antigen receptor engineered T cells. Cancer Lett. 2014, 343, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Ishita, I.J.; Jin, S.E.; Choi, R.J.; Lee, C.M.; Kim, Y.S.; Jung, H.A.; Choi, J.S. Anti-inflammatory activity of edible brown alga Saccharina japonica and its constituents pheophorbide a and pheophytin a in LPS-stimulated RAW 264.7 macrophage cells. Food Chem. Toxicol. 2013, 55, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Lai, C.S.; Wang, Y.J.; Ho, C.T. Acacetin suppressed LPS-induced up-expression of iNOS and COX-2 in murine macrophages and TPA-induced tumor promotion in mice. Biochem. Pharmacol. 2006, 72, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Jomova, K.; Rhodes, C.J.; Kuča, K.; Musílek, K. Redox-and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 2015, 90, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Lue, B.M.; Nielsen, N.S.; Jacobsen, C.; Hellgren, L.; Guo, Z.; Xu, X.B. Antioxidant properties of modified rutin esters by DPPH, reducing power, iron chelation and human low density lipoprotein assays. Food Chem. 2010, 123, 221–230. [Google Scholar] [CrossRef]
- Yun, K.J.; Shin, J.S.; Choi, J.H.; Back, N.I.; Chung, H.G.; Lee, K.T. Quaternary alkaloid, pseudocoptisine isolated from tubers of Corydalis turtschaninovi inhibits LPS-induced nitric oxide, PGE2, and pro-inflammatory cytokines production via the down-regulation of NF-κB in RAW 264.7 murine macrophage cells. Int. Immunopharmacol. 2009, 9, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.W.; Chiou, W.F.; Chao, S.H.; Lee, M.H.; Chen, C.C.; Tsai, Y.C. Ligustilide prevents LPS-induced iNOS expression in RAW 264.7 macrophages by preventing ROS production and down-regulating the MAPK, NF-κB and AP-1 signaling pathways. Int. Immunopharmacol. 2011, 11, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.B.; Reddanna, P. Chebulagic acid (CA) attenuates LPS-induced inflammation by suppressing NF-κB and MAPK activation in RAW 264.7 macrophages. Biochem. Biophys. Res. Commun. 2009, 381, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Mauriz, J.L.; Collado, P.S.; Veneroso, C.; Reiter, R.J.; González-Gallego, J. A review of the molecular aspects of melatonin’s anti-inflammatory actions: Recent insights and new perspectives. J. Pineal Res. 2013, 54, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.T.; Yang, C.M. Role of NADPH oxidase/ROS in pro-inflammatory mediators-induced airway and pulmonary diseases. Biochem. Pharmacol. 2012, 84, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Chiou, Y.S.; Pan, M.H.; Shahidi, F. Anti-inflammatory activity of lipophilic epigallocatechin gallate (EGCG) derivatives in LPS-stimulated murine macrophages. Food Chem. 2012, 134, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Kwon, M.S.; Choi, J.W.; Shin, T.; No, H.K.; Choi, J.S.; Byun, D.S.; Kim, J.I.; Kim, H.R. Anti-inflammatory activities of an ethanol extract of Ecklonia stolonifera in lipopolysaccharide-stimulated RAW 264.7 murine macrophage cells. J. Agric. Food Chem. 2012, 60, 9120–9129. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.G.; Lee, S.G.; Lee, H.H.; Lee, H.J.; Shin, J.S.; Kim, N.J.; An, H.J.; Nam, J.H.; Jang, D.S.; Lee, K.T. α-Chaconine isolated from a Solanum tuberosum L. cv. Jayoung suppresses lipopolysaccharide-induced pro-inflammatory mediators via AP-1 inactivation in RAW 264.7 macrophages and protects mice from endotoxin shock. Chem. Biol. Interact. 2015, 235, 85–94. [Google Scholar] [PubMed]
- Endale, M.; Park, S.C.; Kim, S.; Kim, S.H.; Yang, Y.; Cho, J.Y.; Rhee, M.H. Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and myeloid differentiation factor-88 association, and inhibits MAPK/AP-1 and IKK/NF-κB-induced inflammatory mediators production in RAW 264.7 cells. Immunobiology 2013, 218, 1452–1467. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Yoon, Y.D.; Lee, K.H.; Park, S.K.; Kim, H.M. Costunolide inhibits interleukin-1β expression by down-regulation of AP-1 and MAPK activity in LPS-stimulated RAW 264.7 cells. Biochem. Biophys. Res. Commun. 2014, 313, 171–177. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Hong, S.H.; Park, S.D.; Ahn, S.G.; Yoon, J.H.; Kwon, B.M.; Kim, S.A. 2′-Benzoyloxycinnamaldehyde inhibits nitric oxide production in lipopolysaccharide-stimulated RAW 264.7 cells via regulation of AP-1 Pathway. Eur. J. Pharmacol. 2012, 696, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, G.; Li, P.; Wang, Y.; Si, C.L.; He, J.; Long, W.; Bai, Y.; Feng, Z.; Wang, X. Neuroprotective effects of macranthoin G from Eucommia ulmoides against hydrogen peroxide-induced apoptosis in PC12 cells via inhibiting NF-κB activation. Chem. Biol. Interact. 2014, 224C, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Yang, W.S.; Yi, Y.S.; Sung, G.H.; Rhee, M.H.; Poo, H.; Kim, M.Y.; Kim, K.W.; Kim, J.H.; Cho, J.Y. AP-1/IRF-3 targeted anti-inflammatory activity of andrographolide isolated from Andrographis paniculata. Evid. Based Complement. Altern. Med. 2013, 2013, 1–16. [Google Scholar]
- Shen, T.; Li, X.Q.; Hu, W.C.; Zhang, L.J.; Xu, X.D.; Wu, H.F.; Ji, L.L. Hepatoprotective effect of phenylethanoid glycosides from Incarvillea compacta against CCl4-induced cytotoxicity in HepG2 cells. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 617–625. [Google Scholar] [CrossRef]
- Si, C.L.; Shen, T.; Jiang, Y.Y.; Wu, L.; Yu, G.J.; Ren, X.D.; Xu, G.H.; Hu, W.C. Antioxidant properties and neuroprotective effects of isocampneoside II on hydrogen peroxide-induced oxidative injury in PC12 cell. Food Chem. Toxicol. 2013, 59, 145–152. [Google Scholar] [CrossRef] [PubMed]






| Gene Name | Primer Sequence (5′-3′) | PCR Conditions | PCR Cycles |
|---|---|---|---|
| GAPDH | F: CACTCACGGCAAATTCAACGGCA | Denaturation-94 °C, 30 s | 30 |
| R: GACTCCACGACATACTCAGCAC | Annealing-60 °C, 30 s | ||
| Extension-72 °C, 30 s | |||
| iNOS | F: CCCTTCCGAAGTTTCTGGCAGCAG | Denaturation-94 °C, 30 s | 27 |
| R:GGCTGTCAGAGCCTCGTGGCTTTGG | Annealing-60 °C, 30 s | ||
| Extension-72 °C, 30 s | |||
| COX-2 | F: CACTACATCCTGACCCACTT | Denaturation-94 °C, 30 s | 30 |
| R: ATGCTCCTGCTTGAGTATGT | Annealing-55 °C, 30 s | ||
| Extension-72 °C, 30 s | |||
| TNF-α | F: TGCCTATGTCTCAGCCTCTTC | Denaturation-94 °C, 30 s | 30 |
| R: GAGGCCATTTGGGAACTTCT | Annealing-55 °C, 30 s | ||
| Extension-72 °C, 30 s |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Li, X.; Wu, H.; Long, W.; Jiang, X.; Shen, T.; Qiang, Q.; Si, C.; Wang, X.; Jiang, Y.; et al. 5-Methoxyl Aesculetin Abrogates Lipopolysaccharide-Induced Inflammation by Suppressing MAPK and AP-1 Pathways in RAW 264.7 Cells. Int. J. Mol. Sci. 2016, 17, 315. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17030315
Wu L, Li X, Wu H, Long W, Jiang X, Shen T, Qiang Q, Si C, Wang X, Jiang Y, et al. 5-Methoxyl Aesculetin Abrogates Lipopolysaccharide-Induced Inflammation by Suppressing MAPK and AP-1 Pathways in RAW 264.7 Cells. International Journal of Molecular Sciences. 2016; 17(3):315. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17030315
Chicago/Turabian StyleWu, Lei, Xueqin Li, Haifeng Wu, Wei Long, Xiaojian Jiang, Ting Shen, Qian Qiang, Chuanling Si, Xinfeng Wang, Yunyao Jiang, and et al. 2016. "5-Methoxyl Aesculetin Abrogates Lipopolysaccharide-Induced Inflammation by Suppressing MAPK and AP-1 Pathways in RAW 264.7 Cells" International Journal of Molecular Sciences 17, no. 3: 315. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17030315