Galectin-3: One Molecule for an Alphabet of Diseases, from A to Z
Abstract
:1. Introduction
- Prototypic single-CRD galectins that can form non-covalent homodimers. The following galectins belong to this group: Gal-1, Gal-2, Gal-5, Gal-7, Gal-10, Gal-11, Gal-13, Gal-14 and Gal-15.
- Tandem-repeats of two CRD motifs, similar but not identical, including Gal-4, Gal-6, Gal-8, Gal-9 and Gal-12.
- The chimera-type. The only member of this group is represented by Gal-3, with a single CRD and an intrinsically disordered sequence at the N-terminal domain that promotes oligomerization. In addition, Gal-3 is the only one to be able to pentamerize. It exhibits specific pleiotropic biological function, playing a key role in many physiological and pathological processes (Figure 1).
1.1. Gal-3 Identification
1.2. Gal-3 Tissue Distribution
1.3. Gal-3 Protein/Gene Structure and Carbohydrate Binding
1.4. Gal-3 Subcellular Localization
1.5. Gal-3 Secretion
1.6. Gal-3 Ligands
1.6.1. Gal-3 Extracellular Ligands
1.6.2. Gal-3 Intracellular Ligands
1.6.3. Gal-3 Nuclear Ligands
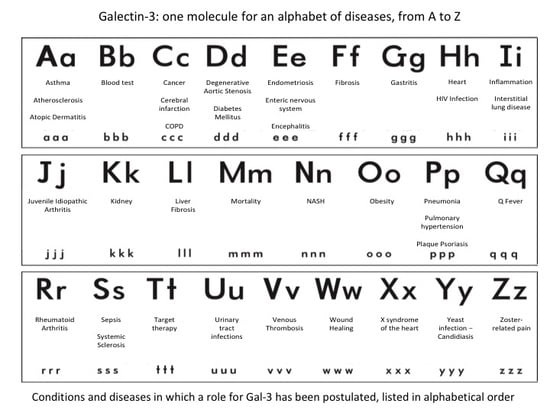
2. Role of Gal-3 in Different Clinical Conditions and Diseases (Listed in Alphabetical Order)
3. A
3.1. Asthma
3.2. Atherosclerosis
3.3. Atopic Dermatitis
4. B
Blood Test
5. C
5.1. Cancer
5.1.1. Gal-3 Subcellular Localization in Tumor Tissues
5.1.2. Gal-3 and Apoptosis in Tumor Tissues
5.1.3. Gal-3 Immune Surveillance and Angiogenesis in Tumor Tissues
5.1.4. Gal-3 in Various Types of Human Cancers
5.2. Cerebral Infarction
5.3. Chronic Obstructive Pulmonary Disease
6. D
6.1. Degenerative Aortic Stenosis
6.2. Diabetes Mellitus
6.2.1. Gal-3 Increases Severity of DM
6.2.2. Gal-3 Decreases Severity of DM
7. E
7.1. Endometriosis
7.2. Enteric Nervous System
7.3. Encephalitis
8. F
Fibrosis
9. G
Gastritis
10. H
10.1. Heart
- •
- assessing the utility of the serum/plasma concentration of the lectin for diagnosis,
- •
- helping in stratifying patients for therapy,
- •
- monitoring therapy response, and
- •
- predicting short- and long-term morbidity and mortality.
10.2. HIV
11. I
11.1. Inflammation
11.2. Interstitial Lung Disease
12. J
Juvenile Idiopathic Arthritis
13. K
Kidney
14. L
Liver Fibrosis
15. M
Mortality
16. N
Non-Alcoholic Steatohepatitis (NASH)
17. O
Obesity
18. P
18.1. Pneumonia
18.2. Pulmonary Hypertension
18.3. Plaque Psoriasis
19. Q
Q Fever
20. R
20.1. Rheumatoid Arthritis
20.1.1. Experimental Studies in Animals
20.1.2. Clinical Studies in Humans
21. S
21.1. Sepsis
21.2. Systemic Sclerosis
22. T
Target Therapy
23. U
Urinary Tract Infections
24. V
Venous Thrombosis
25. W
25.1. Wound Healing
25.1.1. Wound Healing in the Cornea
25.1.2. Wound Healing in the Skin
25.1.3. Wound Healing in the Intestinal Tract
26. X
X Syndrome of the Heart
27. Y
Yeast Infection-Candidiasis
28. Z
Zoster-Related Pain (Allodynia)
29. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cooper, D.N.W. Galectinomics: Finding themes in complexity. Biochim. Biophys. Acta 2002, 1572, 209–231. [Google Scholar] [CrossRef]
- Drickamer, K.; Fadden, A.J. Genomic analysis of C-type lectins. Biochem. Soc. Symp. 2002, 69, 59–72. [Google Scholar] [CrossRef]
- Seetharaman, J.; Kanigsberg, A.; Slaaby, R.; Leffler, H.; Barondes, S.H.; Rini, J.M. X-ray crystal structure of the human galectin-3 carbohydrate recognition domain at 2.1-A resolution. J. Biol. Chem. 1998, 273, 13047–13052. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.C. Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochim. Biophys. Acta 1999, 1473, 172–185. [Google Scholar] [CrossRef]
- Jones, J.L.; Saraswati, S.; Block, A.S.; Lichti, C.F.; Mahadevan, M.; Diekman, A.B. Galectin-3 is associated with prostasomes in human semen. Glycoconj. J. 2010, 27, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Nabi, I.R.; Shankar, J.; Dennis, J.W. The galectin lattice at a glance. J. Cell Sci. 2015, 128, 2213–2219. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.C. The galectin family of mammalian carbohydrate-binding molecules. Biochem. Soc. Trans. 1997, 25, 1194–1198. [Google Scholar] [CrossRef] [PubMed]
- Barondes, S.H.; Castronovo, V.; Cooper, D.N.; Cummings, R.D.; Drickamer, K.; Feizi, T.; Gitt, M.A.; Hirabayashi, J.; Hughes, C.; Kasai, K.; et al. Galectins: A family of animal beta-galactoside-binding lectins. Cell 1994, 76, 597–598. [Google Scholar] [CrossRef]
- Ho, M.K.; Springer, T.A. Mac-2, a novel 32,000 Mr mouse macrophage subpopulation-specific antigen defined by monoclonal antibodies. J. Immunol. 1982, 128, 1221–1228. [Google Scholar] [PubMed]
- Roff, C.F.; Wang, J.L. Endogenous lectins from cultured cells: Isolation and characterization of carbohydrate-binding proteins from 3T3 fibroblasts. J. Biol. Chem. 1983, 258, 10657–10663. [Google Scholar] [PubMed]
- Da, Y.; Leffler, H.; Sakakura, Y.; Kasai, K.; Barondes, S.H. Human breast carcinoma cDNA encoding a galactoside-binding lectin homologous to mouse Mac-2 antigen. Gene 1991, 99, 279–283. [Google Scholar]
- Cherayil, B.J.; Weiner, S.J.; Pillai, S. The Mac-2 antigen is a galactose-specific lectin that binds IgE. J. Exp. Med. 1989, 170, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
- Raz, A.; Pazerini, G.; Carmi, P. Identification of the metastasis-associated, galactoside-binding lectin as a chimeric gene product with homology to an IgE-binding protein. Cancer Res. 1989, 49, 3489–3493. [Google Scholar] [PubMed]
- Raz, A.; Carmi, P.; Raz, T.; Hogan, V.; Mohamed, A.; Wolman, S.R. Molecular cloning and chromosomal mapping of a human galactoside-binding protein. Cancer Res. 1991, 51, 2173–2178. [Google Scholar] [PubMed]
- Sparrow, C.P.; Leffler, H.; Barondes, S.H. Multiple soluble beta-galactoside-binding lectins from human lung. J. Biol. Chem. 1987, 262, 7383–7390. [Google Scholar] [PubMed]
- Leffler, H.; Masiarz, F.R.; Barondes, S.H. Soluble lactose-binding vertebrate lectins: A growing family. Biochemistry 1989, 28, 9222–9229. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.W.; Albrandt, K.; Keller, D.; Liu, F.T. Human IgE-binding protein: A soluble lectin exhibiting a highly conserved interspecies sequence and differential recognition of IgE glycoforms. Biochemistry 1990, 29, 8093–8100. [Google Scholar] [CrossRef] [PubMed]
- Albrandt, K.; Orida, N.K.; Liu, F.T. An IgE-binding protein with a distinctive repetitive sequence and homology with an IgG receptor. Proc. Natl. Acad. Sci. USA 1987, 84, 6859–6863. [Google Scholar] [CrossRef] [PubMed]
- Cerra, R.F.; Gitt, M.A.; Barondes, S.H. Three soluble rat beta-galactoside-binding lectins. J. Biol. Chem. 1985, 260, 10474–10477. [Google Scholar] [PubMed]
- Lotan, R.; Carralero, D.; Lotan, D.; Raz, A. Biochemical and immunological characterization of K-1735P melanoma galactoside-binding lectins and their modulation by differentiation inducers. Cancer Res. 1989, 49, 1261–1268. [Google Scholar] [PubMed]
- Sato, S.; Hughes, R.C. Binding specificity of a baby hamster kidney lectin for H type I and II chains, polylactosamine glycans, and appropriately glycosylated forms of laminin and fibronectin. J. Biol. Chem. 1992, 267, 6983–6990. [Google Scholar] [PubMed]
- Jia, S.; Wang, J.L. Carbohydrate binding protein 35. Complementary DNA sequence reveals homology with proteins of the heterogeneous nuclear RNP. J. Biol. Chem. 1988, 263, 6009–6011. [Google Scholar] [PubMed]
- Woo, H.J.; Shaw, L.M.; Messier, J.M.; Mercurio, A.M. The major nonintegrin laminin binding protein of macrophages is identical to carbohydrate binding protein 35 (Mac-2). J. Biol. Chem. 1990, 265, 7097–7099. [Google Scholar] [PubMed]
- Dumic, J.; Dabelic, S.; Flögel, M. Galectin-3: An open-ended story. Biochim. Biophys. Acta 2006, 1760, 616–635. [Google Scholar] [CrossRef] [PubMed]
- Van den Brûle, F.; Califice, S.; Castronovo, V. Expression of galectins in cancer: A critical review. Glycoconj. J. 2004, 19, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Tang, J.W.; Owusu, L.; Sun, M.Z.; Wu, J.; Zhang, J. Galectin-3 in cancer. Clin. Chim. Acta 2014, 431, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, J.; Kasai, K. The family of metazoan metal-independent beta-galactoside-binding lectins: Structure, function and molecular evolution. Glycobiology 1993, 4, 297–304. [Google Scholar] [CrossRef]
- Wang, J.L.; Laing, J.G.; Anderson, R.L. Lectins in the cell nucleus. Glycobiology 1991, 3, 243–252. [Google Scholar] [CrossRef]
- Hughes, R.C. Mac-2: A versatile galactose-binding protein of mammalian tissues. Glycobiology 1994, 4, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Birdsall, B.; Feeney, J.; Burdett, I.D.J.; Bawumia, S.; Barboni, E.A.M.; Hughes, R.C. NMR solution studies of hamster Gal-3 and electron microscopic visualization of surface-adsorbed complexes: Evidence for interactions between the N- and C-terminal domains. Biochemistry 2001, 40, 4859–4866. [Google Scholar] [CrossRef] [PubMed]
- Huflejt, M.E.; Turck, C.W.; Lindstedt, R.; Barondes, S.H.; Leffler, H. L-29, a soluble lactose-binding lectin, is phosphorylated on serine 6 and serine 12 in vivo and by casein kinase I. J. Biol. Chem. 1993, 268, 26712–26718. [Google Scholar] [PubMed]
- Mazurek, N.; Conklin, J.; Byrd, J.C.; Raz, A.; Bresalier, R.S. Phosphorylation of the β-galactoside-binding protein Gal-3 modulates binding to its ligands. J. Biol. Chem. 2000, 275, 36311–36315. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.P.; Hughes, R.C. Determinants in the N-terminal domains of Gal-3 for secretion by a novel pathway circumventing the endoplasmic reticulum-Golgi complex. Eur. J. Biochem. 1999, 264, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, J.; Hashidate, T.; Arata, Y.; Nishi, N.; Nakamura, T.; Hirashima, M.; Urashima, T.; Oka, T.; Futai, M.; Muller, W.E.G.; et al. Oligosaccharide specificity of galectins: A search by frontal affinity chromatography. Biochim. Biophys. Acta 2002, 1572, 232–254. [Google Scholar] [CrossRef]
- Ochieng, J.; Furtak, V.; Lukyanov, P. Extracellular functions of Gal-3. Glycoconj. J. 2004, 19, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Davidson, P.J.; Davis, M.J.; Patterson, R.J.; Ripoche, M.A.; Poirier, F.; Wang, J.L. Shuttling of Gal-3 between the nucleus and cytoplasm. Glycobiology 2002, 12, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.K.; Liu, F.T. Regulation of cellular homeostasis by galectins. Glycoconj. J. 2004, 19, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Califice, S.; Castronovo, V.; Van Den Brûle, F. Galectin-3 and cancer (Review). Int. J. Oncol. 2004, 25, 983–992. [Google Scholar] [PubMed]
- Moutsatsos, I.K.; Davis, J.M.; Wang, J.L. Endogenous lectins from cultured cells: Subcellular localization of carbohydrate binding protein 35 in 3T3 fibroblasts. J. Cell Biol. 1986, 102, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Moutsatsos, I.K.; Wade, M.; Schindler, M.; Wang, J.L. Endogenous lectins from cultured cells: Nuclear localization of carbohydrate binding protein 35 in proliferating 3T3 fibroblasts. Proc. Natl. Acad. Sci. USA 1987, 84, 6452–6466. [Google Scholar] [CrossRef] [PubMed]
- Agrwal, N.; Wang, J.L.; Voss, P.G. Carbohydrate-binding protein 35. Levels of transcription and mRNA accumulation in quiescent and proliferating cells. J. Biol. Chem. 1989, 264, 17236–17242. [Google Scholar] [PubMed]
- Cowles, E.A.; Moutsatsos, I.K.; Wang, J.L.; Anderson, R.L. Expression of carbohydrate binding protein 35 in human fibroblasts: Comparisons between cells with different proliferative capacities. Exp. Gerontol. 1989, 24, 577–585. [Google Scholar] [CrossRef]
- Hubert, M.; Wang, S.Y.; Wang, J.L.; Seve, A.P.; Hubert, J. Intranuclear distribution of galectin-3 in mouse 3T3 fibroblasts: Comparative analyses by immunofluorescence and immunoelectron microscopy. Exp. Cell Res. 1995, 220, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Burdett, I.; Hughes, R.C. Secretion of the baby hamster kidney 30-kDa galactose-binding lectin from polarized and nonpolarized cells: A pathway independent of the endoplasmic reticulum-Golgi complex. Exp. Cell Res. 1993, 207, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Nickel, W. The mystery of nonclassical protein secretion. A current view on cargo proteins and potential export routes. Eur. J. Biochem. 2003, 270, 2109–2119. [Google Scholar] [CrossRef] [PubMed]
- Mehul, B.; Hughes, R.C. Plasma membrane targeting, vesicular budding and release of galectin 3 from the cytoplasm of mammalian cells during secretion. J. Cell Sci. 1997, 110, 1169–1178. [Google Scholar] [PubMed]
- Gupta, A. Ligands for Galectin-3: Binding interactions. In Animal Lectins: Form, Function and Clinical Applications; Gupta, G.S., Ed.; Springer Verlag Wien: Vienna, Austria; Springer Science & Business Media: Berlin, Germany, 2012; Chapter 12.2; Volume 1, pp. 268–271. [Google Scholar]
- Talaga, M.L.; Fan, N.; Fueri, A.L.; Brown, R.K.; Bandyopadhyay, P.; Dam, T.K. Multitasking Human Lectin Galectin-3 Interacts with Sulfated Glycosaminoglycans and Chondroitin Sulfate Proteoglycans. Biochemistry 2016, 55, 4541–4551. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, I.; Cherayil, B.J.; Isselbacher, K.J.; Pillai, S. Mac-2-binding glycoproteins. Putative ligands for a cytosolic β-galactoside lectin. J. Biol. Chem. 1991, 266, 18731–18736. [Google Scholar] [PubMed]
- Koths, K.; Taylor, E.; Halenbeck, R.; Casipit, C.; Wang, A. Cloning and characterization of a human Mac-2-binding protein, a new member of the superfamily defined by the macrophage scavenger receptor cysteine-rich domain. J. Biol. Chem. 1993, 268, 14245–14249. [Google Scholar] [PubMed]
- Probstmeier, R.; Montag, D.; Schachner, M. Gal-3, a β- galactoside-binding animal lectin, binds to neural recognition molecules. J. Neurochem. 1995, 64, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Ochieng, J.; Warfield, P. Gal-3 binding potentials of mouse tumor RHS and human placental laminins. Biochem. Biophys. Res. Commun. 1995, 217, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.C. Galectins as modulators of cell adhesion. Biochimie 2001, 83, 667–676. [Google Scholar] [CrossRef]
- Dong, S.; Hughes, R.C. Macrophage surface glycoproteins binding to Gal-3 (Mac-2-antigen). Glycoconj. J. 1997, 14, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Ochieng, J.; Leite-Browning, M.L.; Warfield, P. Regulation of cellular adhesion to extracellular matrix proteins by Gal-3. Biochem. Biophys. Res. Commun. 1998, 246, 788–791. [Google Scholar] [CrossRef] [PubMed]
- Goletz, S.; Hanisch, F.G.; Karsten, U. Novel αGalNAc containing glycans on cytokeratins are recognized in vitro by galectins with type II carbohydrate recognition domains. J. Cell Sci. 1997, 110, 1585–1596. [Google Scholar] [PubMed]
- Sève, A.-P.; Felin, M.; Doyennette-Moyne, M.A.; Sahraoui, T.; Aubery, M.; Hubert, J. Evidence for a lactose-mediated association between two nuclear carbohydrate-binding proteins. Glycobiology 1993, 3, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.P.; Strom, M.; Hughes, R.C. Interaction of a novel cysteine and histidine-rich cytoplasmic protein with Gal-3 in a carbohydrate independent manner. FEBS Lett. 2000, 470, 227–231. [Google Scholar] [CrossRef]
- Park, J.W.; Voss, P.G.; Grabski, S.; Wang, J.L.; Patterson, R.J. Association of galectin-1 and Gal-3 with Gemin4 in complexes containing the SMN protein. Nucleic Acids Res. 2001, 27, 3595–3602. [Google Scholar] [CrossRef]
- Liu, F.T.; Patterson, R.J.; Wang, J.L. Intracellular functions of galectins. Biochim. Biophys. Acta 2002, 1572, 263–273. [Google Scholar] [CrossRef]
- Akahani, S.; Nangia-Makker, P.; Inohara, H.; Kim, H.R.C.; Raz, A. Gal-3: A novel antiapoptotic molecule with a functional BH1 (NWGR) domain of Bcl-2 family. Cancer Res. 1997, 57, 5272–5276. [Google Scholar] [PubMed]
- Missotten, M.; Nichols, A.; Rieger, K.; Sadoul, R. Alix, a novel mouse protein undergoing calcium-dependent interaction with the apoptosis-linked-gene 2 (ALG-2) protein. Cell Death Differ. 1999, 6, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Vito, P.; Pellegrini, L.; Guiet, C.; D’Adamio, L. Cloning of AIP1, a novel protein that associates with the apoptosis-linked gene ALG-2 in a Ca2+-dependent reaction. J. Biol. Chem. 1999, 274, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Zhang, M.; Hu, Q.; Zheng, S.; Soh, A.; Zheng, Y.; Yuan, H. Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy (Review). Int. J. Mol. Med. 2018, 41, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Busse, W.W.; Lemanske, R.F., Jr. Asthma. N. Engl. J. Med. 2001, 344, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Stanworth, D.R. The discovery of IgE. Allergy 1993, 48, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Finkelman, F.D.; Vercelli, D. Advances in asthma, allergy mechanisms, and genetics in 2006. J. Allergy Clin. Immunol. 2007, 120, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, A.L.; Grimley, P.M.; Metzger, H. Electron Microscopic Localization of Immunoglobulin E on the Surface Membrane of Human Basophils. J. Exp. Med. 1971, 134, 1403–1416. [Google Scholar] [CrossRef] [PubMed]
- Zuberi, R.I.; Hsu, D.K.; Kalayci, O.; Chen, H.Y.; Sheldon, H.K.; Yu, L.; Apgar, J.R.; Kawakami, T.; Lilly, C.M.; Liu, F.T. Critical role for Gal-3 in airway inflammation and bronchial hyperresponsiveness in a murine model of asthma. Am. J. Pathol. 2004, 165, 2045–2053. [Google Scholar] [CrossRef]
- Ge, X.N.; Bahaie, N.S.; Kang, B.N.; Hosseinkhani, M.R.; Ha, S.G.; Frenzel, E.M.; Liu, F.T.; Rao, S.P.; Sriramarao, P. Allergen-induced airway remodeling is impaired in Gal-3-deficient mice. J. Immunol. 2010, 185, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Lopez, E.; del Pozo, V.; Miguel, T.; Sastre, B.; Seoane, C.; Civantos, E.; Llanes, E.; Baeza, M.L.; Palomino, P.; Cárdaba, B.; et al. Inhibition of Chronic Airway Inflammation and Remodeling by Gal-3 Gene Therapy in a Murine Model. J. Immunol. 2006, 176, 1943–1950. [Google Scholar] [CrossRef] [PubMed]
- López, E.; Zafra, M.P.; Sastre, B.; Gámez, C.; Lahoz, C.; del Pozo, V. Gene Expression Profiling in Lungs of Chronic Asthmatic Mice Treated with Gal-3: Downregulation of Inflammatory and Regulatory Genes. Mediat. Inflamm. 2011, 2011, 823279. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, J.; St-Pierre, C.; Sato, S. Gal-3 interacts with naive and primed neutrophils, inducing innate immune responses. J. Leukoc. Biol. 2005, 78, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, J.; St-Pierre, C.; Bhaumik, P.; Poirier, F.; Sato, S. Role of Gal-3 in leukocyte recruitment in a murine model of lung infection by Streptococcus pneumoniae. J. Immunol. 2008, 180, 2466–2473. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, A.; Christenson, K.; Matlak, M.; Bjorstad, A.; Brown, K.L.; Telemo, E.; Salomonsson, E.; Leffler, H.; Bylund, J. Gal-3 functions as an opsonin and enhances the macrophage clearance of apoptotic neutrophils. Glycobiology 2009, 19, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Gibson, P.G.; Baines, K.J.; Yang, I.A.; Upham, J.W.; Reynolds, P.N.; Hodge, S.; James, A.L.; Jenkins, C.; Peters, M.J.; et al. Anti-inflammatory deficiencies in neutrophilic asthma: Reduced Gal-3 and IL-1RA/IL-1β. Respir. Res. 2015, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- Riccio, A.M.; Mauri, P.; De Ferrari, L.; Rossi, R.; Di Silvestre, D.; Benazzi, L.; Chiappori, A.; Dal Negro, R.W.; Micheletto, C.; Canonica, G.W. Galectin‑3: An early predictive biomarker of modulation of airway remodeling in patients with severe asthma treated with omalizumab for 36 months. Clin. Transl. Allergy 2017, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Simpson, J.L.; Zhang, J.; Gibson, P.G. Galectin-3: Its role in asthma and potential as an anti-inflammatory target. Respir. Res. 2013, 14, 136. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, V.; Rojo, M.; Rubio, M.L.; Cortegano, I.; Cárdaba, B.; Gallardo, S.; Ortega, M.; Civantos, E.; López, E.; Martín-Mosquer, C.; et al. Gene therapy with Galectin-3 inhibits bronchial obstruction and Inflammation in Antigen-challenged rats through Interleukin-5 Gene downregulation. Am. J. Respir. Crit. Care Med. 2002, 166, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature 1993, 362, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Munro, J.M.; Cotran, R.S. The pathogenesis of atherosclerosis: Atherogenesis and inflammation. Lab. Investig. 1988, 58, 249–261. [Google Scholar] [PubMed]
- Geer, J.C.; McGill, H.C.; Strong, J.P. The fine structure of human atherosclerotic lesions. Am. J. Pathol. 1961, 38, 263–287. [Google Scholar] [PubMed]
- Gerrity, R.G. The role of the monocyte in atherogenesis. I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am. J. Pathol. 1981, 103, 181–190. [Google Scholar] [PubMed]
- Faruqi, R.M.; Di Corletto, P.E. Mechanisms of monocyte recruitment and accumulation. Br. Heart J. 1993, 69, S19–S29. [Google Scholar] [CrossRef] [PubMed]
- Stary, H.C.; Blankenhorn, D.H.; Chandler, A.B.; Glagov, S.; Insull, W., Jr.; Richardson, M.; Rosenfeld, M.E.; Shaffer, S.A.; Schwartz, C.J.; Wagner, W.D.; et al. A definition of the intima of human arteries and of its atherosclerosis-prone regions: A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1992, 85, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Nachtigal, M.; Al-Assaad, Z.; Mayer, E.P.; Kim, K.; Monsigny, M. Gal-3 Expression in Human Atherosclerotic Lesions. Am. J. Patol. 1998, 152, 1199–1208. [Google Scholar]
- Konstantinov, K.N.; Shames, B.; Izuno, G.; Liu, F.T. Expression of epsilon BP, a beta- galactoside-binding soluble lectin, in normal and neoplastic epidermis. Exp. Dermatol. 1994, 3, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Kimata, H. Enhancement of IgE production in B cells by neutrophils via Gal-3 in IgE-associated atopic eczema/dermatitis syndrome. Int. Arch. Allergy Immunol. 2002, 128, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Klubal, R.; Osterhoff, B.; Wang, B.; Kinet, J.P.; Maurer, D.; Stingl, G. The high-affinity receptor for Ige is the predominant IgE-binding structure in lesional skin of atopic dermatitis patients. J. Investig. Dermatol. 1997, 108, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Saegusa, J.; Hsu, D.K.; Chen, H.Y.; Yu, L.; Fermin, A.; Fung, M.A.; Liu, F.T. Gal-3 Is Critical for the Development of the Allergic Inflammatory Response in a Mouse Model of Atopic Dermatitis. Am. J. Pathol. 2009, 174, 922–931. [Google Scholar] [CrossRef] [PubMed]
- GR-MD-02 Demonstrates Clinically Significant Effect in Patients with Severe and Refractory Atopic Dermatitis (Eczema). Available online: http://globenewswire.com/news-release/2017/03/14/936280/0/en/GR-MD-02 Demonstrates-Clinically-Significant-Effect-in-Patients-with-Severe-and-Refractory-Atopic-Dermatitis-Eczema.html (accessed on 22 December 2017).
- Sharma, U.C.; Pokharel, S.; van Brakel, T.J.; van Berlo, J.H.; Cleutjens, J.P.; Schroen, B.; André, S.; Crijns, H.J.; Gabius, H.J.; Maessen, J.; et al. Gal-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 2004, 110, 3121–3128. [Google Scholar] [CrossRef] [PubMed]
- De Boer, R.A.; Voors, A.A.; Muntendam, P.; van Gilst, W.H.; van Veldhuisen, D.J. Gal-3: A novel mediator of heart failure development and progression. Eur. J. Heart Fail. 2009, 11, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Lok, D.J.; Van Der Meer, P.; de la Porte, P.W.; Lipsic, E.; Van Wijngaarden, J.; Hillege, H.L.; van Veldhuisen, D.J. Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: Data from the DEAL-HF study. Clin. Res. Cardiol. 2010, 99, 323–328. [Google Scholar] [CrossRef] [PubMed]
- De Boer, R.A.; Lok, D.J.; Jaarsma, T.; van der Meer, P.; Voors, A.A.; Hillege, H.L.; van Veldhuisen, D.J. Predictive value of plasma Gal-3 levels in heart failure with reduced and preserved ejection fraction. Ann. Med. 2011, 43, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Van Kimmenade, R.R.; Januzzi, J.L., Jr.; Ellinor, P.T.; Sharma, U.C.; Bakker, J.A.; Low, A.F.; Martinez, A.; Crijns, H.J.; MacRae, C.A.; Menheere, P.P.; et al. Utility of amino-terminal pro-brain natriuretic peptide, Gal-3, and apelin for the evaluation of patients with acute heart failure. J. Am. Coll. Cardiol. 2006, 48, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Jaarsma, T.; van der Wal, M.H.; Lesman-Leegte, I.; Luttik, M.L.; Hogenhuis, J.; Veeger, N.J.; Sanderman, R.; Hoes, A.W.; van Gilst, W.H.; Lok, D.J.; et al. Effect of moderate or intensive disease management program on outcome in patients with heart failure: Coordinating Study Evaluating Outcomes of Advising and Counseling in Heart Failure (COACH). Arch. Intern. Med. 2008, 168, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Januzzi, J.L., Jr.; Camargo, C.A.; Anwaruddin, S.; Baggish, A.L.; Chen, A.A.; Krauser, D.G.; Tung, R.; Cameron, R.; Nagurney, J.T.; Chae, C.U.; et al. The N-terminal pro- BNP investigation of dyspnea in the emergency department (PRIDE) study. Am. J. Cardiol. 2005, 95, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Meijers, W.C.; Januzzi, J.L.; de Filippi, C.; Adourian, A.S.; Shah, S.J.; van Veldhuisen, D.J.; de Boer, R.A. Elevated plasma Gal-3 is associated with near-term rehospitalization in heart failure: A pooled analysis of 3 clinical trials. Am. Heart J. 2014, 167, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.M.; Fiuzat, M.; Shaw, L.K.; Clare, R.; Whellan, D.J.; Bettari, L.; Shirolkar, S.C.; Donahue, M.; Kitzman, D.W.; Zannad, F.; et al. Galectin-3 in ambulatory patients with heart failure: Results from the HF-ACTION study. Cir. Heart Fail. 2012, 5, 72–78. [Google Scholar] [CrossRef] [PubMed]
- De Boer, R.A.; van Veldhuisen, D.J.; Gansevoort, R.T.; Muller Kobold, A.C.; van Gilst, W.H.; Hillege, H.L.; Bakker, S.J.; van der Harst, P. The fibrosis marker Gal-3 and outcome in the general population. J. Intern. Med. 2012, 272, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.E.; Liu, C.; Lyass, A.; Courchesne, P.; Pencina, M.J.; Vasan, R.S.; Larson, M.G.; Levy, D. Gal-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J. Am. Coll. Cardiol. 2012, 60, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Daniels, L.B.; Clopton, P.; Laughlin, G.A.; Maisel, A.S.; Barrett-Connor, E. Gal-3 is independently associated with cardiovascular mortality in community-dwelling older adults without known cardiovascular disease: The Rancho Bernardo Study. Am. Heart J. 2014, 167, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.E.; Yin, X.; Levy, D.; Vasan, R.S.; Magnani, J.W.; Ellinor, P.T.; McManus, D.D.; Lubitz, S.A.; Larson, M.G.; Benjamin, E.J. Galectin 3 and incident atrial fibrillation in the community. Am. Heart J. 2014, 167, 729–734. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.A.; Olobatoke, A. Gal-3: A novel blood test for the evaluation and management of patients with heart failure. Rev. Cardiovasc. Med. 2011, 12, 200–210. [Google Scholar] [PubMed]
- Danguy, A.; Camby, I.; Kiss, R. Galectins and cancer. Biochim. Biophys. Acta 2002, 1572, 285–293. [Google Scholar] [CrossRef]
- Takenaka, Y.; Fukumori, T.; Raz, A. Gal-3 and metastasis. Glycoconj. J. 2004, 19, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.T.; Rabinovich, G.A. Galectins as modulators of tumour progression. Nat. Rev. Cancer 2005, 5, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Dagher, S.F.; Wang, J.L.; Patterson, R.J. Identification of galectin-3 as a factor in pre-mRNA splicing. Proc. Natl. Acad. Sci. USA 1995, 92, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, S.; Oka, N.; Raz, A. On the role of Gal-3 in cancer apoptosis. Apoptosis 2005, 10, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Ruvolo, P.R. Galectin 3 as a guardian of the tumor microenvironment. Biochim. Biophys. Acta 2016, 1863, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Baldus, S.E.; Zirbes, T.K.; Weingarten, M.; Fromm, S.; Glossmann, J.; Hanisch, F.G.; Mönig, S.P.; Schröder, W.; Flucke, U.; Thiele, J.; et al. Increased Gal-3 expression in gastric cancer: Correlations with histopathological subtypes, galactosylated antigens and tumor cell proliferation. Tumour Biol. 2000, 21, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Radosavljevic, G.; Jovanovic, I.; Majstorovic, I.; Mitrovic, M.; Lisnic, V.J.; Arsenijevic, N.; Jonjic, S.; Lukic, M.L. Deletion of Gal-3 in the host attenuates metastasis of murine melanoma by modulating tumor adhesion and NK cell activity. Clin. Exp. Metastasis 2011, 28, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Prieto, V.G.; Mourad-Zeidan, A.A.; Melnikova, V.; Johnson, M.M.; Lopez, A.; Diwan, A.H.; Lazar, A.J.; Shen, S.S.; Zhang, P.S.; Reed, J.A.; et al. Gal-3 expression is associated with tumor progression and pattern of sun exposure in melanoma. Clin. Cancer Res. 2006, 12, 6709–6715. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Noguchi, T.; Takeno, S.; Takahashi, Y.; Fumoto, S.; Kawahara, K. Impact of nuclear Gal-3 expression on histological differentiation and vascular invasion in patients with esophageal squamous cell carcinoma. Oncol. Rep. 2005, 139, 235–239. [Google Scholar]
- Califice, S.; Castronovo, V.; Bracke, M.; van den Brûle, F. Dual activities of Gal-3 in human prostate cancer: Tumor suppression of nuclear Gal-3 vs tumor promotion of cytoplasmic Gal-3. Oncogene 2004, 23, 7527–7536. [Google Scholar] [CrossRef] [PubMed]
- Van den Brûle, F.A.; Waltregny, D.; Liu, F.T.; Castronovo, V. Alteration of the cytoplasmic/nuclear expression pattern of Gal-3 correlates with prostate carcinoma progression. Int. J. Cancer 2000, 89, 361–367. [Google Scholar] [CrossRef]
- Puglisi, F.; Minisini, A.M.; Barbone, F.; Intersimone, D.; Aprile, G.; Puppin, C.; Damante, G.; Paron, I.; Tell, G.; Piga, A.; et al. Gal-3 expression in non-small cell lung carcinoma. Cancer Lett. 2004, 212, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, S.; Hogan, V.; Inohara, H.; Raz, A. Importin-mediated nuclear translocation of galectin-3. J. Biol. Chem. 2006, 281, 39649–39659. [Google Scholar] [CrossRef] [PubMed]
- Funasaka, T.; Raz, A.; Nangia-Makker, P. Nuclear transport of galectin-3 and its ther- apeutic implications. Semin. Cancer Biol. 2014, 27, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Haudek, K.C.; Spronk, K.J.; Voss, P.G.; Patterson, R.J.; Wang, J.L.; Arnoys, E.J. Dynamics of Galectin-3 in the Nucleus and Cytoplasm. Biochim. Biophys. Acta 2010, 1800, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Kapucuoglu, N.; Basak, P.Y.; Bircan, S.; Sert, S.; Akkaya, V.B. Immunohistochemical galectin-3 expression in non-melanoma skin cancers. Pathol. Res. Pract. 2009, 205, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, V.L.; Heusschen, R.; Caers, J.; Griffioen, A.W. Galectin expression in cancer diagnosis and prognosis: A systematic review. Biochim. Biophys. Acta 2015, 1855, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Fortuna-Costa, A.; Gomes, A.M.; Kozlowski, E.O.; Stelling, M.P.; Pavão, M.S. Extracellular galectin-3 in tumor progression and metastasis. Front. Oncol. 2014, 4, 138. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Dong, X.W.; Guo, X.L. Role of the interaction between galectin-3 and cell adhesion molecules in cancer metastasis. Biomed. Pharmacother. 2015, 69, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Smetana, K., Jr.; André, S.; Kaltner, H.; Kopitz, J.; Gabius, H.J. Context-dependent multifunctionality of galectin-1: A challenge for defining the lectin as therapeutic target. Expert Opin. Ther. Targets 2013, 17, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, T.; Fukumori, T.; Honjo, Y.; Inohara, H.; Kim, H.R.; Raz, A. Galectin-3 phosphorylation is required for its anti-apoptotic function and cell cycle arrest. J. Biol. Chem. 2002, 277, 6852–6857. [Google Scholar] [CrossRef] [PubMed]
- Cecchinelli, B.; Lavra, L.; Rinaldo, C.; Iacovelli, S.; Gurtner, A.; Gasbarri, A.; Ulivieri, A.; Del Prete, F.; Trovato, M.; Piaggio, G.; et al. Repression of the antiapoptotic molecule Gal-3 by homeodomain-interacting protein kinase 2-activated p53 is required for p53-induced apoptosis. Mol. Cell. Biol. 2006, 26, 4746–4757. [Google Scholar] [CrossRef] [PubMed]
- Lavra, L.; Ulivieri, A.; Rinaldo, C.; Dominici, R.; Volante, M.; Luciani, E.; Bartolazzi, A.; Frasca, F.; Soddu, S.; Sciacchitano, S. Gal-3 is stimulated by gain-of-function p53 mutations and modulates chemoresistance in anaplastic thyroid carcinomas. J. Pathol. 2009, 218, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Fukumori, T.; Kanayama, H.O.; Raz, A. The role of galectin-3 in cancer drug resistance. Drug Resist. Updates 2007, 10, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Harazono, Y.; Nakajima, K.; Raz, A. Why anti-Bcl-2 clinical trials fail: A solution. Cancer Metastasis Rev. 2014, 33, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, K.K.; Pang, M.; Gui, D.; Shintaku, I.P.; Kuwabara, I.; Liu, F.T.; Said, J.W.; Baum, L.G.; Teitell, M.A. An Anti-Apoptotic Role for Galectin-3 in Diffuse Large B-Cell Lymphoma. Am. J. Pathol. 2004, 164, 893–902. [Google Scholar] [CrossRef]
- Harazono, Y.; Kho, D.H.; Balan, V.; Nakajima, K.; Zhang, T.; Hogan, V.; Raz, A. Galectin-3 leads to attenuation of apoptosis through Bax heterodimerization in human thyroid carcinoma cells. Oncotarget 2014, 5, 9992–10001. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Ji, B.; Ramachandran, V.; Wang, H.; Hafley, M.; Logsdon, C.; Bresalier, R.S. Overexpressed galectin-3 in pancreatic cancer induces cell proliferation and invasion by binding Ras and activating Ras signaling. PLoS ONE 2012, 7, e42699. [Google Scholar] [CrossRef] [PubMed]
- Elad-Sfadia, G.; Haklai, R.; Balan, E.; Kloog, Y. Galectin-3 augments K-Ras activation and triggers a Ras signal that attenuates ERK but not phosphoinositide 3-kinase activity. J. Biol. Chem. 2004, 279, 34922–34930. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.; Grafi-Cohen, M.; Kraiem, Z.; Kloog, Y. Galectin-3 promotes chronic activation of K-Ras and differentiation block in malignant thyroid carcinomas. Mol. Cancer Ther. 2010, 9, 2208–2219. [Google Scholar] [CrossRef] [PubMed]
- Veschi, V.; Petroni, M.; Cardinali, B.; Dominici, C.; Screpanti, I.; Frati, L.; Bartolazzi, A.; Gulino, A.; Giannini, G. Galectin-3 impairment of MYCN-dependent apoptosis-sensitive phenotype is antagonized by nutlin-3 in neuroblastoma cells. PLoS ONE 2012, 7, e49139. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.M.; Bum-Erdene, K.; Yu, X.; Blanchard, H. Galectin-3 interactions with glycosphingolipids. J. Mol. Biol. 2014, 426, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Hauselmann, I.; Borsig, L. Altered tumor-cell glycosylation promotes metastasis. Front. Oncol. 2014, 4, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du Toit, A. Endocytosis: Bend it like galectin 3. Nat. Rev. Mol. Cell Biol. 2014, 15, 430–431. [Google Scholar] [CrossRef] [PubMed]
- Stanley, P. Galectins CLIC cargo inside. Nat. Cell Biol. 2014, 16, 506–507. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, M.C.; Bengtson, P.; Cucak, H.; Leffler, H. Galectin-3 guides intracellular trafficking of some human serotransferrin glycoforms. J. Biol. Chem. 2013, 288, 28398–28408. [Google Scholar] [CrossRef] [PubMed]
- Lakshminarayan, R.; Wunder, C.; Becken, U.; Howes, M.T.; Benzing, C.; Arumugam, S.; Sales, S.; Ariotti, N.; Chambon, V.; Lamaze, C.; et al. Galectin-3 drives glycosphingolipid-dependent biogenesis of clathrinindependent carriers. Nat. Cell Biol. 2014, 16, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Huergo, S.P.; Blidner, A.G.; Rabinovich, G.A. Galectins: Emerging regulatory checkpoints linking tumor immunity and angiogenesis. Curr. Opin. Immunol. 2017, 45, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Newlaczyl, A.U.; Yu, L.G. Galectin-3—A jack-of-all-trades in cancer. Cancer Lett. 2011, 313, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Cay, T. Immunohistochemical expression of Gal-3 in cancer: A review of the literature. Turk Patoloji Derg. 2012, 28, 1–10. [Google Scholar] [PubMed]
- Ebrahim, A.H.; Alalawi, Z.; Mirandola, L.; Rakhshanda, R.; Dahlbeck, S.; Nguyen, D.; Jenkins, M.; Grizzi, F.; Cobos, E.; Figueroa, J.A.; et al. Galectins in cancer: Carcinogenesis, diagnosis and therapy. Ann. Transl. Med. 2014, 2, 88. [Google Scholar] [CrossRef] [PubMed]
- Irimura, T.; Matsushita, Y.; Sutton, R.C.; Carralero, D.; Ohannesian, D.W.; Cleary, K.R.; Ota, D.M.; Nicolson, G.L.; Lotan, R. Increased content of an endogenous lactose-binding lectin in human colorectal carcinoma progressed to metastatic stages. Cancer Res. 1991, 51, 387–393. [Google Scholar] [PubMed]
- Lotan, R.; Matsushita, Y.; Ohannesian, D.; Carralero, D.; Ota, D.M.; Cleary, K.R.; Nicolson, G.L.; Irimura, T. Lactose-binding lectin expression in human colorectal carcinomas. Relation to tumor progression. Carbohydr. Res. 1991, 213, 47–57. [Google Scholar] [CrossRef]
- Nakamura, M.; Inufusa, H.; Adachi, T.; Aga, M.; Kurimoto, M.; Nakatani, Y.; Wakano, T.; Nakajima, A.; Hida, J.I.; Miyaké, M.; et al. Involvement of galectin-3 expression in colorectal cancer progression and metastasis. Int. J. Oncol. 1999, 15, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Schoeppner, H.L.; Raz, A.; Ho, S.B.; Bresalier, R.S. Expression of an endogenous galactose-binding lectin correlates with neoplastic progression in the colon. Cancer 1995, 75, 2818–2826. [Google Scholar] [CrossRef]
- Castronovo, V.; Campo, E.; van den Brule, F.A.; Claysmith, A.P.; Cioce, V.; Liu, F.T.; Fernandez, P.L.; Sobel, M.E. Inverse modulation of steady-state messenger RNA levels of two nonintegrin laminin-binding proteins in human colon carcinoma. J. Natl. Cancer Inst. 1992, 84, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Lotz, M.M.; Andrews, C.W., Jr.; Korzelius, C.A.; Lee, E.C.; Steele, G.D., Jr.; Clarke, A.; Mercurio, A.M. Decreased expression of Mac-2 (carbohydrate binding protein 35) and loss of its nuclear localization are associated with the neoplastic progression of colon carcinoma. Proc. Natl. Acad. Sci. USA 1993, 90, 3466–3470. [Google Scholar] [CrossRef] [PubMed]
- Sanjuan, X.; Fernandez, P.L.; Castells, A.; Castronovo, V.; van den Brule, F.; Liu, F.T.; Cardesa, A.; Campo, E. Differential expression of galectin 3 and galectin 1 in colorectal cancer progression. Gastroenterology 1997, 113, 1906–1915. [Google Scholar] [CrossRef]
- Xu, X.C.; el-Naggar, A.K.; Lotan, R. Differential expression of galectin-1 and Gal-3 in thyroid tumors. Potential diagnostic implications. Am. J. Pathol. 1995, 147, 815–822. [Google Scholar] [PubMed]
- Orlandi, F.; Saggiorato, E.; Pivano, G.; Puligheddu, B.; Termine, A.; Cappia, S.; De Giuli, P.; Angeli, A. Gal-3 is a presurgical marker of human thyroid carcinoma. Cancer Res. 1998, 58, 3015–3020. [Google Scholar] [PubMed]
- Gasbarri, A.; Martegani, M.P.; Del Prete, F.; Lucante, T.; Natali, P.G.; Bartolazzi, A. Gal-3 and CD44v6 isoforms in the preoperative evaluation of thyroid nodules. J. Clin. Oncol. 1999, 17, 3494–3502. [Google Scholar] [CrossRef] [PubMed]
- Inohara, H.; Honjo, Y.; Yoshii, T.; Akahani, S.; Yoshida, J.; Hattori, K.; Okamoto, S.; Sawada, T.; Raz, A.; Kubo, T. Expression of Gal-3 in fine-needle aspirates as a diagnostic marker differentiating benign from malignant thyroid neoplasms. Cancer 1999, 85, 2475–2484. [Google Scholar] [CrossRef]
- Cvejic, D.; Savin, S.; Golubovic, S.; Paunovic, I.; Tatic, S.; Havelka, M. Gal-3 and carcinoembryonic antigen expression in medullary thyroid carcinoma: Possible relation to tumour progression. Histopathology 2000, 37, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Bartolazzi, A.; Gasbarri, A.; Papotti, M.; Bussolati, G.; Lucante, T.; Khan, A.; Inohara, H.; Marandino, F.; Orlandi, F.; Nardi, F.; et al. Application of an immunodiagnostic method for improving preoperative diagnosis of nodular thyroid lesions. Lancet 2001, 357, 1644–1650. [Google Scholar] [CrossRef]
- Saggiorato, E.; Cappia, S.; De Giuli, P.; Mussa, A.; Pancani, G.; Caraci, P.; Angeli, A.; Orlandi, F. Gal-3 as a presurgical immunocytodiagnostic marker of minimally invasive follicular thyroid carcinoma. J. Clin. Endocrinol. Metab. 2001, 86, 5152–5158. [Google Scholar] [CrossRef] [PubMed]
- Bresalier, R.S.; Yan, P.S.; Byrd, J.C.; Lotan, R.; Raz, A. Expression of the endogenous galactose-binding protein Gal-3 correlates with the malignant potential of tumors in the central nervous system. Cancer 1997, 80, 776–787. [Google Scholar] [CrossRef]
- Borges, C.B.; Bernardes, E.S.; Latorraca, E.F.; Becker, A.P.; Neder, L.; Chammas, R.; Roque-Barreira, M.C.; Machado, H.R.; de Oliveira, R.S. Gal-3 expression: A useful tool in the differential diagnosis of posterior fossa tumors in children. Childs Nerv. Syst. 2011, 27, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Veschi, V.; Petroni, M.; Bartolazzi, A.; Altavista, P.; Dominici, C.; Capalbo, C.; Boldrini, R.; Castellano, A.; McDowell, H.P.; Pizer, B.; et al. Galectin-3 is a marker of favorable prognosis and a biologically relevant molecule in neuroblastic tumors. Cell. Death Dis. 2014, 5, e1100. [Google Scholar] [CrossRef] [PubMed]
- Gillenwater, A.; Xu, X.C.; el-Naggar, A.K.; Clayman, G.L.; Lotan, R. Expression of galectins in head and neck squamous cell carcinoma. Head Neck 1996, 18, 422–432. [Google Scholar] [CrossRef]
- Schaffert, C.; Pour, P.M.; Chaney, W.G. Localization of Gal-3 in normal and diseased pancreatic tissue. Int. J. Pancreatol. 1998, 23, 1–9. [Google Scholar] [PubMed]
- Cindolo, L.; Benvenuto, G.; Salvatore, P.; Pero, R.; Salvatore, G.; Mirone, V.; Prezioso, D.; Altieri, V.; Bruni, C.B.; Chiariotti, L. Galectin-1 and Gal-3 expression in human bladder transitional-cell carcinomas. Int. J. Cancer 1999, 84, 39–43. [Google Scholar] [CrossRef]
- Dancer, J.Y.; Truong, L.D.; Zhai, Q.; Shen, S.S. Expression of Gal-3 in renal neoplasms: A diagnostic, possible prognostic marker. Arch. Pathol. Lab. Med. 2010, 134, 90–94. [Google Scholar] [PubMed]
- Young, A.N.; Amin, M.B.; Moreno, C.S.; Lim, S.D.; Cohen, C.; Petros, J.A.; Marshall, F.F.; Neish, A.S. Expression profiling of renal epithelial neoplasms: A method for tumor classification and discovery of diagnostic molecular markers. Am. J. Pathol. 2001, 158, 1639–1651. [Google Scholar] [CrossRef]
- Hsu, D.K.; Dowling, C.A.; Jeng, K.C.G.; Chen, J.T.; Yang, R.Y.; Liu, F.T. Gal-3 expression is induced in cirrhotic liver and hepatocellular carcinoma. Int. J. Cancer 1999, 81, 519–526. [Google Scholar] [CrossRef]
- Karaarslan, S.; Yurum, F.N.; Kumbaraci, B.S.; Pala, E.E.; Sivrikoz, O.N.; Akyildiz, M.; Bugdayci, M.H. The role of parafibromin, Gal-3, HBME-1, and Ki-67 in the differential diagnosis of parathyroid tumors. Oman Med. J. 2015, 30, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Saggiorato, E.; Bergero, N.; Volante, M.; Bacillo, E.; Rosas, R.; Gasparri, G.; Orlandi, F.; Papotti, M. Gal-3 and Ki-67 expression in multiglandular parathyroid lesions. Am. J. Clin. Pathol. 2006, 126, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Remmelink, M.; de Leval, L.; Decaestecker, C.; Duray, A.; Crompot, E.; Sirtaine, N.; André, S.; Kaltner, H.; Leroy, X.; Gabius, H.J.; et al. Quantitative immunohistochemical fingerprinting of adhesion/growth-regulatory galectins in salivary gland tumours: Divergent profiles with diagnostic potential. Histopathology 2011, 58, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Lee, S.J.; Sung, H.J.; Choi, I.K.; Choi, C.W.; Kim, B.S.; Kim, J.S.; Yu, W.; Hwang, H.S.; Kim, I.S. Increased serum 90 K and Gal-3 expression are associated with advanced stage and a worse prognosis in diffuse large B-cell lymphomas. Acta Haematol. 2008, 120, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Abdou, A.G.; Hammam, M.A.; Farargy, S.E.; Farag, A.G.; El Shafey, E.N.; Farouk, S.; Elnaidany, N.F. Diagnostic and Prognostic Role of Galectin 3 Expression in Cutaneous Melanoma. Am. J. Dermatopathol. 2010, 32, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Bartolazzi, A.; Bellotti, C.; Sciacchitano, S. Methodology and technical requirements of the Gal-3 test for the preoperative characterization of thyroid nodules. Appl. Immunohistochem. Mol. Morphol. 2012, 20, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Carpi, A.; Naccarato, A.G.; Iervasi, G.; Nicolini, A.; Bevilacqua, G.; Viacava, P.; Collecchi, P.; Lavra, L.; Marchetti, C.; Sciacchitano, S.; et al. Large needle aspiration biopsy and Gal-3 determination in selected thyroid nodules with indeterminate FNA-cytology. Br. J. Cancer 2006, 95, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Carpi, A.; Di Coscio, G.; Iervasi, G.; Antonelli, A.; Mechanick, J.; Sciacchitano, S.; Nicolini, A. Thyroid fine needle aspiration: How to improve clinicians' confidence and performance with the technique. Cancer Lett. 2008, 264, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Bartolazzi, A.; Orlandi, F.; Saggiorato, E.; Volante, M.; Arecco, F.; Rossetto, R.; Palestini, N.; Ghigo, E.; Papotti, M.; Bussolati, G.; et al. Italian Thyroid Cancer Study Group (ITCSG). Gal-3-expression analysis in the surgical selection of follicular thyroid nodules with indeterminate fine-needle aspiration cytology: A prospective multicentre study. Lancet Oncol. 2008, 9, 543–549. [Google Scholar] [CrossRef]
- De Matos, L.L.; Del Giglio, A.B.; Matsubayashi, C.O.; de Lima Farah, M.; Del Giglio, A.; da Silva Pinhal, M.A. Expression of ck-19, Gal-3 and hbme-1 in the differentiation of thyroid lesions: Systematic review and diagnostic meta-analysis. Diagn. Pathol. 2012, 7, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sciacchitano, S.; Lavra, L.; Ulivieri, A.; Magi, F.; De Francesco, G.P.; Bellotti, C.; Salehi, L.B.; Trovato, M.; Drago, C.; Bartolazzi, A. Comparative analysis of diagnostic performance, feasibility and cost of different test-methods for thyroid nodules with indeterminate cytology. Oncotarget 2017, 8, 49421–49442. [Google Scholar] [CrossRef] [PubMed]
- Sciacchitano, S.; Lavra, L.; Ulivieri, A.; Magi, F.; Porcelli, T.; Amendola, S.; De Francesco, G.P.; Bellotti, C.; Trovato, M.C.; Salehi, L.B.; et al. Combined clinical and ultrasound follow-up assists in malignancy detection in Gal-3 negative Thy-3 thyroid nodules. Endocrine 2016, 54, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Bartolazzi, A.; D’Alessandria, C.; Parisella, M.G.; Signore, A.; Del Prete, F.; Lavra, L.; Braesch-Andersen, S.; Massari, R.; Trotta, C.; Soluri, A.; et al. Thyroid cancer imaging in vivo by targeting the anti-apoptotic molecule Gal-3. PLoS ONE 2008, 3, e3768. [Google Scholar] [CrossRef] [PubMed]
- Iurisci, I.; Tinari, N.; Natoli, C.; Angelucci, D.; Cianchetti, E.; Iacobelli, S. Concentrations of Gal-3 in the sera of normal controls and cancer patients. Clin. Cancer Res. 2000, 6, 1389–1393. [Google Scholar] [PubMed]
- Chiu, C.G.; Strugnell, S.S.; Griffith, O.L.; Jones, S.J.; Gown, A.M.; Walker, B.; Nabi, I.R.; Wiseman, S.M. Diagnostic utility of Gal-3 in thyroid cancer. Am. J. Pathol. 2010, 176, 2067–2081. [Google Scholar] [CrossRef] [PubMed]
- Balan, V.; Wang, Y.; Nangia-Makker, P.; Kho, D.; Bajaj, M.; Smith, D.; Heilbrun, L.; Raz, A.; Heath, E. Gal-3: A possible complementary marker to the PSA blood test. Oncotarget 2013, 4, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, I. The role of Gal-3 as a marker of cancer and inflammation in a stage IV ovarian cancer patient with underlying pro-inflammatory comorbidities. Case Rep. Oncol. 2013, 6, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Lalancette-Hébert, M.; Swarup, V.; Beaulieu, J.M.; Bohacek, I.; Abdelhamid, E.; Weng, Y.C.; Sato, S.; Kriz, J. Gal-3 is required for resident microglia activation and proliferation in response to ischemic injury. J. Neurosci. 2012, 32, 10383–10395. [Google Scholar] [CrossRef] [PubMed]
- Doverhag, C.; Hedtjärn, M.; Poirier, F.; Mallard, C.; Hagberg, H.; Karlsson, A.; Sävman, K. Gal-3 contributes to neonatal hypoxic-ischemic brain injury. Neurobiol. Dis. 2010, 38, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Ekingen, E.; Yilmaz, M.; Yildiz, M.; Atescelik, M.; Goktekin, M.C.; Gurger, M.; Alatas, O.D.; Basturk, M.; Ilhan, N. Utilization of glial fibrillary acidic protein and Gal-3 in the dagnosis of cerebral infarction patients with normal cranial tomography. Niger. J. Clin. Pract. 2017, 20, 433–437. [Google Scholar] [PubMed]
- Agoston-Coldea, L.; Lupu, S.; Petrovai, D.; Mocan, T.; Mousseaux, E. Correlations between echocardiographic parameters of right ventricular dysfunction and Galectin-3 in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Med. Ultrason. 2015, 17, 487–495. [Google Scholar] [PubMed]
- Pilette, C.; Colinet, B.; Kiss, R.; André, S.; Kaltner, H.; Gabius, H.J.; Delos, M.; Vaerman, J.P.; Decramer, M.; Sibille, Y. Increased galectin-3 expression and intra-epithelial neutrophils in small airways in severe COPD. Eur. Respir. J. 2007, 29, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Mukaro, V.R.; Bylund, J.; Hodge, G.; Holmes, M.; Jersmann, H.; Reynolds, P.N.; Hodge, S. Lectins offer new perspectives in the development of macrophage-targeted therapies for COPD/emphysema. PLoS ONE 2013, 8, e56147. [Google Scholar] [CrossRef] [PubMed]
- Hodge, S.; Dean, M.; Eisen, D.P. Lectins as Potential Adjunct Therapeutics for COPD/Emphysema? J. Pulm. Respir. Med. 2013, 3, 5. [Google Scholar] [CrossRef]
- Feng, W.; Wu, X.; Li, S.; Zhai, C.; Wang, J.; Shi, W.; Li, M. Association of serum Galectin-3 with the acute exacerbation of chronic obstructive pulmonary disease. Med. Sci. Monit. 2017, 23, 4612–4618. [Google Scholar] [CrossRef] [PubMed]
- Bobrowska, B.; Wieczorek-Surdacka, E.; Kruszelnicka, O.; Chyrchel, B.; Surdacki, A.; Dudek, D. Clinical correlates and prognostic value of plasma Gal-3 levels in degenerative aortic stenosis: A single-center prospective study of patients referred for invasive treatment. Int. J. Mol. Sci. 2017, 18, 947. [Google Scholar] [CrossRef] [PubMed]
- Henderson, N.C.; Sethi, T. The regulation of inflammation by Gal-3. Immunol. Rev. 2009, 230, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Liu, F.T. Gal-3 and inflammation. Glycobiol. Insights 2016, 6, 1–9. [Google Scholar]
- Weigert, J.; Neumeier, M.; Wanninger, J.; Bauer, S.; Farkas, S.; Scherer, M.N.; Schnitzbauer, A.; Schäffler, A.; Aslanidis, C.; Schölmerich, J.; et al. Serum Gal-3 is elevated in obesity and negatively correlates with glycosylated hemoglobin in type 2 diabetes. J. Clin. Endocrinol. Metab. 2010, 95, 1404–1411. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.H.; Lou, Y.F.; Li, T.L.; Chen, H.H.; Liu, Q.; He, X.J. Serum Gal-3: A risk factor for vascular complications in type 2 diabetes mellitus. Chin. Med. J. 2013, 126, 2109–2115. [Google Scholar] [PubMed]
- Yilmaz, H.; Cakmak, M.; Inan, O.; Darcin, T.; Akcay, A. Increased levels of Gal-3 were associated with prediabetes and diabetes: New risk factor? J. Endocrinol. Investig. 2014, 38, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Seferovic, J.P.; Lalic, N.M.; Floridi, F.; Tesic, M.; Seferovic, P.M.; Giga, V.; Lalic, K.; Jotic, A.; Jovicic, S.; Colak, E.; et al. Structural myocardial alterations in diabetes and hypertension: The role of Gal-3. Clin. Chem. Lab. Med. 2014, 52, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, D.; Celik, O.; Satilmis, S.; Aslan, S.; Erturk, M.; Cakmak, H.A.; Kalkan, A.K.; Ozyilmaz, S.; Diker, V.; Gul, M. Association between serum Gal-3 levels and coronary atherosclerosis and plaque burden/structure in patients with type 2 diabetes mellitus. Coron. Artery Dis. 2015, 26, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Darrow, A.L.; Shohet, R.V.; Maresh, J.G. Transcriptional analysis of the endothelial response to diabetes reveals a role for Gal-3. Physiol. Genom. 2011, 43, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Kiwaki, K.; Novak, C.M.; Hsu, D.K.; Liu, F.T.; Levine, J.A. Gal-3 stimulates preadipocyte proliferation and is up-regulated in growing adipose tissue. Obesity 2007, 15, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Kim, S.J.; Kang, H.G.; Lee, H.W.; Kim, J.H.; Hwang, K.A.; Song, J.; Chun, K.H. Gal-3 activates PPARgamma and supports white adipose tissue formation and high-fat diet-induced obesity. Endocrinology 2015, 156, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Chou, H.C.; Chen, Y.H.; Chan, H.L. High glucose-induced proteome alterations in hepatocytes and its possible relevance to diabetic liver disease. J. Nutr. Biochem. 2013, 24, 1889–1910. [Google Scholar] [CrossRef] [PubMed]
- Mensah-Brown, E.P.; Al Rabesi, Z.; Shahin, A.; Al Shamsi, M.; Arsenijevic, N.; Hsu, D.K.; Liu, F.T.; Lukic, M.L. Targeted disruption of the Gal-3 gene results in decreased susceptibility to multiple low dose streptozotocin-induced diabetes in mice. Clin. Immunol. 2009, 130, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, G.; Iacobini, C.; Ricci, C.; Blasetti Fantauzzi, C.; Menini, S. Gal-3 in diabetic patients. Clin. Chem. Lab. Med. 2014, 52, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Ohkura, T.; Fujioka, Y.; Nakanishi, R.; Shiochi, H.; Sumi, K.; Yamamoto, N.; Matsuzawa, K.; Izawa, S.; Ohkura, H.; Ueta, E.; et al. Low serum Gal-3 concentrations are associated with insulin resistance in patients with type 2 diabetes mellitus. Diabetol. Metab. Syndr. 2014, 6, 106. [Google Scholar] [CrossRef] [PubMed]
- Pejnovic, N.N.; Pantic, J.M.; Jovanovic, I.P.; Radosavljevic, G.D.; Milovanovic, M.Z.; Nikolic, I.G.; Zdravkovic, N.S.; Djukic, A.L.; Arsenijevic, N.N.; Lukic, M.L. Gal-3 deficiency accelerates high-fat diet-induced obesity and amplifies inflammation in adipose tissue and pancreatic islets. Diabetes 2013, 62, 1932–1944. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Rhodes, D.H.; Pini, M.; Akasheh, R.T.; Castellanos, K.J.; Cabay, R.J.; Cooper, D.; Perretti, M.; Fantuzzi, G. Increased adiposity, dysregulated glucose metabolism and systemic inflammation in Gal-3 KO mice. PLoS ONE 2013, 8, e57915. [Google Scholar] [CrossRef] [PubMed]
- Darrow, A.L.; Shohet, R.V. Gal-3 deficiency exacerbates hyperglycemia and the endothelial response to diabetes. Cardiovasc. Diabetol. 2015, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, D.H.; Pini, M.; Castellanos, K.J.; Montero-Melendez, T.; Cooper, D.; Perretti, M.; Fantuzzi, G. Adipose Tissue-Specific Modulation of Galectin Expression in Lean and Obese Mice: Evidence for Regulatory Function. Obesity 2013, 21, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, G.; Pricci, F.; Iacobini, C.; Leto, G.; Amadio, L.; Barsotti, P.; Frigeri, L.; Hsu, D.K.; Vlassara, H.; Liu, F.T.; et al. Accelerated diabetic glomerulopathy in Gal-3/AGE receptor-3 knockout mice. FASEB J. 2001, 15, 2471–2479. [Google Scholar] [CrossRef] [PubMed]
- Iacobini, C.; Menini, S.; Oddi, G.; Ricci, C.; Amadio, L.; Pricci, F.; Olivieri, A.; Sorcini, M.; Di Mario, U.; Pesce, C.; et al. Gal-3/AGE-receptor 3 knockout mice show accelerated AGE-induced glomerular injury. Evidence for a protective role of Gal-3 as an AGE-receptor. FASEB J. 2004, 18, 1773–1775. [Google Scholar] [CrossRef] [PubMed]
- Lebovic, D.I.; Mueller, M.D.; Taylor, R.N. Immunobiology of endometriosis. Fertil. Steril. 2001, 75, 1–10. [Google Scholar] [CrossRef]
- Omwandho, C.O.; Konrad, L.; Halis, G.; Oehmke, F.; Tinneberg, H.R. Role of TGF-betas in normal human endometrium and endometriosis. Hum. Reprod. 2010, 25, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Noël, J.C.; Chapron, C.; Borghese, B.; Fayt, I.; Anaf, V. Gal-3 is over- expressed in various forms of endometriosis. Appl. Immunohistochem. Mol. Morphol. 2011, 19, 253–257. [Google Scholar] [PubMed]
- Caserta, D.; Di Benedetto, L.; Bordi, G.; D’Ambrosio, A.; Moscarini, M. Levels of Gal-3 and stimulation expressed gene 2 in the peritoneal fluid of women with endometriosis: A pilot study. Gynecol. Endocrinol. 2014, 30, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Liao, F.; Lin, T.N.; Liu, F.T. Galectins and neuroinflammation. Adv. Neurobiol. 2014, 9, 517–542. [Google Scholar] [PubMed]
- Borghese, B.; Vaiman, D.; Mondon, F.; Mbaye, M.; Anaf, V.; Noël, J.C.; de Ziegler, D.; Chapron, C. Neurotrophins and pain in endometriosis. Gynecol. Obstet. Fertil. 2010, 38, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B. The Enteric Nervous System; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 35–38. [Google Scholar]
- Young, E. Gut instincts: The secrets of your second brain. New Sci. 2012, 216, 38–42. [Google Scholar] [CrossRef]
- Gershon, M. The Second Brain: The Scientific Basis of Gut Instinct and a Groundbreaking New Understanding of Nervous Disorders of the Stomach and Intestines; Harper Collins: New York, NY, USA, 1998. [Google Scholar]
- Jiang, K.; Rankin, C.R.; Nava, P.; Sumagin, R.; Kamekura, R.; Stowell, S.R.; Feng, M.; Parkos, C.A.; Nusrat, A. Gal-3 regulates desmoglein-2 and intestinal epithelial intercellular adhesion. J. Biol. Chem. 2014, 289, 10510–10517. [Google Scholar] [CrossRef] [PubMed]
- Delacour, D.; Koch, A.; Ackermann, W.; Eude-Le Parco, I.; Elsässer, H.P.; Poirier, F.; Jacob, R. Loss of Gal-3 impairs membrane polarisation of mouse enterocytes in vivo. J. Cell Sci. 2008, 121, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Dudas, S.P.; Yunker, C.K.; Sternberg, L.R.; Byrd, J.C.; Bresalier, R.S. Expression of human intestinal mucin is modulated by the beta-galactoside binding protein Gal-3 in colon cancer. Gastroenterology 2002, 123, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Lippert, E.; Gunckel, M.; Brenmoehl, J.; Bataille, F.; Falk, W.; Scholmerich, J.; Obermeier, F.; Rogler, G. Regulation of Gal-3 function in mucosal fibroblasts: Potential role in mucosal inflammation. Clin. Exp. Immunol. 2008, 152, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Boza-Serrano, A.; Turesson, M.F.; Deierborg, T.; Ekblad, E.; Voss, U. Gal-3 causes enteric neuronal loss in mice after left sided permanent middle cerebral artery occlusion, a model of stroke. Sci. Rep. 2016, 6, 32893. [Google Scholar] [CrossRef] [PubMed]
- He, X.-W.; Li, W.L.; Li, C.; Liu, P.; Shen, Y.G.; Zhu, M.; Jin, X.P. Serum levels of galectin-1, galectin-3, and galectin-9 are associated with large artery atherosclerotic stroke. Sci Rep. 2017, 7, 40994. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, L.A.; Millet, V.; Hoyos, H.C.; Giannoni, J.P.; Croci, D.O.; Marder, M.; Liu, F.T.; Rabinovich, G.A.; Pasquini, J.M. Galectin-3 drives oligodendrocyte differentiation to control myelin integrity and function. Cell Death Differ. 2011, 18, 1746–1756. [Google Scholar] [CrossRef] [PubMed]
- Comte, I.; Kim, Y.; Young, C.C.; van der Harg, J.M.; Hockberger, P.; Bolam, P.J.; Poirier, F.; Szele, F.G. Galectin-3 maintains cell motility from the subventricular zone to the olfactory bulb. J. Cell Sci. 2011, 124, 2438–2447. [Google Scholar] [CrossRef] [PubMed]
- Jaquenod De Giusti, C.; Alberdi, L.; Frik, J.; Ferrer, M.F.; Scharrig, E.; Schattner, M.; Gomez, R.M. Gal-3 is upregulated in activated glia during Junin virus-induced murine encephalitis. Neurosci. Lett. 2011, 501, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Niwa, M.; Hoshi, M.; Saito, K.; Hisamatsu, K.; Hatano, Y.; Tomita, H.; Miyazaki, T.; Hara, A. Early microlesion of viral encephalitis confirmed by Gal-3 expression after a virus inoculation. Neurosci. Lett. 2015, 592, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, I.; Taniguchi, K.; Komatsu, F.; Sasaki, A.; Andoh, T.; Nojima, H.; Shiraki, K.; Hsu, D.K.; Liu, F.T.; Kato, I.; et al. Contribution of spinal galectin-3 to acute herpetic allodynia in mice. Pain 2012, 153, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Insel, P.A.; Murray, F.; Yokoyama, U.; Romano, S.; Yun, H.; Brown, L.; Snead, A.; Lu, D.; Aroonsakool, N. cAMP and Epac in the regulation of tissue fibrosis. Br. J. Pharmacol. 2012, 166, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Speca, S.; Giusti, I.; Rieder, F.; Latella, G. Cellular and molecular mechanisms of intestinal fibrosis. World J. Gastroenterol. 2012, 18, 3635–3661. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L.; Sheppard, D.; Duffield, J.S.; Violette, S. Therapy for fibrotic diseases: Nearing the starting line. Sci. Transl. Med. 2013, 5, 167sr1. [Google Scholar] [CrossRef] [PubMed]
- Henderson, N.C.; Mackinnon, A.C.; Rooney, C.; Sethi, T. Gal-3: A Central Regulator of Chronic Inflammation and Tissue Fibrosis. In Galectins and Disease Implications for Targeted Therapeutics; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2012; Chapter 22; Volume 1115, pp. 377–390. [Google Scholar]
- Henderson, N.C.; Mackinnon, A.C.; Farnworth, S.L.; Poirier, F.; Russo, F.P.; Iredale, J.P.; Haslett, C.; Simpson, K.J.; Sethi, T. Gal-3 regulates myofibroblast activation and hepatic fibrosis. Proc. Natl. Acad. Sci. USA 2006, 103, 5060–5065. [Google Scholar] [CrossRef] [PubMed]
- Henderson, N.C.; Mackinnon, A.C.; Farnworth, S.L.; Kipari, T.; Haslett, C.; Iredale, J.P.; Liu, F.T.; Hughes, J.; Sethi, T. Gal-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am. J. Pathol. 2008, 172, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Dang, Z.; MacKinnon, A.; Marson, L.P.; Sethi, T. Tubular atrophy and interstitial fibrosis after renal transplantation is dependent on Gal-3. Transplantation 2012, 93, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Mackinnon, A.C.; Gibbons, M.A.; Farnworth, S.L.; Leffler, H.; Nilsson, U.J.; Delaine, T.; Simpson, A.J.; Forbes, S.J.; Hirani, N.; Gauldie, J.; et al. Regulation of transforming growth factor-beta1-driven lung fibrosis by Gal-3. Am. J. Respir. Crit. Care Med. 2012, 185, 537–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishi, Y.; Sano, H.; Kawashima, T.; Okada, T.; Kuroda, T.; Kikkawa, K.; Kawashima, S.; Tanabe, M.; Goto, T.; Matsuzawa, Y.; et al. Role of Gal-3 in human pulmonary fibrosis. Allergol. Int. 2007, 56, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Thandavarayan, R.A.; Watanabe, K.; Ma, M.; Veeraveedu, P.T.; Gurusamy, N.; Palaniyandi, S.S.; Zhang, S.; Muslin, A.J.; Kodama, M.; Aizawa, Y. 14-3-3 Protein regulates Ask1 signaling and protects against diabetic cardiomyopathy. Biochem. Pharmacol. 2008, 75, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Rhaleb, N.E.; Pokharel, S.; Harding, P.; Rasoul, S.; Peng, H.; Carretero, O.A. Novel anti-inflammatory mechanisms of Nacetyl-Ser-Asp-Lys-Pro in hypertension-induced target organ damage. Am. J. Physiol. 2008, 294, H1226–H1232. [Google Scholar]
- Burguillos, M.A.; Svensson, M.; Schulte, T.; Boza-Serrano, A.; Garcia-Quintanilla, A.; Kavanagh, E.; Santiago, M.; Viceconte, N.; Oliva-Martin, M.J.; Osman, A.M.; et al. Microglia-Secreted Gal-3 Acts as a Toll-like Receptor 4 Ligand and Contributes to Microglial Activation. Cell Rep. 2015, 10, 1626–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taniguchi, T.; Asano, Y.; Akamata, K.; Noda, S.; Masui, Y.; Yamada, D.; Takahashi, T.; Ichimura, Y.; Toyama, T.; Tamaki, Z.; et al. Serum Levels of Gal-3: Possible Association with Fibrosis, Aberrant Angiogenesis, and Immune Activation in Patients with Systemic Sclerosis. J. Rheumatol. 2012, 39, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Koca, S.S.; Akbas, F.; Ozgen, M.; Yolbas, S.; Ilhan, N.; Gundogdu, B.; Isik, A. Serum Gal-3 level in systemic sclerosis. Clin. Rheumatol. 2014, 33, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Fichtner-Feigl, S.; Strober, W.; Kawakami, K.; Puri, R.K.; Kitani, A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat. Med. 2006, 12, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Jin, H.; Ullenbruch, M.; Hu, B.; Hashimoto, N.; Moore, B.; McKenzie, A.; Lukacs, N.W.; Phan, S.H. Regulation of found in inflammatory zone 1 expression in bleomycin-induced lung fibrosis: Role of IL-4/IL-13 and mediation via STAT-6. J. Immunol. 2004, 173, 3425–3431. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat. Rev. Immunol. 2004, 4, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Faludi, R.; Nagy, G.; Tőkés-Füzesi, M.; Kovács, K.; Czirják, L.; Komócsi, A. Gal-3 is an independent predictor of survival in systemic sclerosis. Int. J. Cardiol. 2017, 233, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Vray, B.; Camby, I.; Vercruysse, V.; Mijatovic, T.; Bovin, N.V.; Ricciardi-Castagnoli, P.; Kaltner, H.; Salmon, I.; Gabius, H.J.; Kiss, R. Up-regulation of Gal-3 and its ligands by Trypanosoma cruzi infection with modulation of adhesion and migration of murine dendritic cells. Glycobiology 2004, 14, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Fogel, S.; Guittaut, M.; Legrand, A.; Monsigny, M.; Hebert, E. The tat protein of HIV-1 induces galectin-3 expression. Glycobiology 1999, 9, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.; Hammes, S.R.; Kuwabara, I.; Greene, W.C.; Liu, F.T. Human T lymphotropic virus-I infection of human T lymphocytes induces expression of the beta- galactoside-binding lectin, Gal-3. Am. J. Pathol. 1996, 148, 1661–1670. [Google Scholar] [PubMed]
- Fowler, M.; Thomas, R.J.; Atherton, J.; Roberts, I.S.; High, N.J. Gal-3 binds to Helicobacter pylori O-antigen: It is upregulated and rapidly secreted by gastric epithelial cells in response to H. pylori adhesion. Cell. Microbiol. 2006, 8, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Park, A.M.; Hagiwara, S.; Hsu, D.K.; Liu, F.T.; Yoshie, O. Gal-3 Plays an important role in innate immunity to gastric infection by helicobacter pylori. Infect. Immun. 2016, 84, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Subhash, V.V.; Ling, S.S.; Ho, B. Extracellular galectin-3 counteracts adhesion and exhibits chemoattraction in Helicobacter pylori infected gastric cancer cells. Microbiology 2016, 162, 1360–1366. [Google Scholar] [CrossRef] [PubMed]
- Parsonnet, J.; Friedman, G.D.; Vandersteen, D.P.; Chang, Y.; Vogelman, J.H.; Orentreich, N.; Sibley, R.K. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 1991, 325, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Uemura, N.; Okamoto, S.; Yamamoto, S.; Matsumura, N.; Yamaguchi, S.; Yamakido, M.; Taniyama, K.; Sasaki, N.; Schlemper, R.J. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 2001, 345, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Subhash, V.V.; Ho, B. Galectin 3 acts as an enhancer of survival responses in H. pylori-infected gastric cancer cells. Cell Biol. Toxicol. 2016, 32, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; D’Ambrosio, M.; Liao, T.D.; Peng, H.; Rhaleb, N.E.; Sharma, U.; André, S.; Gabius, H.J.; Carretero, O.A. N-acetyl-seryl-aspartyllysyl-proline prevents cardiac remodeling and dysfunction induced by Gal-3, a mammalian adhesion/growth-regulatory lectin. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H404–H412. [Google Scholar] [CrossRef] [PubMed]
- Creemers, E.E.; Pinto, Y.M. Molecular mechanisms that control interstitial fibrosis in the pressure overloaded heart. Cardiovasc. Res. 2011, 89, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Kramer, F. Galectin-3: Clinical utility and prognostic value in patients with heart failure. Res. Rep. Clin. Cardiol. 2013, 4, 13–22. [Google Scholar] [CrossRef]
- Shah, R.V.; Chen-Tournoux, A.A.; Picard, M.H.; van Kimmenade, R.R.; Januzzi, J.L. Galectin-3, cardiac structure and function, and long-term mortality in patients with acutely decompensated heart failure. Eur. J. Heart Fail. 2010, 12, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Gullestad, L.; Ueland, T.; Kjekshus, J.; Nymo, S.H.; Hulthe, J.; Muntendam, P.; Adourian, A.; Böhm, M.; van Veldhuisen, D.J.; Komajda, M.; et al. Galectin-3 predicts response to statin therapy in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA). Eur. Heart J. 2012, 33, 2290–2296. [Google Scholar] [CrossRef] [PubMed]
- Stolen, C.M.; Adourian, A.; Meyer, T.E.; Stein, K.M.; Solomon, S.D. Plasma Galectin-3 and Heart Failure Outcomes in MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy). J. Card. Fail. 2014, 20, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Milting, H.; Ellinghaus, P.; Seewald, M.; Cakar, H.; Bohms, B.; Kassner, A.; Körfer, R.; Klein, M.; Krahn, T.; Kruska, L.; et al. Plasma biomarkers of myocardial fibrosis and remodeling in terminal heart failure patients supported by mechanical circulatory support devices. J. Heart Lung Transplant. 2008, 27, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Erkilet, G.; Özpeker, C.; Böthig, D.; Kramer, F.; Röfe, D.; Bohms, B.; Morshuis, M.; Gummert, J.; Milting, H. The biomarker plasma galectin-3 in advanced heart failure and survival with mechanical circulatory support devices. J. Heart Lung Transplant. 2013, 32, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Grandin, E.W.; Jarolim, P.; Murphy, S.A.; Ritterova, L.; Cannon, C.P.; Braunwald, E.; Morrow, D.A. Galectin-3 and the development of heart failure after acute coronary syndrome: Pilot experience from PROVE IT-TIMI 22. Clin. Chem. 2012, 58, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Lok, D.J.; Lok, S.I.; Bruggink-André de la Porte, P.W.; Badings, E.; Lipsic, E.; van Wijngaarden, J.; de Boer, R.A.; van Veldhuisen, D.J.; van der Meer, P. Galectin-3 is an independent marker for ventricular remodeling and mortality in patients with chronic heart failure. Clin. Res. Cardiol. 2013, 102, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Peacock, W.F.; Di Somma, S. Emergency department use of Galectin-3. Crit. Pathw. Cardiol. 2014, 13, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Teichman, S.L.; Maisel, A.S.; Storrow, A.B. Challenges in acute heart failure clinical management: Optimizing care despite incomplete evidence and imperfect drugs. Crit. Pathw. Cardiol. 2015, 14, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013, 128, e240–e327. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J. Am. Coll. Cardiol. 2017, 70, 776–803. [Google Scholar] [PubMed]
- Wang, S.F.; Tsao, C.H.; Lin, Y.T.; Hsu, D.K.; Chiang, M.L.; Lo, C.H.; Chien, F.C.; Chen, P.; Arthur Chen, Y.M.; Chen, H.Y.; et al. Galectin-3 promotes HIV-1 budding via association with Alix and Gag p6. Glycobiology 2014, 24, 1022–1035. [Google Scholar] [CrossRef] [PubMed]
- Abbas, W.; Herbein, G. Plasma membrane signaling in HIV-1 infection. Biochim. Biophys. Acta 2014, 1838, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.C.; Ushijima, H.; Theis, C.; Sève, A.P.; Hubert, J.; Müller, W.E.G. Expression of nuclear lectin carbohydrate-binding protein 35 in human immunodeficiency virus type 1-infected molt-3 cells. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1995, 9, 340–348. [Google Scholar] [PubMed]
- Ouellet, M.; Mercier, S.; Pelletier, I.; Bounou, S.; Roy, J.; Hirabayashi, J.; Sato, S.; Tremblay, M.J. Galectin-1 Acts as a Soluble Host Factor That Promotes HIV-1 Infectivity through Stabilization of Virus Attachment to Host Cells. J. Immunol. 2005, 174, 4120–4126. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Fu, C.; Cong, Z.; Peng, L.; Peng, Z.; Chen, T.; Wang, W.; Jiang, H.; Wei, Q.; Qin, C. Galectin-3 promotes caspase-independent cell death of HIV-1-infected macrophages. FEBS J. 2017, 284, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Dabelic, S.; Supraha, S.; Dumic, J. Gal-3 in macrophage-like cells exposed to immunomodulatory drugs. Biochim. Biophys. Acta 2006, 1760, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Sano, H.; Hsu, D.K.; Yu, L.; Apgar, J.R.; Kuwabara, I.; Yamanaka, T.; Hirashima, M.; Liu, F.T. Human Gal-3 is a novel chemoattractant for monocytes and macrophages. J. Immunol. 2000, 165, 2156–2164. [Google Scholar] [CrossRef] [PubMed]
- Swarte, V.V.; Mebius, R.E.; Joziasse, D.H.; Van den Eijnden, D.H.; Kraal, G. Lymphocyte triggering via L-selectin leads to enhanced Gal-3-mediated binding to dendritic cells. Eur. J. Immunol. 1998, 28, 2864–2871. [Google Scholar] [CrossRef]
- Truong, M.J.; Gruart, V.; Liu, F.T.; Prin, L.; Capron, A.; Capron, M. IgE-binding molecules (Mac-2/epsilon BP) expressed by human eosinophils. Implication in IgE-dependent eosinophil cytotoxicity. Eur. J. Immunol. 1993, 23, 3230–3235. [Google Scholar] [CrossRef] [PubMed]
- Frigeri, L.G.; Liu, F.T. Surface expression of function- al IgE binding protein, an endogenous lectin, on mast cells and macrophages. J. Immunol. 1992, 148, 861–867. [Google Scholar] [PubMed]
- Tsuboi, S.; Sutoh, M.; Hatakeyama, S.; Hiraoka, N.; Habuchi, T.; Horikawa, Y.; Hashimoto, Y.; Yoneyama, T.; Mori, K.; Koie, T.; et al. A novel strategy for evasion of NK cell immunity by tumours expressing core2 O-glycans. EMBO J. 2011, 30, 3173–3185. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Wang, H.Y.; Miyahara, Y.; Peng, G.; Wang, R.F. Tumor-associated Gal-3 modulates the function of tumor-reactive T cells. Cancer Res. 2008, 68, 7228–7236. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Rodriguez, E.V.; Montes, C.L.; Motran, C.C.; Zuniga, E.I.; Liu, F.T.; Rabinovich, G.A.; Gruppi, A. Gal-3 mediates IL-4-induced survival and differentiation of B cells: Functional cross-talk and implications during Trypanosoma cruzi infection. J. Immunol. 2004, 172, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.J.; Strasser, A.; Metcalf, D. Selective up-regulation of macrophage function in granulocyte-macrophage colony-stimulating factor transgenic mice. J. Immunol. 1991, 147, 2957–2963. [Google Scholar] [PubMed]
- Rabinovich, G.A.; Toscano, M.A. Turning ‘sweet’ on immunity: Galectin–glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 2009, 9, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Thiemann, S.; Baum, L.G. Galectins and immune responses-just how do they do those things they do? Annu. Rev. Immunol. 2016, 34, 243–264. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Liu, F.T.; Yang, R.Y. Roles of Gal-3 in immune responses. Arch. Immunol. Ther. Exp. 2005, 53, 497–504. [Google Scholar]
- Kuwabara, I.; Liu, F.T. Gal-3 promotes adhesion of human neutrophils to laminin. J. Immunol. 1996, 156, 3939–3944. [Google Scholar] [PubMed]
- Frigeri, L.G.; Zuberi, R.I.; Liu, F.T. Epsilon BP, a beta-galactoside-binding animal lectin, recognizes IgE receptor (Fc epsilon RI) and activates mast cells. Biochemistry 1993, 32, 7644–7649. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.T.; Hsu, D.K.; Zuberi, R.I.; Kuwabara, I.; Chi, E.Y.; Henderson, W.R., Jr. Expression and function of Gal-3, a beta-galactoside binding lectin, in human monocytes and macrophages. Am. J. Pathol. 1995, 147, 1016–1028. [Google Scholar] [PubMed]
- Dietz, A.B.; Bulur, P.A.; Knutson, G.J.; Matasic, R.; Vuk-Pavlovic, S. Maturation of human monocyte-derived dendritic cells studied by microarray hybridization. Biochem. Biophys. Res. Commun. 2000, 275, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Helming, L.; Gordon, S. Macrophage fusion induced by IL-4 alternative activation is a multistage process involving multiple target molecules. Eur. J. Immunol. 2007, 37, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Ouellet, N.; Pelletier, I.; Simard, M.; Rancourt, A.; Bergeron, M.G. Role of Gal-3 as an adhesion molecule for neutrophil extravasation during streptococcal pneumonia. J. Immunol. 2002, 168, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Kohatsu, L.; Hsu, D.K.; Jegalian, A.G.; Liu, F.T.; Baum, L.G. Gal-3 induces death of Candida species expressing specific beta-1,2-linked mannans. J. Immunol. 2006, 177, 4718–4726. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Mizuno, K.; Horio, T. Monocyte derived multinucleated giant cells and sarcoidosis. J. Dermatol. Sci. 2003, 31, 119–128. [Google Scholar] [CrossRef]
- Friedman, S.L. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J. Biol. Chem. 2000, 275, 2247–2250. [Google Scholar] [CrossRef] [PubMed]
- Duffield, J.S.; Forbes, S.J.; Constandinou, C.M.; Clay, S.; Partolina, M.; Vuthoori, S.; Wu, S.; Lang, R.; Iredale, J.P. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J. Clin. Investig. 2005, 115, 56–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inohara, H.; Akahani, S.; Raz, A. Gal-3 stimulates cell proliferation. Exp. Cell Res. 1998, 245, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, D.B.; Kawada, N.; Imamura, K.; Miyamoto, Y.; Tateno, C.; Seki, S.; Kuroki, T.; Yoshizato, K. Proteome analysis of rat hepatic stellate cells. Hepatology 2000, 32, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Kasper, M.; Hughes, R.C. Immunocytochemical evidence for a modulation of galectin 3 (Mac-2), a carbohydrate binding protein, in pulmonary fibrosis. J. Pathol. 1996, 179, 309–316. [Google Scholar] [CrossRef]
- Ho, J.E.; Gao, W.; Levy, D.; Santhanakrishnan, R.; Araki, T.; Rosas, I.O.; Hatabu, H.; Latourelle, J.C.; Nishino, M.; Dupuis, J.; et al. Galectin-3 Is Associated with Restrictive Lung Disease and Interstitial Lung Abnormalities. Am. J. Respir. Crit. Care Med. 2016, 194, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Piguet, P.F.; Collart, M.A.; Grau, G.E.; Kapanci, Y.; Vassalli, P. Tumor necrosis factor/cachectin plays a key role in bleomycin-induced pneumopathy and fibrosis. J. Exp. Med. 1989, 170, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Piguet, P.F.; Ribaux, C.; Karpuz, V.; Grau, G.E.; Kapanci, Y. Expression and localization of tumor necrosis factor-alpha and its mRNA in idiopathic pulmonary fibrosis. Am. J. Pathol. 1993, 143, 651–655. [Google Scholar] [PubMed]
- Turner-warwick, M. Precapillary systemic-pulmonary anastomoses. Thorax 1963, 18, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Nangia-Makker, P.; Honjo, Y.; Sarvis, R.; Akahani, S.; Hogan, V.; Pienta, K.J.; Raz, A. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am. J. Pathol. 2000, 156, 899–909. [Google Scholar] [CrossRef]
- Harjacek, M.; Diaz-Cano, S.; De Miguel, M.; Wolfe, H.; Maldonado, C.A.; Rabinovich, G.A. Expression of galectins-1 and -3 correlates with defective mononuclear cell apoptosis in patients with juvenile idiopathic arthritis. J. Rheumatol. 2001, 28, 1914–1922. [Google Scholar] [PubMed]
- Ezzat, M.H.; El-Gammasy, T.M.; Shaheen, K.Y.; Osman, A.O. Elevated production of Gal-3 is correlated with juvenile idiopathic arthritis disease activity, severity, and progression. Int. J. Rheum. Dis. 2011, 14, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Bullock, S.L.; Johnson, T.M.; Bao, Q.; Hughes, R.C.; Winyard, P.J.; Woolf, A.S. Gal-3 modulates ureteric bud branching in organ culture of the developing mouse kidney. J. Am. Soc. Nephrol. 2001, 12, 515–523. [Google Scholar] [PubMed]
- Bichara, M.; Attmane-Elakeb, A.; Brown, D.; Essig, M.; Karim, Z.; Muffat-Joly, M.; Micheli, L.; Eude-Le Parco, I.; Cluzeaud, F.; Peuchmaur, M.; et al. Exploring the role of galectin 3 in kidney function: A genetic approach. Glycobiology 2006, 16, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Z.; Kuo, P.L. The Role of Gal-3 in the Kidneys. Int. J. Mol. Sci. 2016, 17, 565. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, J.; Kobayashi, S.; Ishida, A.; Nakabayashi, I.; Tajima, O.; Miura, S.; Katayama, M.; Nogami, H. Up-regulation of Gal-3 in acute renal failure of the rat. Am. J. Pathol. 2000, 157, 815–823. [Google Scholar] [CrossRef]
- Sasaki, S.; Bao, Q.; Hughes, R.C. Gal-3 modulates rat mesangial cell proliferation and matrix synthesis during experimental glomerulonephritis induced by anti-Thy1.1 antibodies. J. Pathol. 1999, 187, 481–489. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Kobayashi, S.; Hemmi, N.; Ikee, R.; Hyodo, N.; Saigusa, T.; Namikoshi, T.; Yamada, M.; Suzuki, S.; Miura, S. Gal-3-positive cell infiltration in human diabetic nephropathy. Nephrol. Dial. Transplant. 2004, 19, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Winkelmann, B.R.; März, W.; Boehm, B.O.; Zotz, R.; Hager, J.; Hellstern, P.; Senges, J.; LURIC Study Group (LUdwigshafen RIsk and Cardiovascular Health). Rationale and design of the LURIC study-a resource for functional genomics, pharmacogenomics and long-term prognosis of cardiovascular disease. Pharmacogenomics 2001, 2 (Suppl. 1), S1–S73. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C.; Krane, V.; März, W.; Olschewski, M.; Asmus, H.G.; Krämer, W.; Kühn, K.W.; Kütemeyer, H.; Mann, J.F.; Ruf, G.; et al. Randomized controlled trial on the efficacy and safety of atorvastatin in patients with type 2 diabetes on hemodialysis (4D study): Demographic and baseline characteristics. Kidney Blood Press. Res. 2004, 27, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Drechsler, C.; Delgado, G.; Wanner, C.; Blouin, K.; Pilz, S.; Tomaschitz, A.; Kleber, M.E.; Dressel, A.; Willmes, C.; Krane, V.; et al. Gal-3, Renal Function, and Clinical Outcomes: Results from the LURIC and 4D Studies. J. Am. Soc. Nephrol. 2015, 26, 2213–2221. [Google Scholar] [CrossRef] [PubMed]
- Kolatsi-Joannou, M.; Price, K.L.; Winyard, P.J.; Long, D.A. Modified citrus pectin reduces Gal-3 expression and disease severity in experimental acute kidney injury. PLoS ONE 2011, 6, e18683. [Google Scholar] [CrossRef] [PubMed]
- La Jolla Begins Phase IIb Trial of GCS-100 to Treat Advanced CKD. Available online: http://www.drugdevelopment-technology.com/news/newsla-jolla-begins-phase-iib-trial-of-gcs-100-totreat-advanced-ckd-4535972 (accessed on 22 December 2017).
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Friess, H.; Zhu, Z.; Frigeri, L.; Zimmermann, A.; Korc, M.; Berberat, P.O.; Buchler, M.W. Galectin-1 and galectin-3 in chronic pancreatitis. Lab. Investig. 2000, 80, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L.; Roll, F.J.; Boyles, J.; Bissell, D.M. Hepatic lipocytes: The principal collagen-producing cells of normal rat liver. Proc. Natl. Acad. Sci. USA 1985, 82, 8681–8685. [Google Scholar] [CrossRef] [PubMed]
- Galectin Therapeutics Announces Results from Phase 2b NASH-CX Trial. Available online: http://investor.galectintherapeutics.com/news-releases/news-release-details/galectin-therapeutics-announces-results-phase-2b-nash-cx-trial (accessed on 22 December 2017).
- Djoussé, L.; Matsumoto, C.; Petrone, A.; Weir, N.L.; Tsai, M.Y.; Gaziano, J.M. Plasma galectin 3 and heart failure risk in the Physicians’ Health Study. Eur. J. Heart Fail. 2014, 16, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Fukusato, T. Histopathology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J. Gastroenterol. 2014, 20, 15539–15548. [Google Scholar] [CrossRef] [PubMed]
- Li, L.C.; Li, J.; Gao, J. Functions of Galectin-3 and Its Role in Fibrotic Diseases. J. Pharmacol. Exp. Ther. 2014, 351, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Iacobini, C.; Menini, S.; Ricci, C.; Blasetti Fantauzzi, C.; Scipioni, A.; Salvi, L.; Cordone, S.; Delucchi, F.; Serino, M.; Federici, M.; et al. Gal-3 ablation protects mice from diet-induced NASH: A major scavenging role for Gal-3 in liver. J. Hepatol. 2011, 54, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Nomoto, K.; Tsuneyama, K.; Abdel Aziz, H.O.; Takahashi, H.; Murai, Y.; Cui, Z.G.; Fujimoto, M.; Kato, I.; Hiraga, K.; Hsu, D.K.; et al. Disrupted Gal-3 causes non-alcoholic fatty liver disease in male mice. J. Pathol. 2006, 210, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Jeftic, I.; Jovicic, N.; Pantic, J.; Arsenijevic, N.; Lukic, M.L.; Pejnovic, N. Gal-3 ablation enhances liver steatosis, but attenuates Inflammation and IL-33-dependent fibrosis in obesogenic mouse model of nonalcoholic steatohepatitis. Mol. Med. 2015, 21, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Traber, P.G.; Zomer, E. Therapy of experimental NASH and fibrosis with galectin inhibitors. PLoS ONE 2013, 8, e83481. [Google Scholar] [CrossRef] [PubMed]
- Traber, P.G.; Chou, H.; Zomer, E.; Hong, F.; Klyosov, A.; Fiel, M.I.; Friedman, S.L. Regression of fibrosis and reversal of cirrhosis in rats by galectin inhibitors in thioacetamide-induced liver disease. PLoS ONE 2013, 8, e75361. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Marri, S.R.; Chalasani, N.; Kohli, R.; Aronstein, W.; Thompson, G.A.; Irish, W.; Miles, M.V.; Xanthakos, S.A.; Lawitz, E.; et al. Randomised clinical study: GR-MD-02, a Gal-3 inhibitor, vs. placebo in patients having non-alcoholic steatohepatitis with advanced fibrosis. Aliment. Pharmacol. Ther. 2016, 44, 1183–1198. [Google Scholar] [CrossRef] [PubMed]
- Galectin Tanks on Failure of NASH Treatment (GALT). Available online: https://www.investopedia.com/news/galectin-tanks-failure-nash-treatment-galt/ (accessed on 22 December 2017).
- Results from the NASH-FX Study Underscore the Importance of Completing the NASH-CX Clinical Trial for Patients with NASH Cirrhosis. Available online: http://perspectives.galectintherapeutics.com/importance-completing-nash-cx-trial-for-patients-with-nash-cirrhosis/ (accessed on 22 December 2017).
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Van Greevenbroek, M.M.; Schalkwijk, C.G.; Stehouwer, C.D. Obesity-associated low-grade inflammation in type 2 diabetes mellitus: Causes and consequences. Neth. J. Med. 2013, 71, 174–187. [Google Scholar] [PubMed]
- Xu, L.; Kitade, H.; Ni, Y.; Ota, T. Roles of chemokines and chemokine receptors in obesity-associated insulin resistance and nonalcoholic fatty liver disease. Biomolecules 2015, 5, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Herrero, L.; Shapiro, H.; Nayer, A.; Lee, J.; Shoelson, S.E. Inflammation and adipose tissue macrophages in lipodystrophic mice. Proc. Natl. Acad. Sci. USA 2010, 107, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Chiang, M.T.; Yet, S.F.; Chau, L.Y. Myeloid heme oxygenase-1 haploin- sufficiency reduces high fat diet-induced insulin resistance by affecting adipose macrophage infiltration in mice. PLoS ONE 2012, 7, e38626. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Del Proposto, J.B.; Westcott, D.J.; Saltiel, A.R. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes 2008, 57, 3239–3246. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, S.; Lu, M.; Bandyopadhyay, G.; Oh, D.; Imamura, T.; Johnson, A.M.F.; Sears, D.; Shen, Z.; Cui, B.; et al. Hematopoietic-derived Galectin-3 Causes Cellular and Systemic Insulin Resistance. Cell 2016, 167, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Rudan, I.; Tomaskovic, L.; Boschi-Pinto, C.H.C. Global estimate of the incidence of clinical pneumonia among children under five years of age. Bull. World Health Org. 2004, 82, 895–903. [Google Scholar] [PubMed]
- Farnworth, S.L.; Henderson, N.C.; Mackinnon, A.C.; Atkinson, K.M.; Wilkinson, T.; Dhaliwal, K.; Hayashi, K.; Simpson, A.J.; Rossi, A.G.; Haslett, C.; et al. Galectin-3 Reduces the Severity of Pneumococcal Pneumonia by Augmenting Neutrophil Function. Am. J. Pathol. 2008, 172, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.D.; Souza, P.R.; Flak, M.B.; Thedchanamoorthy, P.; Norling, L.V.; Cooper, D. Galectin-3–null mice display defective neutrophil clearance during acute inflammation. J. Leukoc. Biol. 2017, 101, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Morrell, N.W.; Adnot, S.; Archer, S.L.; Dupuis, J.; Jones, P.L.; MacLean, M.R.; McMurtry, I.F.; Stenmark, K.R.; Thistlethwaite, P.A.; Weissmann, N.; et al. Cellular and Molecular Basis of Pulmonary Arterial Hypertension. J. Am. Coll. Cardiol. 2009, 54 (Suppl. 1), S20–S31. [Google Scholar] [CrossRef] [PubMed]
- Markowska, A.I.; Liu, F.T.; Panjwani, N. Galectin-3 is an important mediator of VEGF- and bFGF-mediated angiogenic response. J. Exp. Med. 2010, 207, 1981–1993. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, X.; Liu, B.; Li, T.; Yu, Z. Galectin-3: A potential biomarker in pulmonary arterial hypertension. Cardiol. Plus 2016, 1, 14–20. [Google Scholar]
- Calvier, L.; Legchenko, E.; Grimm, L.; Sallmon, H.; Hatch, A.; Plouffe, B.D.; Schroeder, C.; Bauersachs, J.; Murthy, S.K.; Hansmann, G. Galectin-3 and aldosterone as potential tandem biomarkers in pulmonary arterial hypertension. Heart 2016, 102, 390–396. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, X.; Luo, H.; Li, T.; Zhao, L.; Qi, Q.; Liu, Y.; Yu, Z. Galectin-3 mediates the pulmonary arterial hypertension-induced right ventricular remodeling through interacting with NADPH oxidase 4. J. Am. Soc. Hypertens. 2017, 11, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Fenster, B.E.; Lasalvia, L.; Schroeder, J.D.; Smyser, J.; Silveira, L.J.; Buckner, J.K.; Brown, K.K. Galectin-3 levels are associated with right ventricular functional and morphologic changes in pulmonary arterial hypertension. Heart Vessels 2016, 31, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.E. Elevated Galectin-3 Level Predicts Pulmonary Artery Hypertension. Biol. Mark. Guid. Ther. 2016, 3, 89–97. [Google Scholar] [CrossRef]
- Berezin, A.E. Is elevated circulating galectin-3 level a predictor of pulmonary artery hypertension development and progression? Clin. Med. Biochem. 2016, 2, 114. [Google Scholar] [CrossRef]
- Barman, S.A.; Chen, F.; Su, Y.; Stepp, D.; Zhou, J.; Meadows, L.; Wang, Y.; Li, X.; Haigh, S.; Bordan, Z.; et al. Gal-3 mediates vascular remodeling in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2016, 193, A7918. [Google Scholar]
- Larsen, L.; Chen, H.-Y.; Saegusa, J.; Liu, F.T. Gal-3 and the skin. J. Dermatolol. Sci. 2011, 64, 85–91. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, H.; Perez-Gala, S.; Bonay, P.; Cruz-Adalia, A.; Cibrian, D.; Sanchez-Cuellar, S.; Dauden, E.; Fresno, M.; García-Diez, A.; Sanchez-Madrid, F. Psoriasis in humans is associated with down-regulation of galectins in dendritic cells. J. Pathol. 2012, 228, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Lacina, L.; Plzáková, Z.; Smetana, K., Jr.; Štork, J.; Kaltner, H.; André, S. Glycophenotype of Psoriatic Skin. Folia Biol. 2006, 52, 10–15. [Google Scholar]
- Voth, D.E.; Heinzen, R.A. Lounging in a lysosome: The intracellular lifestyle of Coxiella burnetii. Cell. Microbiol. 2007, 9, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Dupont, N.; Lafont, F. How autophagy regulates the host cell signaling associated with the postpartum bacteria cocoon experienced as a danger signal. Autophagy 2009, 5, 1222–1223. [Google Scholar] [CrossRef] [PubMed]
- Paz, I.; Sachse, M.; Dupont, N.; Mounier, J.; Cederfur, C.; Enninga, J.; Leffler, H.; Poirier, F.; Prevost, M.C.; Lafont, F.; et al. Gal-3, a marker for vacuole lysis by invasive pathogens. Cell. Microbiol. 2010, 12, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Thurston, T.L.M.; Wandel, M.P.; vonMuhlinen, N.; Foeglein, A.; Randow, F. Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature 2012, 482, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Southwick, F.S.; Purich, D.L. Listeria and Shigella actin-based motility in host cells. Trans. Am. Clin. Climatol. Assoc. 1998, 109, 160–173. [Google Scholar] [PubMed]
- Maejima, I.; Takahashi, A.; Omori, H.; Kimura, T.; Takabatake, Y.; Saitoh, T.; Yamamoto, A.; Hamasaki, M.; Noda, T.; Isaka, Y.; et al. Autophagy sequesters damaged lysosomes to control lysosomal biogenesis and kidney injury. EMBO J. 2013, 32, 2336–2347. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yu, Y.; Koehn, C.D.; Zhang, Z.; Su, K. Galectins in the pathogenesis of rheumatoid arthritis. J. Clin. Cell. Immunol. 2013, 4, 1000164. [Google Scholar] [CrossRef] [PubMed]
- Shou, J.; Bull, C.M.; Li, L.; Qian, H.R.; Wei, T.; Luo, S.; Perkins, D.; Solenberg, P.J.; Tan, S.L.; Chen, X.Y.; et al. Identification of blood biomarkers of rheumatoid arthritis by transcript profiling of peripheral blood mononuclear cells from the rat collagen induced arthritis model. Arthritis Res. Ther. 2006, 8, R28. [Google Scholar] [CrossRef] [PubMed]
- Forsman, H.; Islander, U.; Andreasson, E.; Andersson, A.; Onnheim, K.; Karlstrom, A.; Savman, K.; Magnusson, M.; Brown, K.L.; Karlsson, A. Galectin 3 aggravates joint inflammation and destruction in antigen-induced arthritis. Arthritis Rheum. 2011, 63, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, S.; Kuchen, S.; Seemayer, C.A.; Kyburz, D.; Hirt, A.; Klinzing, S.; Michel, B.A.; Gay, R.E.; Liu, F.T.; Gay, S.; et al. Galectin 3 and its binding protein in rheumatoid arthritis. Arthritis Rheum. 2003, 48, 2788–2795. [Google Scholar] [CrossRef] [PubMed]
- Neidhart, M.; Zaucke, F.; von Knoch, R.; Jungel, A.; Michel, B.A.; Gay, R.E.; Gay, S. Gal-3 is induced in rheumatoid arthritis synovial fibroblasts after adhesion to cartilage oligomeric. Ann. Rheum. Dis. 2005, 64, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Neidhart, M.; Rethage, J.; Kuchen, S.; Künzler, P.; Crowl, R.M.; Billingham, M.E.; Gay, R.E.; Gay, S. Retrotransposable L1 elements expressed in rheumatoid arthritis synovial tissue: Association with genomic DNA hypomethylation and influence on gene expression. Arthritis Rheum. 2000, 43, 2634–2647. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kang, S.W.; Song, J.K.; Park, J.J.; Bae, Y.D.; Lee, E.Y.; Lee, E.B.; Song, Y.W. Serum Gal-3 and Gal-3 binding protein levels in Behçet’s disease and their association with disease activity. Clin. Exp. Rheumatol. 2007, 25, S41–S45. [Google Scholar] [PubMed]
- Hu, C.Y.; Chang, S.K.; Wu, C.S.; Tsai, W.I.; Hsu, P.N. Gal-3 gene (LGALS3) +292C allele is a genetic predisposition factor for rheumatoid arthritis in Taiwan. Clin. Rheumatol. 2011, 30, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Neidhart, M.; Seemayer, C.A.; Hummel, K.M.; Michel, B.A.; Gay, R.E.; Gay, S. Functional characterization of adherent synovial fluid cells in rheumatoid arthritis: Destructive potential in vitro and in vivo. Arthritis Rheum. 2003, 4, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Dasuri, K.; Antonovici, M.; Chen, K.; Wong, K.; Standing, K.; Ens, W.; El-Gabalawy, H.; Wilkins, J.A. The synovial proteome: Analysis of fibroblast-like synoviocytes. Arthritis Res. Ther. 2004, 6, R161–R168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filer, A.; Bik, M.; Parsonage, G.N.; Fitton, J.; Trebilcock, E.; Howlett, K.; Cook, M.; Raza, K.; Simmons, D.L.; Thomas, A.M.; et al. Galectin 3 induces a distinctive pattern of cytokine and chemokine production in rheumatoid synovial fibroblasts via selective signaling pathways. Arthritis Rheum. 2009, 60, 1604–1614. [Google Scholar] [CrossRef] [PubMed]
- Stowell, S.R.; Qian, Y.; Karmakar, S.; Koyama, N.S.; Dias-Baruffi, M.; Leffler, H.; McEver, R.P.; Cummings, R.D. Differential roles of galectin-1 and Gal-3 in regulating leukocyte viability and cytokine secretion. J. Immunol. 2008, 180, 3091–4102. [Google Scholar] [CrossRef] [PubMed]
- Issa, S.F.; Duer, A.; Østergaard, M.; Hørslev-Petersen, K.; Hetland, M.L.; Hansen, M.S.; Junker, K.; Lindegaard, H.M.; Møller, J.M.; Junker, P. Increased galectin-3 may serve as a serologic signature of pre-rheumatoid arthritis while markers of synovitis and cartilage do not differ between early undifferentiated arthritis subsets. Arthritis Res. Ther. 2017, 19, 80. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.R.; Shiau, A.L.; Chen, S.Y.; Cheng, Z.S.; Li, Y.T.; Lee, C.H.; Yo, Y.T.; Lo, C.W.; Lin, Y.S.; Juan, H.Y.; et al. Intra-articular lentivirus-mediated delivery of Gal-3 shRNA and galectin-1 gene ameliorates collagen-induced arthritis. Gene Ther. 2010, 17, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yéléhé-Okouma, M.; Ea, H.K.; Jouzeau, J.Y.; Reboul, P. Gal-3: A key player in arthritis. Joint Bone Spine 2017, 84, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.B.; Li, Q.; Steichen, A.L.; Binstock, B.J.; Metzger, D.W.; Teale, J.M.; Sharma, J. Galectin-3 Functions as an Alarmin: Pathogenic role for sepsis development in murine respiratory tularemia. PLoS ONE 2013, 8, e59616. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Komai-Koma, M.; Gilchrist, D.S.; Hsu, D.K.; Liu, F.T.; Springall, T.; Xu, D. Gal-3 is a negative regulator of lipopolysaccharide-mediated inflammation. J. Immunol. 2008, 181, 2781–2789. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, G.A.; Baum, L.G.; Tinari, N.; Paganelli, R.; Natoli, C.; Liu, F.T.; Iacobelli, S. Galectins and their ligands: Amplifiers, silencers or tuners of the inflammatory response? Trends Immunol. 2002, 23, 313–320. [Google Scholar] [CrossRef]
- Sato, S.; Hughes, R.C. Regulation of secretion and surface expression of Mac-2, a galactoside-binding protein of macrophages. J. Biol. Chem. 1994, 269, 4424–4430. [Google Scholar] [PubMed]
- Kim, H.; Hur, M.; Moon, H.W.; Yun, Y.M.; Di Somma, S.; On behalf of GREAT Network. Multi-marker approach using procalcitonin, presepsin, Gal-3, and soluble suppression of tumorigenicity 2 for the prediction of mortality in sepsis. Ann. Intensive Care 2017, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, A.; Avvedimento, E.V.; Krieg, T. Scleroderma. N. Engl. J. Med. 2009, 360, 1989–2003. [Google Scholar] [CrossRef] [PubMed]
- Van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An American college of rheumatology/European league against rheumatism collaborative initiative. Ann. Rheum. Dis. 2013, 72, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Glinsky, V.V.; Raz, A. Modified citrus pectin anti-metastatic properties: One bullet, multiple targets. Carbohydr. Res. 2009, 344, 1788–1791. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elsaad, N.M.; Elkashef, W.F. Modified citrus pectin stops progression of liver fibrosis by inhibiting Gal-3 and inducing apoptosis of stellate cells. Can. J. Physiol. Pharmacol. 2016, 94, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Delphi, L.; Sepehri, H.; Khorramizadeh, M.R.; Mansoori, F. Pectic-oligoshaccharides from apples induce apoptosis and cell cycle arrest in MDA-MB-231 cells, a model of human breast cancer. Asian Pac. J. Cancer Prev. 2015, 16, 5265–5271. [Google Scholar] [CrossRef] [PubMed]
- Grous, J.J.; Redfern, C.H.; Mahadevan, D.; Schindler, J. GCS-100, a galectin-3 antagonist, in refractory solid tumors: A phase I study. J. Clin. Oncol. 2006, 24 (Suppl. 18), 13023. [Google Scholar] [CrossRef]
- Ahmad, N.; Gabius, H.J.; Andre, S.; Kaltner, H.; Sabesan, S.; Roy, R.; Liu, B.; Macaluso, F.; Brewer, C.F. Gal-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J. Biol. Chem. 2004, 279, 10841–10847. [Google Scholar] [CrossRef] [PubMed]
- Streetly, M.J.; Maharaj, L.; Joel, S.; Schey, S.A.; Gribben, J.G.; Cotter, F.E. GCS-100, a novel galectin-3 antagonist, modulates MCL-1, NOXA, and cell cycle to induce myeloma cell death. Blood 2010, 115, 3939–3948. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.; Li, G.; Podar, K.; Hideshima, T.; Neri, P.; He, D.; Mitsiades, N.; Richardson, P.; Chang, Y.; Schindler, J.; et al. A novel carbohydrate-based therapeutic GCS-100 overcomes bortezomib resistance and enhances dexamethasone-induced apoptosis in multiple myeloma cells. Cancer Res. 2005, 65, 8350–8358. [Google Scholar] [CrossRef] [PubMed]
- De Boer, R.A.; van der Velde, A.R.; Mueller, C.; van Veldhuisen, D.J.; Anker, S.D.; Peacock, W.F.; Adams, K.F.; Maisel, A.S. Gal-3: A Modifiable Risk Factor in Heart Failure. Cardiovasc. Drugs Ther. 2014, 28, 237–246. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health (NIH). Available online: https://www.clinicaltrials.gov/ct2/search (accessed on 22 December 2017).
- Sato, S.; St-Pierre, C.; Bhaumik, P.; Nieminen, J. Galectins in innate immunity: Dual functions of host soluble beta-galactoside-binding lectins as damage-associated molecular patterns (DAMPs) and as receptors for pathogen-associated molecular patterns (PAMPs). Immunol. Rev. 2009, 230, 172–187. [Google Scholar] [CrossRef] [PubMed]
- John, C.M.; Jarvis, G.A.; Swanson, K.V.; Leffler, H.; Cooper, M.D.; Huflejt, M.E.; Griffiss, J.M. Gal-3 binds lactosaminylated lipooligosaccharides from Neisseria gonorrhoeae and is selectively expressed by mucosal epithelial cells that are infected. Cell. Microbiol. 2002, 4, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, G.A.; Gruppi, A. Galectins as immunoregulators during infectious processes: From microbial invasion to the resolution of the disease. Parasite Immunol. 2005, 27, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Kasamatsu, A.; Uzawa, K.; Shimada, K.; Shiiba, M.; Otsuka, Y.; Seki, N.; Abiko, Y.; Tanzawa, H. Elevation of galectin-9 as an in ammatory response in the periodontal ligament cells exposed to Porphylomonas gingivalis lipopolysaccharide in vitro and in vivo. Int. J. Biochem. Cell Biol. 2005, 37, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Ouellet, M.; St-Pierre, C.; Tremblay, M.J. Glycans, galectins, and HIV-1 infection. Ann. N.Y. Acad. Sci. 2012, 1253, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S.; Pelletier, I.; Ouellet, M.; Vargas, A.; Tremblay, M.J.; Sato, S.; Barbeau, B. Induction of galectin-1 expression by HTLV-I Tax and its impact on HTLV-I infectivity. Retrovirology 2008, 5, 105. [Google Scholar] [CrossRef] [PubMed]
- Okumura, C.Y.; Baum, L.G.; Johnson, P.J. Galectin-1 on cervical epithelial cells is a receptor for the sexually transmitted human parasite Trichomonas vaginalis. Cell. Microbiol. 2008, 10, 2078–2090. [Google Scholar] [CrossRef] [PubMed]
- Fichorova, R.N. Impact of T. vaginalis infection on innate immune responses and reproductive outcome. J. Reprod. Immunol. 2009, 83, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Hepojoki, J.; Strandin, T.; Hetzel, U.; Sironen, T.; Klingström, J.; Sane, J.; Mäkelä, S.; Mustonen, J.; Meri, S.; Lundkvist, A.; et al. Acute hantavirus infection induces Gal-3-binding protein. J. Gen. Virol. 2014, 95, 2356–2364. [Google Scholar] [CrossRef] [PubMed]
- Artini, M.; Natoli, C.; Tinari, N.; Costanzo, A.; Marinelli, R.; Balsano, C.; Porcari, P.; Angelucci, D.; D’Egidio, M.; Levrero, M.; et al. Elevated serum levels of 90K/MAC-2 BP predict unresponsiveness to alpha-interferon therapy in chronic HCV hepatitis patients. J. Hepatol. 1996, 25, 212–217. [Google Scholar] [CrossRef]
- Longo, G.; Natoli, C.; Rafanelli, D.; Tinari, N.; Morfini, M.; Rossi-Ferrini, P.; D’Ostilio, N.; Iacobelli, S. Prognostic value of a novel circulating serum 90K antigen in HIV-infected haemophilia patients. Br. J. Haematol. 1993, 85, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Altman, E.; Harrison, B.A.; Latta, R.K.; Lee, K.K.; Kelly, J.F.; Thibault, P. Gal-3-mediated adherence of Proteus mirabilis to Madin-Darby canine kidney cells. Biochem. Cell Biol. 2001, 79, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Desmedt, V.; Desmedt, S.; Delanghe, J.R.; Speeckaert, R.; Speeckaert, M.M. Gal-3 in renal pathology: More than just an innocent bystander. Am. J. Nephrol. 2016, 43, 305–317. [Google Scholar] [CrossRef] [PubMed]
- DeRoo, E.P.; Wrobleski, S.K.; Shea, E.M.; Al-Khalil, R.K.; Hawley, A.E.; Henke, P.K.; Myers, D.D., Jr.; Wakefield, T.W.; Diaz, J.A. The role of Gal-3 and Gal-3–binding protein in venous thrombosis. Blood 2015, 125, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Said, N.; Amin, S.; Wu, H.K.; Bruce, A.; Garate, M.; Hsu, D.K.; Kuwabara, I.; Liu, F.T.; Panjwani, N. Galectins-3 and -7, but not galectin-1, play a role in re- epithelialization of wounds. J. Biol. Chem. 2002, 277, 42299–42305. [Google Scholar] [CrossRef] [PubMed]
- Ochieng, J.; Green, B.; Evans, S.; James, O.; Warfield, P. Modulation of the biological functions of Gal-3 by matrix metalloproteinases. Biochim. Biophys. Acta 1998, 1379, 97–106. [Google Scholar] [CrossRef]
- Saravanan, C.; Cao, Z.; Head, S.R.; Panjwani, N. Detection of differentially expressed wound-healing-related glycogenes in Gal-3-deficient mice. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5690–5696. [Google Scholar] [CrossRef] [PubMed]
- Paret, C.; Bourouba, M.; Beer, A.; Miyazaki, K.; Schnölzer, M.; Fiedler, S.; Zöller, M. Ly6 family member C4.4A binds laminins 1 and 5, associates with Gal-3 and supports cell migration. Int. J. Cancer 2005, 115, 724–733. [Google Scholar]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Sivamani, R.K.; Garcia, M.S.; Isseroff, R.R. Wound re-epithelialization: Modulating keratinocyte migration in wound healing. Front. Biosci. 2007, 12, 2849–2868. [Google Scholar]
- Fonder, M.A.; Lazarus, G.S.; Cowan, D.A.; Aronson-Cook, B.; Kohli, A.R.; Mamelak, A.J. Treating the chronic wound: A practical approach to the care of nonhealing wounds and wound care dressings. J. Am. Acad. Dermatol. 2008, 58, 185–206. [Google Scholar] [CrossRef] [PubMed]
- Eaglstein, W.H.; Falanga, V. Chronic wounds. Surg. Clin. N. Am. 1997, 77, 689–700. [Google Scholar] [CrossRef]
- Ljubimov, A.V.; Saghizadeh, M. Progress in corneal wound healing. Prog. Retin. Eye Res. 2015, 49, 17–45. [Google Scholar] [CrossRef] [PubMed]
- Seiler, W.O.; Stahelin, H.B.; Zolliker, R.; Kallenberger, A.; Lüscher, N.J. Impaired migration of epidermal cells from decubitus ulcers in cell cultures. A cause of protracted wound healing? Am. J. Clin. Pathol. 1989, 92, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Panjwani, N. Role of galectins in re-epithelialization of wounds. Ann. Transl. Med. 2014, 2, 89. [Google Scholar] [CrossRef] [PubMed]
- Yabuta, C.; Yano, F.; Fujii, A.; Shearer, T.R.; Azuma, M. Gal-3 enhances epithelial cell adhesion and wound healing in rat cornea. Ophthalmic Res. 2014, 51, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Fujii, A.; Shearer, T.R.; Azuma, M. Gal-3 enhances extracellular matrix associations and wound healing in monkey corneal epithelium. Exp. Eye Res. 2015, 137, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.M.; Gaudino, G. Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp. Cell Res. 2005, 304, 274–286. [Google Scholar] [CrossRef] [PubMed]
- McCawley, L.J.; O'Brien, P.; Hudson, L.G. Epidermal growth factor (EGF)- and scatter factor/hepatocyte growth factor (SF/HGF)-mediated keratinocyte migration is coincident with induction of matrix metalloproteinase (MMP)-9. J. Cell. Physiol. 1998, 176, 255–265. [Google Scholar] [CrossRef]
- Shirakata, Y.; Kimura, R.; Nanba, D.; Iwamoto, R.; Tokumaru, S.; Morimoto, C.; Yokota, K.; Nakamura, M.; Sayama, K.; Mekada, E.; et al. Heparin-binding EGF-like growth factor accelerates keratinocyte migration and skin wound healing. J. Cell Sci. 2005, 118, 2363–2370. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Hsu, D.K.; Chen, H.Y.; Yang, R.Y.; Carraway, K.L., 3rd.; Isseroff, R.R.; Liu, F.T. Galectin-3 regulates intracellular trafficking of egfr through alix and promotes keratinocyte migration. J. Investig. Dermatol. 2012, 132, 2828–2837. [Google Scholar] [CrossRef] [PubMed]
- Puthenedam, M.; Wu, F.; Shetye, A.; Michaels, A.; Rhee, K.J.; Kwon, J.H. Matrilysin-1 (MMP7) cleaves Gal-3 and inhibits wound healing in intestinal epithelial cells. Inflamm. Bowel Dis. 2011, 17, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Kemp, H.G. Left ventricular function in patients with anginal syndrome and normal coronary arteriograms. Am. J. Cardiol. 1973, 32, 375–376. [Google Scholar] [CrossRef]
- Vermeltfoort, I.A.; Raijmakers, P.G.; Riphagen, I.I.; Odekerken, D.A.M.; Kuijper, A.F.M.; Zwijnenburg, A.; Teule, G.J.J. Definitions and incidence of cardiac syndrome X: Review and analysis of clinical data. Clin. Res. Cardiol. 2010, 99, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Kaski, J.C.; Rosano, G.M.; Collins, P.; Nihoyannopoulos, P.; Maseri, A.; Poole-Wilson, P.A. Cardiac syndrome X: Clinical characteristics and left ventricular function. Long-term follow-up study. J. Am. Coll. Cardiol. 1995, 25, 807–814. [Google Scholar] [CrossRef]
- Lamendola, P.; Lanza, G.A.; Spinelli, A.; Sgueglia, G.A.; Di Monaco, A.; Barone, L.; Sestito, A.; Crea, F. Long-term prognosis of patients with cardiac syndrome X. Int. J. Cardiol. 2010, 140, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Vermeltfoort, I.A.C.; Teule, G.J.J.; van Dijk, A.B.; Muntinga, H.J.; Raijmakers, P.G.H.M. Long-term prognosis of patients with cardiac syndrome X: A review. Neth. Heart J. 2012, 20, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Cooper-DeHoff, R.M.; McClure, C.; Johnson, B.D.; Shaw, L.J.; Handberg, E.M.; Zineh, I.; Kelsey, S.F.; Arnsdorf, M.F.; Black, H.R.; et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: A report from the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch. Intern. Med. 2009, 169, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Zhu, C.G.; Nan, J.L.; Li, J.; Li, Z.C.; Zeng, H.S.; Gao, Z.; Qin, X.W.; Zhang, C.Y. Elevated circulating inflammatory markers in female patients with cardiac syndrome X. Cytokine 2007, 40, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Espliguero, R.; Mollichelli, N.; Avanzas, P.; Zouridakis, E.; Newey, V.R.; Nassiri, D.K.; Kaski, J.C. Chronic inflammation and increased arterial stiffness in patients with cardiac syndrome X. Eur. Heart J. 2003, 24, 2006–2011. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, S.; Sade, L.E.; Yildirir, A.; Demir, O.; Bozbas, H.; Muderrisoglu, H. Serum levels of C-reactive protein and uric acid in patients with cardiac syndrome X. Acta Cardiol. 2009, 64, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Bozcali, E.; Polat, V.; Aciksari, G.; Opan, S.; Bayrak, I.H.; Paker, N.; Karakaya, O. Serum concentrations of galectin-3 in patients with cardiac syndrome X. Atherosclerosis 2014, 237, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, R.P.; John, E. Bennett Forum on Deep Mycoses Study Design 2004: Candidiasis and salvage therapy for aspergillosis. Clin. Infect. Dis. 2005, 41 (Suppl. 6), S369–S370. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Mikulska, M.; Viscoli, C. Bench-to-bedside review: Therapeutic management of invasive candidiasis in the intensive care unit. Crit. Care 2010, 14, 244. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, D.K., Jr.; Stoll, B.J.; Gantz, M.G.; Walsh, M.C.; Sanchez, P.J.; Das, A.; Shankaran, S.; Higgins, R.D.; Auten, K.J.; Miller, N.A.; et al. Eunice Kennedy shriver national institute of child health and human development neonatal research network. Neonatal candidiasis: Epidemiology, risk factors, and clinical judgment. Pediatrics 2010, 126, e865–e873. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D. Pathogenesis and treatment of recurrent vulvovaginal candidiasis. Clin. Infect. Dis. 1992, 14 (Suppl. 1), S148–S153. [Google Scholar] [CrossRef] [PubMed]
- Holikova, Z.; Hrdlickova-Cela, E.; Plzak, J.; Smetana, K., Jr.; Betka, J.; Dvorankova, B.; Esner, M.; Wasano, K.; Andre, S.; Kaltner, H.; et al. Defining the glycophenotype of squamous epithelia using plant and mammalian lectins: Differentiation-dependent expression of 2,6- and 2,3-linked N-acetylneura-minic acid in squamous epithelia and carcinomas, and its differential effect on binding of the endogenous lectins galectins-1 and -3. APMIS 2002, 110, 845–856. [Google Scholar] [PubMed]
- Hrdlickova-Cela, E.; Plzak, J.; Smetana, K.; Melkova, Z.; Kaltner, H.; Filipec, M.; Liu, F.; Gabius, H. Detection of Gal-3 in tear fluid at disease states and immunohistochemical and lectin histochemical analysis in human corneal and conjunctival epithelium. Br. J. Ophthalmol. 2001, 85, 1336–1340. [Google Scholar] [CrossRef] [PubMed]
- Honjo, Y.; Inohara, H.; Akahani, S.; Yoshii, T.; Takenaka, Y.; Yoshida, J.; Hattori, K.; Tomiyama, Y.; Raz, A.; Kubo, T. Expression of cytoplasmic Gal-3 as a prognostic marker in tongue carcinoma. Clin. Cancer Res. 2000, 6, 4635–4640. [Google Scholar] [PubMed]
- Jensen-Jarolim, E.; Gscheidlinger, R.; Oberhuber, G.; Neuchrist, C.; Lucas, T.; Bises, G.; Radauer, C.; Willheim, M.; Scheiner, O.; Liu, F.T.; et al. The constitutive expression of Gal-3 is downregulated in the intestinal epithelia of Crohn’s disease patients, and tumour necrosis factor decreases the level of Gal-3-specific mRNA in HCT-8 cells. Eur. J. Gastroenterol. Hepatol. 2002, 14, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Linden, J.R.; Kunkel, D.; Laforce-Nesbitt, S.S.; Bliss, J.M. The Role of Gal-3 in Phagocytosis of Candida albicans and Candida parapsilosis by Human Neutrophils. Cell. Microbiol. 2013, 15, 1127–1142. [Google Scholar] [CrossRef] [PubMed]
- Linden, J.R.; De Paepe, M.E.; Laforce-Nesbitt, S.S.; Bliss, J.M. Galectin-3 plays an important role in protection against disseminated candidiasis. Med. Mycol. 2013, 51, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Plantinga, T.S.; Johnson, M.D.; Scott, W.K.; Joosten, L.A.; van der Meer, J.W.; Perfect, J.R.; Kullberg, B.J.; Netea, M.G. Human genetic susceptibility to Candida infections. Med. Mycol. 2012, 50, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Smeekens, S.P.; van de Veerdonk, F.L.; Kullberg, B.J.; Netea, M.G. Genetic susceptibility to Candida infections. EMBO Mol. Med. 2013, 5, 805–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.Y.; Huang, J.H.; Chen, W.Y.; Chan, Y.C.; Lin, C.H.; Chen, Y.C.; Liu, F.T.; Wu-Hsieh, B.A. Cell Intrinsic Gal-3 Attenuates Neutrophil ROS-Dependent Killing of Candida by Modulating CR3 Downstream Syk Activation. Front. Immunol. 2017, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.J.; Lin, H.Y.; Tu, Z.; Lin, T.C.; Wu, S.C.; Tseng, Y.Y.; Liu, F.T.; Hsu, S.T.; Lin, C.H. Dual thio-digalactoside-binding modes of human galectins as the structural basis for the design of potent and selective inhibitors. Sci. Rep. 2016, 6, 29457. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, U.; Leffler, H.; Mukhopadhyay, B.; Rajput, V.; Inventor; Galecto Biotech Ab, Assignee. Galectoside Inhibitors of Galectins. U.S. Patent 9,353,141, 2016. Available online: http://www.google.com/patents/US9353141 (accessed on 22 December 2017).
- Sampathkumar, P.; Drage, L.A.; Martin, D.P. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin. Proc. 2009, 84, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Gershon, A.A.; Gershon, M.D.; Breuer, J.; Levin, M.J.; Oaklander, A.L.; Griffiths, P.D. Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J. Clin. Virol. 2010, 48, S2–S7. [Google Scholar] [CrossRef]
- Park, J.; Jang, W.S.; Park, K.Y.; Li, K.; Seo, S.J.; Hong, C.K.; Lee, J.B. Thermography as a predictor of postherpetic neuralgia in acute herpes zoster patients: A preliminary study. Skin Res. Technol. 2012, 18, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Yawn, B.P.; Gilden, D. The global epidemiology of herpes zoster. Neurology 2013, 81, 928–930. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Gebremeskel, B.G.; Acosta, C.J. Systematic review of incidence and complications of herpes zoster: Towards a global perspective. BMJ Open 2014, 4, e004833. [Google Scholar] [CrossRef] [PubMed]
- Rowbotham, M.C.; Fields, H.L. The relationship of pain, allodynia and thermal sensation in post-herpetic neuralgia. Brain 1996, 119, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Regan, L.J.; Dodd, J.; Barondes, S.H.; Jessell, T.M. Selective expression of endogenous lactose-binding lectins and lactoseries glycoconjugates in subsets of rat sensory neurons. Proc. Natl. Acad. Sci. USA 1986, 83, 2248–2252. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Chung, K. Endogenous lectin (RL-29) expression of the autonomic preganglionic neurons in the rat spinal cord. Anat. Rec. 1999, 254, 53–60. [Google Scholar] [CrossRef]
- Ma, Z.; Han, Q.; Wang, X.; Ai, Z.; Zheng, Y. Galectin-3 Inhibition Is Associated with Neuropathic Pain Attenuation after Peripheral Nerve Injury. PLoS ONE 2016, 11, e0148792. [Google Scholar]



| Letter Names | Disease/Condition | Possible Role in Pathogenesis | Possible Role in Diagnosis/Prognosis | Possible Role in Patients Stratification for Therapy | Possible Role in Therapy |
|---|---|---|---|---|---|
| A | Asthma | Gal-3 positive patients are more responsive to omalizumab | |||
| Atherosclerosis | Gal-3 is expressed in advanced atherosclerotic lesions | ||||
| Atopic Dermatitis | Gal-3 released by PMNs amplifies IgE production | Gal-3 inhibitors used in a phase 2 controlled trial for treatment of severe atopic dermatitis | |||
| B | Blood test | Blood assays (ELISA) to predict outcome of acute and chronic heart failure | |||
| C | Cancer | Gal-3 controls oncogenesis, cancer progression and metastasis | Diagnostic role in thyroid, prostate, and ovarian cancers | Clinical trials using Gal-3 inhibitors for melanoma and large B-cell lymphoma | |
| Cerebral infarction | Serum levels of Gal-3 are increased in ischemic brain damage | Measurement of serum Gal-3 levels may help in diagnosis of cerebral infarction | |||
| COPD | Gal-3 levels measured in BAL are reduced in healthy smokers | In patients with COPD serum Gal-3 levels may predict right ventricular dysfunction and acute exacerbation of COPD | |||
| D | Degenerative Aortic Stenosis | Serum Gal-3 levels may be useful in stratification for therapy in elderly patients affected by degenerative aortic stenosis | |||
| Diabetes Mellitus | Conflicting results: Gal-3 plays a rotective role; Gal-3 is a risk factor for vascular/renal/cardiac complications | ||||
| E | Endometriosis | Gal-3 is overexpressed in endometriotic tissue | |||
| Enteric nervous system | Gal-3 is crucial mediator of the GI damage observed after brain injuries, at the level of enteric neuronal cells | ||||
| Encephalitis | Gal-3 is involved in various types of CNS inflammation and in particular in viral encephalitis | ||||
| F | Fibrosis | Gal-3 is key regulator of chronic inflammation and tissue fibrogenesis | Serum Gal-3 levels are increased in fibrosis affecting the liver, the kidney, the lung, the heart, the nervous system and in the systemic sclerosis. In such conditions Gal-3 has been proposed as a new prognostic factor | ||
| G | Gastritis | Gal-3 is upregulated in gastric epithelial cells following adhesion of Helicobacter pylori | Experimental evidence indicates that administration of extracellular recombinant Gal-3 (rGal-3) is able to inhibit the adhesion of Helicobacter pylori to the gastric epithelial cells | ||
| H | Heart | Serum Gal-3 levels are associated with mortality in patients with chronic heart failure | Gal-3 is useful as a marker for stratification for therapy (rosuvastatin and cardiac resynchronization) in patient with heart failure | ||
| HIV infection | Gal-3 is associated with early stages of HIV infection and is involved in the host response to HIV infection. | ||||
| I | Inflammation | Gal-3 is involved in many processes during both acute and chronic inflammatory response | |||
| Interstitial lung disease | Gal-3 is increased in the serum of patients affected by IPF, and is associated with decreased lung volumes and altered gas exchange | A novel target therapy for IPF was proposed using a Gal-3 inhibitor (TD139) formulated for inhalation and a clinical trial process was started | |||
| J | Juvenile Idiopathic Arthritis | Gal-3 serum levels are increased in children affected by JIA and correlate with disease activity | |||
| K | Kidney | Gal-3 serum levels are upregulated in diabetic nephropathy and acute renal failure | Experimental evidence indicates that pharmacologic inhibition of Gal-3 with MCP is protective toward nephropathy. Another Gal-3 inhibitor, the GCS-100, significantly improved the eGFR in patients with CKD stage 3b. | ||
| L | Liver fibrosis | Gal-3 plays a critical role in hepatic stellate cells activation to a myofibroblast phenotype, a critical event in extracellular matrix deposition and in the development of cirrhosis | A Gal-3 antagonist, named GR-MD-02, is current on a phase 2 Clinical Trial to prevent progression toward cirrhosis in patients with NASH | ||
| M | Mortality | Gal-3 is associated with risk of coronary heart disease, CVD mortality, and all-cause mortality | |||
| N | Non-alcoholic steatohepatitis (NASH) | Experimental evidence indicates that mice lacking the Gal-3 gene are resistant to liver fibrosis | The specific Gal-3 inhibitor, named GR-MB-02, is on Phase 2 Clinical Trial for the treatment of advanced fibrosis due to NASH | ||
| O | Obesity | Gal-3 is expressed in all types of macrophages that permeate white adipose tissue and it is an important regulator of their polarization and activity. Gal3 can bind directly to the insulin receptor (IR) and inhibit downstream IR signaling. | Experimental evidence in mice indicates that inhibition of Gal-3 improves insulin sensitivity | ||
| P | Pneumonia | Gal-3 is involved in the recruitment of neutrophils during lung infections with Streptococcus pneumoniae | Experimental evidence indicates that exogenous administration of Gal-3 to mice has a direct antimicrobial, bacteriostatic effect on Streptococcus pneumonia | ||
| Pulmonary hypertension | Conflicting results:Gal-3 plasma levels are decreased in patients with PAH;Gal-3 plasma levels are increased in PAH independently to etiology and are are associated with severity of PAH | ||||
| Plaque Psoriasis | Gal-3 is expressed in a high proportion of Langerhans cells from skin punch biopsies, obtained from lesional plaque-type psoriatic skin | An open-label phase 2a clinical trial is ongoing using the Gal-3 antagonist GR-MD-02 for the treatment of psoriasis | |||
| Q | Q Fever | Glactin-3 is a sensor of damage caused by the Coxiella burnetii | |||
| R | Rheumatoid Arthritis | Gal-3 plays a key role as pro-inflammatory mediator in RA | Gal-3 serum level has been proposed as a predictive marker of future RA | Experimental evidence indicates that therapeutic intra-articularly administration of lentiviral vectors encoding Gal-3 small hairpin RNA (shRNA), in rats with collagen-induced arthritis, significantly ameliorates disease activity | |
| S | Sepsis | Serum Gal-3 levels may represent a useful marker to predict mortality in patients affected by sepsis | Experimental evidence indicates that Gal-3 may represent a potential target for treatment of sepsis, at least for that induced by Francisella novicida | ||
| Systemic Sclerosis | Serum Gal-3 levels correlate with the developmental process of skin sclerosis and of digital ulcers in diffuse cutaneous SSc | Gal-3 appears to be a reliable biomarker to predict all-cause mortality in patients affected by SSc | |||
| T | Target therapy | Gal-3 is involved in numerous degenerative processes within the body, including cancer proliferation/metastasis, heart failure, atherosclerosis, diabetes, chronic inflammation, fibrosis and related organ failure. | Numerous compounds that are able to inhibit Gal-3 function have been proposed and are currently in Clinical Trials for many different diseases/conditions | ||
| U | Urinary tract infections | Gal-3 plays a role in urinary tract viral and bacterial infections in humans | |||
| V | Venous Thrombosis | Serum levels of Gal-3 and of Gal-3 binding protein are elevated in the course of venous thrombosis | |||
| W | Wound Healing | Gal-3 plays a key player in the re-epithelialization of wounds in many different tissues, including the cornea, the skin and the GI tract | |||
| X | X syndrome of the heart | Serum Gal-3 levels are significantly higher in the CSX patients in comparison with the healthy controls | |||
| Y | Yeast infection-Candidiasis | Gal-3 is present in the fungal granulomata, it binds to Candida albicans in a carbohydrate-specific manner and it displays a direct fungicidal activity | Experimental evidence indicates that the inhibition of Gal-3, obtained by knocking down the gene, by silencing its expression or by administration of the specific inhibitor TD139 is able to inhibit neutrophil reactive oxygen species (ROS) production and enhance the ability of neutrophils to kill Candida albicans | ||
| Z | Zoster-related pain (allodynia) | Gal-3 is involved in the process of Wallerian degeneration distal to the injury and subsequent axonal regrowth and functional re-innervation | Experimental evidence indicates that in mice the intrathecal administration of the Gal-3 inhibitor modified citrus pectin (MCP) is able to significantly attenuate neurophathic pain |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sciacchitano, S.; Lavra, L.; Morgante, A.; Ulivieri, A.; Magi, F.; De Francesco, G.P.; Bellotti, C.; Salehi, L.B.; Ricci, A. Galectin-3: One Molecule for an Alphabet of Diseases, from A to Z. Int. J. Mol. Sci. 2018, 19, 379. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19020379
Sciacchitano S, Lavra L, Morgante A, Ulivieri A, Magi F, De Francesco GP, Bellotti C, Salehi LB, Ricci A. Galectin-3: One Molecule for an Alphabet of Diseases, from A to Z. International Journal of Molecular Sciences. 2018; 19(2):379. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19020379
Chicago/Turabian StyleSciacchitano, Salvatore, Luca Lavra, Alessandra Morgante, Alessandra Ulivieri, Fiorenza Magi, Gian Paolo De Francesco, Carlo Bellotti, Leila B. Salehi, and Alberto Ricci. 2018. "Galectin-3: One Molecule for an Alphabet of Diseases, from A to Z" International Journal of Molecular Sciences 19, no. 2: 379. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19020379