Hepatoprotective Limonoids from Andiroba (Carapa guianensis)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation
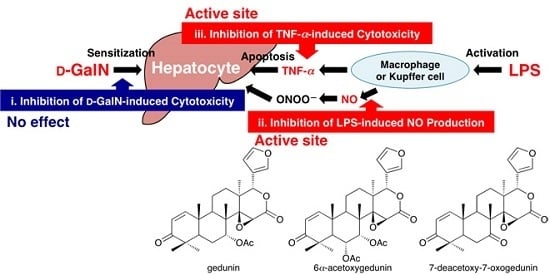
2.2. Protective Effects of Principal Limonoids (1, 2, and 3) on Liver Injury Induced by d-GalN/LPS in Mice
2.3. Effects on d-GalN-induced Cytotoxicity in Primary Cultured Mouse Hepatocytes
2.4. Effects on LPS-induced NO Production in Mouse Peritoneal Macrophages
2.5. Effects on TNF-α-induced Cytotoxicity in L929 Cells
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Material
3.3. Isolation of Compounds 1–3 from the Seed Oil of C. Guianensis
3.4. Reagents
3.5. Animals
3.6. Effects on d-GalN/LPS-induced Liver Injury in Mice
3.7. Effects on Cytotoxicity Induced by d-GalN in Primary Cultured Mouse Hepatocytes
3.8. Effects on Production of NO in LPS-induced Mouse Peritoneal Macrophages
3.9. Effects on Cytotoxicity Induced by TNF-α in L929 Cells
3.10. Statistics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liao, S.G.; Chen, H.D.; Yue, J.M. Plant orthoesters. Chem. Rev. 2009, 109, 1092–1140. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Di, Y.T.; Hao, X.J. The advances in the limonoid chemistry of the Meliaceae family. Curr. Org. Chem. 2011, 15, 1363–1391. [Google Scholar]
- Tan, Q.G.; Luo, X.D. Meliaceous limonoids: Chemistry and biological activities. Chem. Rev. 2011, 111, 7437–7522. [Google Scholar] [CrossRef] [PubMed]
- Pereira da Silva, V.P.; Oliveira, R.R.; Figueiredo, M.R. Isolation of limonoids from seeds of Carapa guianensis Aublet (Meliaceae) by high-speed countercurrent chromatography. Phytochem. Anal. 2009, 20, 70–81. [Google Scholar]
- Nakanishi, K.; Sasaki, S.; Kiang, A.K.; Goh, J.; Kakisawa, H.; Ohashi, M.; Goto, M.; Watanabe, J.; Yokotani, H.; Matsumura, C.; et al. Phytochemical survey of Malaysian plants preliminary chemical and pharmacological screening. Chem. Pharm. Bull. 1965, 55, 457–464. [Google Scholar] [CrossRef]
- Waterman, A.M. The effect of water-soluble extracts from the heartwood of tropical American woods on the growth of two wood-decay fungi. Trop. Woods 1946, 88, 1–11. [Google Scholar]
- Prophiro, J.S.; da Silva Mario, A.N.; Kanis, L.A.; da Rocha, L.C.B.P.; Duque-Luna, J.E.; da Silva, O.S. First report on susceptibility of wild Aedes aegypty (Diptera: Culicidae) using Carapa guianensis (Meliaceae) and Copaifera sp. (Leguminosae). Parasitol. Res. 2012, 110, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Penido, C.; Costa, K.A.; Pennaforte, R.J.; Costa, M.F.; Pereira, J.F.; Siani, A.C.; Henriques, M.G. Anti-allergic effects of natural tetranortriterpenoids isolated from Carapa guianensis Aublet on allergen-induced vascular permeability and hyperalgesia. Inflamm. Res. 2005, 54, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Bickii, J.; Njifutie, N.; Foyere, J.A.; Basco, L.K.; Ringwald, P.J. In vitro antimalarial activity of limonoids from Khaya grandifoliola C.D.C. (Meliaceae). J. Ethnopharmacol. 2000, 69, 27–33. [Google Scholar] [CrossRef]
- Penido, C.; Conte, F.P.; Chagas, M.S.S.; Rodrigue, C.A.B.; Pereira, J.F.G.; Henriques, M.G.M.O. Antiinflammatory effects of natural tetranortriterpenoids isolated from Carapa guianensis Aublet on zymosan-induced arthritis in mice. Inflamm. Res. 2006, 55, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, F.K.; Rodrigues, R.; da Silva, V.P.; Figueiredo, R.; Penido, C.; Henriques, M.G.M.O. Modulation of T lymphocyte and eosinophil functions in vitro by natural tetranortriterpenoids isolated from Carapa guianensis Aublet. Int. Immunopharmacol. 2011, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Miranda, R.N.C., Jr.; Dolabela, M.F.; da Silva, M.N.; Povoa, M.M.; Maia, J.G.S. Antiplasmoidal activity of the andiroba (Carapa guianensis Aublet., Meliaceae) oil and its limonoid-rich fraction. J. Ethnopharmacol. 2012, 142, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Costa-Silva, H.; Lima, C.R.; Silva, E.J.; Araújo, A.V.; Fraga, M.C.; Ribeiro E Ribeiro, A.; Arruda, A.C.; Lafayette, S.S.; Wanderley, A.G. Acute and subacute toxicity of the Carapa guianensis Aublet (Meliaceae) seed oil. J. Ethnopharmacol. 2008, 116, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Yamada, T.; In, Y.; Muraoka, O.; Kajimoto, T.; Tanaka, R. Absolute stereostructure of andirolides A–G from the flower of Carapa guianensis. Tetrahedron 2011, 67, 782–792. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sakamoto, A.; Inoue, T.; Yamada, T.; Kikuchi, T.; Kajimoto, T.; Muraoka, O.; Sato, A.; Wataya, Y.; Kim, H.-S.; et al. Andirolides H–P from the flower of andiroba (Carapa guianensis, Meliaceae). Tetrahedron 2012, 68, 3669–3677. [Google Scholar] [CrossRef]
- Sakamoto, A.; Tanaka, Y.; Inoue, T.; Kikuchi, T.; Kajimoto, T.; Muraoka, O.; Yamada, T.; Tanaka, R. Andirolides Q–V from the flower of andiroba. Fitoterapia 2013, 90, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, A.; Tanaka, Y.; Yamada, T.; Kikuchi, T.; Muraoka, O.; Ninomiya, K.; Morikawa, T.; Tanaka, R. Andirolides W–Y from the flower oil of andiroba. Fitoterapia 2015, 100, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Nagai, Y.; Mitooka, A.; Ujike, R.; Muraoka, O.; Yamada, T.; Tanaka, R. Carapanolides A and B: Unusal 9,10-seco-mexicanolides having a 2R,9S-oxygen bridge from the seeds of Carapa guianensis. Tetrahedron Lett. 2012, 53, 6685–6688. [Google Scholar] [CrossRef]
- Inoue, T.; Matsui, Y.; Kikuchi, T.; In, Y.; Muraoka, O.; Yamada, T.; Tanaka, R. Carapanolides C–I from the seeds of andiroba (Carapa guianensis, Miliaceae). Fitoterapia 2014, 96, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Kikuchi, T.; Inoue, T.; Muraoka, O.; Yamada, T.; Tanaka, R. Carapanolides J–L from seeds of Carapa guianensis (andiroba) and their effects on LPS-activated NO production. Molecules 2014, 19, 17130–17140. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Matsui, Y.; Kikuchi, T.; Yamada, T.; In, Y.; Muraoka, O.; Sakai, C.; Ninomiya, K.; Morikawa, T.; Tanaka, R. Carapanolides M–S from seeds of andiroba (Carapa guianensis, Meliaceae) and triglyceride metabolism-promoting activity in high glucose-pretreated HepG2 cells. Tetrahedron 2015, 71, 2753–2760. [Google Scholar] [CrossRef]
- Miyake, T.; Ishimoto, S.; Higuchi, K.; Minoura, K.; Kikuchi, T.; Yamada, T.; Muraoka, O.; Tanaka, R. Carapanolides T–X from Carapa guianensis (andiroba) seeds. Molecules 2015, 20, 20955–20966. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Matsui, Y.; Kikuchi, T.; In, Y.; Yamada, T.; Muraoka, O.; Matsunaga, S.; Tanaka, R. Guianolides A and B, new carbon skeletal limonoids from the seeds of Carapa guianensis. Org. Lett. 2013, 15, 3018–3021. [Google Scholar] [CrossRef] [PubMed]
- Freudenberg, M.A.; Galanos, C. Tumor necrosis factor-α mediates lethal activity of killed gram-negative and gram-positive bacteria in d-galactosamine-treated mice. Infect. Immun. 1991, 59, 2110–2115. [Google Scholar] [PubMed]
- Josephs, M.D.; Bahjat, F.R.; Fukuzuka, K.; Ksontini, R.; Solorzano, C.C.; Edwards, C.K., 3rd; Tannahill, C.L.; MacKay, S.L.; Copeland, E.M., 3rd; Moldawer, L.L. Lipopolysaccharide and d-galactosamine-induced hepatic injury is mediated by TNF-α and not by Fas ligand. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R1196–R1201. [Google Scholar] [PubMed]
- Matsuda, H.; Ninomiya, K.; Morikawa, T.; Yoshikawa, M. Inhibitory effect and action mechanism of sesquiterpenes from Zedoariae Rhizoma on d-galactosamine/lipopolysaccharide-induced liver injury. Bioorg. Med. Chem. Lett. 1998, 8, 339–344. [Google Scholar] [CrossRef]
- Matsuda, H.; Morikawa, T.; Ninomiya, K.; Yoshikawa, M. Hepatoprotective constituents from Zedoariae Rhizoma: Absolute stereostructures of three new carabrane-type sesquiterpenes, curcumenolactonea A, B, and C. Bioorg. Med. Chem. 2001, 9, 909–916. [Google Scholar] [CrossRef]
- Morikawa, T.; Matsuda, H.; Ninomiya, K.; Yoshikawa, M. Medicinal foodstuffs. XXIX. potent protective effects of sesquiterpenes and curcumin from Zedoariae Rhizoma on liver injury induced by d-galactosamine/lipopolysaccharide or tumor necrosis factor-α. Biol. Pharm. Bull. 2002, 25, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T. Search for bioactive constituents from several medicinal food: Hepatoprotective, antidiabetic, and antiallergic activities. J. Nat. Med. 2007, 61, 112–126. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Morikawa, T.; Kashima, Y.; Ninomiya, K.; Matsuda, H. Structures of new dammarane-type triterpene saponins from the flower buds of Panax notoginseng and hepatoprotective effects of principal ginseng saponins. J. Nat. Prod. 2003, 66, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Nishida, N.; Ninomiya, K.; Ohgushi, T.; Kubo, M.; Morikawa, T.; Matsuda, H. Inhibitory effects of coumarin and acetylene constituents from the roots of Angellica furcijuga on d-galactosamine/lipopolysaccharide-induced liver injury in mice and on nitric oxide production in lipopolysaccharide-activated mouse peritoneal macrophages. Bioorg. Med. Chem. 2006, 14, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Ninomiya, K.; Morikawa, T.; Yasuda, D.; Yamaguchi, I.; Yoshikawa, M. Protective effects of amide constituents from the fruit of Piper chaba on d-galactosamine/TNF-α-induced cell death in mouse hepatocytes. Bioorg. Med. Chem. Lett. 2008, 18, 2038–2042. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Ninomiya, K.; Morikawa, T.; Yasuda, D.; Yamaguchi, I.; Yoshikawa, M. Hepatoprotective amide constituents from the fruit of Piper chaba: Structural requirements, mode of action, and new amides. Bioorg. Med. Chem. 2009, 17, 7313–7323. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T. Search for TNF-α sensitivety degradation principles from medicinal foods—Hepatoprotective amide constituents from Thai natural medicine Piper chaba. Yakugaku Zasshi 2010, 130, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Pan, Y.; Ninomiya, K.; Imura, K.; Matsuda, H.; Yoshikawa, M.; Yuan, D.; Muraoka, O. Acylated phenylethanoid oligoglycosides with hepatoprotective activity from the desert plant Cistanche tubulosa. Bioorg. Med. Chem. 2010, 18, 1882–1890. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Ninomiya, K.; Imura, K.; Yamaguchi, T.; Aakgi, Y.; Yoshikawa, M.; Hayakawa, T.; Muraoka, O. Hepatoprotective triterpene from traditional Tibetan medicine Potentilla anserina. Phytochemistry 2014, 102, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Osumi, W.; Jin, D.; Imai, Y.; Tashiro, K.; Li, Z.-L.; Otsuki, Y.; Maemura, K.; Komeda, K.; Hirokawa, F.; Hayashi, M.; et al. Recombinant human soluble thrombomodulin improved lipopolysaccharide/d-galactosamine-induced acute liver failure in mice. J. Pharmacol. Sci. 2015, 129, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.I.; Hong, J.M.; Choi, J.W.; Choi, H.S.; Kwak, J.H.; Lee, D.U.; Lee, S.K.; Lee, S.M. β-Caryophyllene alleviates d-galactosamine and lipopolysaccharide-induced hepatic injury through suppression of the TLR4 and RAGE signaling pathways. Eur. J. Pharmacol. 2015, 764, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Sass, G.; Heinlein, S.; Agli, A.; Bang, R.; Schümann, J.; Tiegs, G. Cytokine expression in three mouse models of experimental hepatitis. Cytokine 2002, 19, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.I.; McGregor, R.A.; Choi, M.S.; Seo, K.I.; Jung, U.J.; Yeo, J.; Kim, M.J.; Lee, M.K. Low doses of curcumin protect alcohol-induced liver damage by modulation of the alcohol metabolic pathway, CYE2E1 and AMPK. Life Sci. 2013, 93, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Daglia, M.; Moghaddam, A.H.; Habtemariam, S.; Nabavi, S.M. Curcumin and liver disease: From chemistry to meidicne. Comp. Rev. Food Sci. Food Saf. 2013, 13, 62–77. [Google Scholar] [CrossRef]
- Palipoch, S.; Punsawad, C.; Koomhin, P.; Suwannalert, P. Hepatoprotective effect of curcumin and α-tocopherol against cisplatin-induced oxidative stress. BMC Comp. Alt. Med. 2014, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- Fehér, J.; Deák, G.; Muzes, G.; Láng, I.; Niederland, V.; Nékám, K.; Kárteszi, M. Liver-protective action of silymarin therapy in chronic alcoholic liver diseases. Orv. Hetil. 1989, 130, 2723–2727. [Google Scholar] [PubMed]
- Skottová, N.; Krecman, V. Silymarin as a potential hypocholesterolaemic drug. Physiol. Res. 1998, 47, 1–7. [Google Scholar] [PubMed]
- Yoshikawa, M.; Xu, F.; Morikawa, T.; Ninomiya, K.; Matsuda, H. Anastatins A and B, new skeletal flavonoids with hepatoprotective activities from the desert plant Anastatica hierochuntica. Bioorg. Med. Chem. Lett. 2003, 13, 1045–1049. [Google Scholar] [CrossRef]
- Xu, F.; Morikawa, T.; Matsuda, H.; Ninomiya, K.; Yoshikawa, M. Structures of sesquiterpenes and hepatoprotective constituents from the Egyptian herbal medicine Cyperus longus. J. Nat. Prod. 2004, 67, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Morikawa, T.; Xu, F.; Ninomiya, K.; Yoshikawa, M. New isoflavones and pterocarpanes with hepatoprotective activity from the stems of Erycibe expansa. Planta Med. 2004, 70, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Morikawa, T.; Matsuda, H.; Ninomiya, K.; Li, X.; Yoshikawa, M. New flavanone oligoglycosides, theaflavanosides I, II, III, and IV, with hepatoprotective activity from the seeds of tea plant (Camellia sinensis). Heterocycles 2007, 71, 1193–1201. [Google Scholar]
- Ninomiya, K.; Morikawa, T.; Zhang, Y.; Nakamura, S.; Matsuda, H.; Muraoka, O.; Yoshikawa, M. Bioactive constituents from Chinese natural medicines. XXIII. Absolute structures of new megastigmane glycosides, sedumosides A4, A5, A6, H, and I, and hepatoprotective megastigmanes from Sedum sarmentosum. Chem. Pharm. Bull. 2007, 55, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Morikawa, T.; Nakamura, S.; Ninomiya, K.; Matsuda, H.; Muraoka, O.; Yoshikawa, M. Bioactive constituents from Chinese natural medicines. XXV. New flavonol bisdesmosides, sarmenosides I, II, III, and IV, with hepatoprotective activity from Sedum sarmentosum. Heterocycles 2007, 71, 1565–1576. [Google Scholar]
- Ninomiya, K.; Morikawam, T.; Xie, H.; Matsuda, H.; Yoshikawa, M. Bioactive constituents from Chinese natural medicines. XXXI. Hepatoprotective principles from Sinocrassula indica: Structres of sinocrassosides A8, A9, A10, A11, and A12. Heterocycles 2008, 75, 1983–1995. [Google Scholar]
- Nakamura, S.; Li, X.; Matsuda, H.; Ninomiya, K.; Morikawa, T.; Yamaguti, K.; Yoshikawa, M. Bioactive constituents from Chinese natural medicines. XXVI. chemical structures and hepatoprotective effects of constituents from roots of Rhodiola sachalinensis. Chem. Pharm. Bull. 2007, 55, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Okazaki, Y.; Ninomiya, K.; Morikawa, T.; Matsuda, H.; Yoshikawa, M. Medicinal flowers. XXIV. Chemical structures and hepatoprotective effects of constituents from flowers of Hedychium coronarium. Chem. Pharm. Bull. 2008, 56, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Chaipech, S.; Morikawa, T.; Ninomiya, K.; Yoshikawa, M.; Pongpiriyadacha, Y.; Hayakawa, T.; Muraoka, O. Structures of two new phenolic glycosides, kaempferiaosides A and B, and hepatoprotective constituents from the rhizomes of Kaempferia parviflora. Chem. Pharm. Bull. 2012, 60, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Xu, F.; Ninomiya, K.; Nakashima, S.; Oda, Y.; Morikawa, T.; Muraoka, O.; Yoshikawa, M.; Matsuda, H. Chemical structures and hepatoprotective effects of constituents from Cassia auriculata leaves. Chem. Pharm. Bull. 2014, 62, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Kouroku, Y.; Fujita, E.; Jimbo, A.; Mukasa, T.; Tsuru, T.; Momoi, M.Y.; Momoi, T. Locallization of active from of caspase-8 in mouse L929 cells induced by TNF treatment and polyglutamine aggregates. Biochem. Biophys. Res. Commun. 2000, 270, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Tiegs, G.; Wolter, M.; Wendel, A. Tumor necrosis factor is a terminal mediator in galactosamine/endotoxin-induced hepatitis in mice. Biochem. Pharmacol. 1989, 38, 627–631. [Google Scholar] [CrossRef]
- Peng, X.X.; Zhang, S.H.; Wang, X.L.; Ye, T.J.; Li, H.; Yan, X.F.; Wei, L.; Wu, Z.P.; Hu, J.; Zou, C.P.; et al. Panax Notoginseng flower saponins (PNFS) inhibit LPS-stimulated NO overproduction and iNOS gene overexpression via the suppression of TLR4-mediated MAPK/NF-kappa B signaling pathways in RAW264.7 macrophages. Chin. Med. 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, X.; Fang, L.; Liu, F.; Cai, R.; Peng, C.; Qi, Y. Rhein exerts pro- and anti-inflammatory actions by targeting IKKβ inhibition in LPS-activated macrophages. Free Radic. Biol. Med. 2014, 72, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Borges, P.V.; Moret, K.H.; Maya-Monteiro, C.M.; Souza-Silva, F.; Alves, C.R.; Batista, P.R.; Caffarena, E.R.; Pacheco, P.; das Graças Henriques, M.; Penido, C. Gedunin binds to myeloid differentiation protein 2 and impairs lipopolysaccharide-induced toll-like receptor 4 signaling in macrophages. Mol. Pharmacol. 2015, 88, 949–961. [Google Scholar] [CrossRef] [PubMed]

| Treatment | Dose (mg/kg, p.o.) | N | sAST | sALT | Mortality | ||
|---|---|---|---|---|---|---|---|
| (Karmen Unit) | Inhibition (%) | (Karmen Unit) | Inhibition (%) | ||||
| Normal (vehicle) | – | 8 | 107 ± 9 ** | – | 20 ± 2 ** | – | 0/8 |
| Control | – | 12 | 5237 ± 1,000 | – | 8533 ± 1795 | – | 4/16 |
| Gedunin (1) | 25 | 7 | 2304 ± 651 * | 56.2 | 2950 ± 710 * | 65.7 | 0/7 |
| 50 | 7 | 1923 ± 576 * | 63.5 | 2824 ± 754 * | 67.2 | 0/7 | |
| 6α-Acetoxygedunin (2) | 25 | 7 | 2384 ± 579 * | 54.7 | 3120 ± 830 * | 63.7 | 0/7 |
| 50 | 7 | 1696 ± 580 ** | 67.9 | 2397 ± 873 ** | 72.2 | 0/7 | |
| 7-Deacetoxy-7-oxo- | 25 | 7 | 2093 ± 742 * | 60.3 | 2899 ± 1024 * | 66.3 | 0/7 |
| gedunin (3) | 50 | 6 | 1759 ± 579 * | 66.7 | 2572 ± 903 ** | 70.2 | 1/7 |
| Control | – | 10 | 6033 ± 1647 | – | 6605 ± 1985 | – | 6/16 |
| Curcumin [36] | 12.5 | 10 | 4770 ± 1218 | 21.1 | 5024 ± 1189 | 24.0 | 0/10 |
| 25 | 10 | 3177 ± 979 | 47.8 | 3253 ± 981 | 50.9 | 0/10 | |
| 50 | 9 | 2220 ± 563 * | 63.8 | 1916 ± 483 * | 71.2 | 1/10 | |
| Control | – | 10 | 4709 ± 461 | – | 7088 ± 917 | – | 4/14 |
| Silybin | 500 | 8 | 1361 ± 191 ** | 71.1 | 1990 ± 439 ** | 71.9 | 0/8 |
| Treatment | Inhibition (%) | ||||
|---|---|---|---|---|---|
| 0 µM | 3 µM | 10 µM | 30 µM | 100 µM | |
| Gedunin (1) | 0.0 ± 1.8 | −8.4 ± 1.9 | −3.9 ± 0.4 | −3.2 ± 0.9 | −5.2 ±0.2 |
| 6α-Acetoxygedunin (2) | 0.0 ± 2.4 | −2.6 ± 1.0 | −1.9 ± 0.3 | −2.5 ± 0.7 | −1.8 ± 0.6 |
| 7-Deacetoxy-7-oxogedunin (3) | 0.0 ± 2.2 | −4.6 ± 0.5 | −8.2 ± 0.9 | −8.3 ± 1.1 | −8.9 ± 0.6 |
| 7-Deacetoxy-7α-hydroxygedunin (4) | 0.0 ± 1.6 | −2.0 ± 1.4 | −6.3 ± 0.5 | −8.0 ± 0.4 | −4.1 ± 0.7 |
| Andirolide H (5) | 0.0 ± 2.5 | −6.2 ± 2.6 | −9.0 ± 0.5 | −9.2 ± 0.8 | −0.6 ± 0.8 |
| 6α-Hydroxygedunin (6) | 0.0 ± 2.0 | −7.2 ± 2.4 | −9.4 ± 0.8 | −0.1 ± 0.7 | −2.8 ± 0.5 |
| Methyl angolensate (7) | 0.0 ± 2.2 | −2.1 ± 1.2 | −7.5 ± 0.9 | −6.3 ± 0.7 | −6.6 ± 1.0 |
| Epoxyazadiradione (8) | 0.0 ± 2.1 | −3.1 ± 8.9 | −2.2 ± 2.5 | −10.8 ± 0.6 | −11.9 ± 0.3 |
| 17β-Hydroxyazadiradione (9) | 0.0 ± 2.0 | 15.3 ± 2.3 | −4.1 ± 1.2 | −7.5 ± 1.4 | −7.5 ± 4.0 |
| Carapanolide C (10) | 0.0 ± 1.4 | 8.0 ± 4.3 | 3.4 ± 4.2 | 6.7 ± 2.1 | −7.7 ± 4.4 |
| Carapanolide R (11) | 0.0 ± 2.1 | 21.5 ± 2.8 ** | 27.8 ± 5.0 ** | 46.0 ± 4.7 ** | 36.0 ± 3.2 ** |
| Carapanolide S (12) | 0.0 ± 2.1 | −7.8 ± 3.2 | −3.8 ± 4.1 | −3.7 ± 3.1 | −7.1 ± 3.2 |
| Carapanolide M (13) | 0.0 ± 1.6 | −7.0 ± 0.5 | −7.3 ± 0.7 | 1.0 ± 0.4 | −9.9 ± 1.0 |
| Carapanolide Q (14) | 0.0 ± 1.6 | 2.7 ± 1.9 | −3.5 ± 2.9 | −2.5 ± 2.1 | −6.2 ± 1.7 |
| Carapanolide O (15) | 0.0 ± 1.9 | 7.5 ± 3.9 | −5.3 ± 5.6 | −5.2 ± 3.9 | −2.1 ± 1.7 |
| Guianolide A (16) | 0.0 ± 3.7 | 9.2 ± 4.2 | 11.0 ± 5.3 | 9.8 ± 2.8 | 23.5 ± 3.5 ** |
| Carapanolide A (17) | 0.0 ± 2.0 | −6.8 ± 1.2 | −8.3 ± 0.7 | −4.5 ± 0.6 | −7.0 ± 0.6 |
| Curcumin [26,27,29] | 0.0 ± 3.7 | 0.1 ± 3.8 | 1.1 ± 2.2 | −17.7 ± 1.3 | −44.3 ± 0.3 |
| Silybin [33,35,36] | 0.0 ± 0.3 | 4.8 ± 1.1 | 7.7 ± 0.7 | 45.2 ± 8.8 ** | 77.0 ± 5.5 ** |
| Treatment | Inhibition (%) | IC50 | ||||
|---|---|---|---|---|---|---|
| 0 µM | 3 µM | 10 µM | 30 µM | 100 µM | (µM) | |
| Gedunin (1) [17] | 0.0 ± 5.6 (100.0 ± 4.1) | 25.1 ± 2.5 ** (102.2 ± 5.3) | 84.5 ± 2.3 ** (119.5 ± 5.3) | 101.8 ± 0.6 ** (94.8 ± 1.4) | 100.9 ± 0.4 ** (3.0 ± 0.4 #) | 4.6 |
| 6α-Acetoxygedunin (2) [17] | 0.0 ± 1.5 (100.0 ± 1.6) | 16.9 ± 1.7 ** (96.8 ± 1.2) | 67.6 ± 4.6 ** (102.3 ± 2.2) | 88.4 ± 3.5 ** (92.5 ± 1.7) | 99.6 ± 0.2 ** (53.6 ± 5.1 #) | 7.9 |
| 7-Deacetoxy-7-oxogedunin (3) [17] | 0.0 ± 6.5 (100.0 ± 5.1) | 7.4 ± 5.2 (100.3 ± 3.9) | 40.9 ± 4.7 ** (98.9 ± 3.2) | 94.0 ± 0.8 ** (98.8 ± 7.4) | 88.1 ± 2.1 ** (83.7 ± 1.2) | 12.8 |
| 7-Deacetoxy-7α-hydroxy-gedunin (4) [17] | 0.0 ± 2.4 (100.0 ± 4.4) | 15.7 ± 4.6 ** (110.3 ± 5.9) | 55.7 ± 4.0 ** (106.6 ± 3.1) | 98.8 ± 0.4 ** (96.3 ± 4.6) | 100.2 ± 0.2 ** (2.6 ± 0.5 #) | 8.7 |
| Andirolide H (5) | 0.0 ± 5.6 (100.0 ± 1.8) | 5.8 ± 6.1 (99.8 ± 4.5) | 63.9 ± 3.0 ** (103.9 ± 6.9) | 97.2 ± 0.9 ** (108.9 ± 2.4) | 99.7 ± 0.5 ** (4.9 ± 0.5 #) | 9.4 |
| 6α-Hydroxygedunin (6) [17] | 0.0 ± 6.2 (100.0 ± 4.5) | 7.7 ± 7.1 (88.4 ± 3.0) | 20.7 ± 4.3 ** (87.6 ± 4.0) | 64.0 ± 3.1 ** (90.4 ± 2.6) | 97.3 ± 0.3 ** (82.2 ± 4.2) | 19.1 |
| Methyl angolensate (7) [17] | 0.0 ± 5.9 (100.0 ± 2.4) | 10.1 ± 4.2 (108.8 ± 11.0) | 20.0 ± 8.1 (108.8 ± 5.5) | 42.2 ± 3.5 ** (111.0 ± 4.5) | 24.0 ± 4.2 * (78.1 ± 5.3 #) | > 100 |
| Epoxyazadiradione (8) | 0.0 ± 0.8 (100.0 ± 4.1) | 10.5 ± 0.9 * (99.6 ± 2.9) | 56.0 ± 4.0 ** (94.8 ± 2.3) | 102.6 ±4.0 ** (81.9 ± 2.7) | 112.3 ± 0.7 ** (10.0 ± 0.5 #) | 8.2 |
| 17β-Hydroxyazadiradione (9) | 0.0 ± 4.9 (100.0 ± 1.8) | −10.4 ± 6.8 (95.4 ± 5.2) | 9.4 ± 7.1 (94.4 ± 1.4) | 65.1 ± 4.5 ** (94.8 ± 4.9) | 97.4 ± 0.7 ** (84.8 ± 3.6) | 20.3 |
| Carapanolide C (10) | 0.0 ± 2.6 (100.0 ± 3.4) | 4.2 ± 8.8 (96.0 ± 4.1) | 20.8 ± 5.0 ** (98.9 ± 3.4) | 20.2 ± 4.9 (91.8 ± 2.8) | 13.2 ± 1.9 (80.0 ± 4.4) | >100 |
| Carapanolide R (11) | 0.0 ± 1.3 (100.0 ± 1.0) | 4.0 ± 2.2 (95.1 ± 1.8) | 8.9 ± 2.3 (98.0 ± 1.7) | 17.4 ± 1.3 (106.0 ± 1.9) | 75.6 ± 1.2 ** (118.4 ± 1.0) | 68.3 |
| Carapanolide S (12) | 0.0 ± 2.8 (100.0 ± 0.7) | 2.5 ± 1.2 (97.9 ± 2.7) | 15.9 ± 1.3 ** (93.8 ± 2.0) | 72.2 ± 3.6 ** (96.8 ± 4.4) | 99.8 ± 0.4 ** (73.7 ± 3.8 #) | 15.5 |
| Carapanolide M (13) | 0.0 ± 2.2 (100.0 ± 2.3) | −1.1 ± 2.7 (99.2 ± 0.6) | 3.2 ± 2.8 (95.1 ± 1.3) | 30.3 ± 3.1 ** (94.2 ± 2.9) | 85.1 ± 1.5 ** (111.9 ± 1.6) | 41.6 |
| Carapanolide Q (14) | 0.0 ± 2.4 (100.0 ± 2.8) | 1.9 ± 0.7 (99.8 ± 1.6) | 14.4 ± 2.2 (96.3 ± 1.6) | 44.3 ± 1.1 ** (95.5 ± 4.1) | 75.3 ± 3.5 ** (115.5 ± 2.2) | 38.0 |
| Carapanolide O (15) | 0.0 ± 2.5 (100.0 ± 4.6) | −2.5 ± 5.4 (104.1 ± 2.5) | 14.7 ± 8.2 (107.9 ± 2.7) | 6.9 ± 5.0 (106.7 ± 2.5) | 102.5 ± 3.2 ** (108.7 ± 5.2) | 46.0 |
| Guianolide A (16) | 0.0 ± 1.8 (100.9 ± 0.9) | 1.9 ± 3.2 (96.9 ± 1.8) | 3.2 ± 1.4 (101.5 ± 1.9) | 12.7 ± 1.6 (98.7 ± 1.8) | 71.3 ± 3.2 ** (106.2 ± 1.6) | 77.9 |
| Carapanolide A (17) | 0.0 ± 1.5 (100.0 ± 1.8) | −0.6 ± 2.1 (103.0 ± 2.6) | 1.2 ± 2.0 (103.9 ± 4.3) | 41.1 ± 1.0 ** (109.5 ± 4.3) | 4.9 ± 1.8 (91.3 ± 1.6) | > 100 |
| l-NMMA [33,36] | 0.0 ± 3.1 (100.0 ± 0.9) | 1.4 ± 2.8 (101.1 ± 5.7) | 19.9 ± 2.8 ** (100.7 ± 6.2) | 43.0 ± 2.1 ** (102.6 ± 4.2) | 70.9 ± 1.6 ** (106.4 ± 4.6) | 36.0 |
| CAPE [33,36] | 0.0 ± 2.1 (100.0 ± 1.5) | 5.9 ± 5.2 (95.4 ± 0.7) | 44.4 ± 3.2 ** (70.0 ± 4.0 #) | 86.2 ± 1.1 ** (71.4 ± 6.0 #) | 99.6 ± 0.1 ** (53.0 ± 1.4 #) | 11.0 |
| Treatment | Inhibition (%) | ||||
|---|---|---|---|---|---|
| 0 µM | 1 µM | 3 µM | 10 µM | 30 µM | |
| Gedunin (1) | 0.0 ± 2.1 | 4.5 ± 1.9 | 21.8 ± 3.7 ** | 38.1 ± 3.8 ** | 36.5 ± 4.1 ** |
| 6α-Acetoxygedunin (2) | 0.0 ± 1.6 | 10.9 ± 1.0 ** | 23.2 ± 1.8 ** | 36.3 ± 2.1 ** | 37.3 ± 1.4 ** |
| 7-Deacetoxy-7-oxogedunin (3) | 0.0 ± 1.1 | 5.8 ± 1.5 | 26.7 ± 4.5 ** | 58.6 ± 7.2 ** | 68.7 ± 4.8 ** |
| 7-Deacetoxy-7α-hydroxygedunin (4) | 0.0 ± 0.3 | −6.5 ± 2.4 | 2.7 ± 2.3 | 36.5 ± 1.8 ** | |
| Andirolide H (5) | 0.0 ± 0.8 | −6.6 ± 3.6 | −0.7 ± 1.2 | 7.6 ± 1.1 | 39.2 ± 1.7 ** |
| 6α-Hydroxygedunin (6) | 0.0 ± 1.3 | 8.1 ± 1.9 | 6.7 ± 1.5 | 12.1 ± 3.0 | 28.3 ± 1.7 ** |
| Methyl angolensate (7) | 0.0 ± 1.4 | −0.5 ± 3.5 | 0.6 ± 2.9 | 13.3 ± 2.6 * | 24.6 ± 2.9 ** |
| Epoxyazadiradione (8) | 0.0 ± 5.3 | 13.7 ± 3.9 | 39.1 ± 6.5 ** | 91.5 ± 11.4 ** | |
| 17β-Hydroxyazadiradione (9) | 0.0 ± 1.5 | 14.1 ± 3.4 | 23.9 ± 3.9 ** | 64.0 ± 3.3 ** | 91.3 ± 8.2 ** |
| Carapanolide C (10) | 0.0 ± 3.7 | 4.9 ± 2.1 | 14.2 ± 3.2 | 27.7 ± 4.3 ** | 54.5 ± 5.5 ** |
| Carapanolide R (11) | 0.0 ± 4.1 | −6.3 ± 4.7 | −1.3 ± 3.8 | 31.7 ± 3.8 ** | |
| Carapanolide S (12) | 0.0 ± 1.5 | −5.5 ± 2.2 | −1.4 ± 1.5 | −2.5 ± 1.2 | |
| Carapanolide M (13) | 0.0 ± 6.5 | −1.5 ± 7.1 | 7.0 ± 4.4 | −5.1 ± 6.2 | |
| Carapanolide Q (14) | 0.0 ± 5.5 | 8.6 ± 4.4 | 1.3 ± 4.2 | 9.2 ± 2.5 | |
| Carapanolide O (15) | 0.0 ± 6.5 | 6.3 ± 4.3 | 1.0 ± 6.4 | 1.5 ± 4.1 | |
| Guianolide A (16) | 0.0 ± 2.9 | −6.2 ± 5.2 | −4.5 ± 1.9 | −7.3 ± 3.0 | |
| Carapanolide A (17) | 0.0 ± 3.7 | 8.8 ± 6.5 | 21.5 ± 5.5 ** | 58.2 ± 4.7 ** | |
| Treatment | Inhibition (%) | ||||
| 0 µM | 3 µM | 10 µM | 30 µM | 100 µM | |
| Silybin [36] | 0.0 ± 2.6 | 5.3 ± 2.8 | 22.0 ± 3.8 ** | 48.0 ± 4.1 ** | 50.8 ± 3.9 ** |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ninomiya, K.; Miyazawa, S.; Ozeki, K.; Matsuo, N.; Muraoka, O.; Kikuchi, T.; Yamada, T.; Tanaka, R.; Morikawa, T. Hepatoprotective Limonoids from Andiroba (Carapa guianensis). Int. J. Mol. Sci. 2016, 17, 591. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17040591
Ninomiya K, Miyazawa S, Ozeki K, Matsuo N, Muraoka O, Kikuchi T, Yamada T, Tanaka R, Morikawa T. Hepatoprotective Limonoids from Andiroba (Carapa guianensis). International Journal of Molecular Sciences. 2016; 17(4):591. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17040591
Chicago/Turabian StyleNinomiya, Kiyofumi, Seiya Miyazawa, Kaiten Ozeki, Natsuko Matsuo, Osamu Muraoka, Takashi Kikuchi, Takeshi Yamada, Reiko Tanaka, and Toshio Morikawa. 2016. "Hepatoprotective Limonoids from Andiroba (Carapa guianensis)" International Journal of Molecular Sciences 17, no. 4: 591. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17040591