Vitamin K Dependent Proteins in Kidney Disease
Abstract
:1. Introduction
2. Methodology
2.1. Search Strategy and Selection of Studies
2.2. Inclusion and Exclusion Criteria
2.3. Identification, Selection, Screening and Inclusion
3. Functional and Molecular Background
3.1. Matrix Gla Protein
3.2. Osteocalcin
3.3. Growth Arrest Specific Protein 6
3.4. Gla-Rich Protein
4. VKDPs in Kidney Disease
4.1. Vitamin K Insufficiency in Kidney Disease
4.2. Matrix Gla Protein in Kidney Disease
4.2.1. Human Studies on Circulating MGP
4.2.2. MGP in Experimental Studies
4.2.3. Studies Assessing MGP in Tissues
4.3. Osteocalcin in Kidney Disease
4.3.1. Osteocalcin in CKD and Renal Transplant
4.3.2. Osteocalcin in Dialysis and Interventional Studies
4.4. Gas6 in Kidney Disease
4.4.1. Gas6 in CKD and Acute Kidney Disease
4.4.2. Gas6 in Renal Cancer
4.4.3. Gas6 in Experimental Studies
4.5. GRP in Kidney Disease
5. Discussions
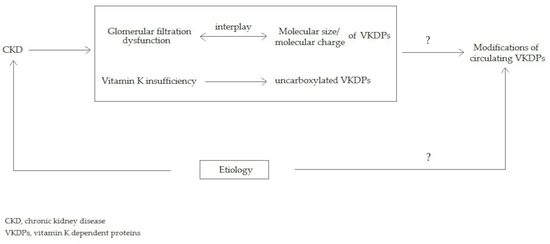
5.1. The Interplay between Molecular Charge and Weight Could Play a Role in Glomerular Filtration of VKDPs
5.2. The Relationship between the Etiologies of CKD and the Modifications of Circulating VKDPs
5.3. VKDPs as Potential Markers in Kidney Disease
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| %ucOC | Percentage of total osteocalcin that is uncarboxylated |
| CCRC | Clear cell renal carcinoma |
| CKD | Chronic kidney disease |
| cOC | Carboxylated osteocalcin |
| cMGP | Carboxylated matrix Gla protein |
| DN | Diabetic nephropathy |
| dp-cMGP | Dephospho-carboxylated matrix Gla protein |
| dp-ucMGP | Dephospho-uncarboxylated matrix Gla protein |
| ESRD | End-stage renal disease |
| Gas6 | Growth-arrest specific protein 6 |
| Gla | Carboxy glutamic acid |
| Glu | Glutamic acid |
| GRP | Gla-rich protein |
| HCO | High Cut-Off dialysis |
| HD | Hemodialysis |
| HF | Conventional High Flow dialysis |
| HRO | High Retention Onset dialysis |
| MCO | Medium Cut-Off dialysis |
| MDCK | Madin-Darby Canine Kidney |
| MGP | Matrix Gla protein |
| OC | Osteocalcin |
| p-cMGP | Phospho-carboxylated matrix Gla protein |
| Ser | Serine |
| t-ucMGP | Total-uncarboxylated matrix Gla protein |
| ucMGP | Uncarboxylated matrix Gla protein |
| ucOC | Uncarboxylated osteocalcin |
| VC | Vascular calcification |
| VKDPs | Vitamin K dependent proteins |
| VSMCs | Vascular smooth muscle cells |
References
- Lopez-Giacoman, S.; Madero, M. Biomarkers in chronic kidney disease, from kidney function to kidney damage. World J. Nephrol. 2015, 4, 57–73. [Google Scholar] [CrossRef]
- Rysz, J.; Gluba-Brzózka, A.; Franczyk, B.; Jabłonowski, Z.; Ciałkowska-Rysz, A. Novel Biomarkers in the Diagnosis of Chronic Kidney Disease and the Prediction of Its Outcome. Int. J. Mol. Sci. 2017, 18, 1702. [Google Scholar] [CrossRef] [PubMed]
- Chatrou, M.L.L.; Reutelingsperger, C.P.; Schurgers, L.J. Role of vitamin K-dependent proteins in the arterial vessel wall. Hamostaseologie 2011, 31, 251–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schurgers, L.J.; Dissel, P.E.; Spronk, H.M.; Soute, B.A.; Dhore, C.R.; Cleutjens, J.P.; Vermeer, C. Role of vitamin K and vitamin K-dependent proteins in vascular calcification. Z. Kardiol. 2001, 90 (Suppl. 3), 57–63. [Google Scholar] [CrossRef] [PubMed]
- London, G.M.; Guérin, A.P.; Marchais, S.J.; Métivier, F.; Pannier, B.; Adda, H. Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol. Dial. Transplant. 2003, 18, 1731–1740. [Google Scholar] [CrossRef]
- Stitt, T.N.; Conn, G.; Gore, M.; Lai, C.; Bruno, J.; Radziejewski, C.; Mattsson, K.; Fisher, J.; Gies, D.R.; Jones, P.F. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell 1995, 80, 661–670. [Google Scholar] [CrossRef] [Green Version]
- Varnum, B.C.; Young, C.; Elliott, G.; Garcia, A.; Bartley, T.D.; Fridell, Y.W.; Hunt, R.W.; Trail, G.; Clogston, C.; Toso, R.J. Axl receptor tyrosine kinase stimulated by the vitamin K-dependent protein encoded by growth-arrest-specific gene 6. Nature 1995, 373, 623–626. [Google Scholar] [CrossRef]
- Davidsen, K.T.; Haaland, G.S.; Lie, M.K.; Lorens, J.B.; Engelsen, A.S.T. The Role of Axl Receptor Tyrosine Kinase in Tumor Cell Plasticity and Therapy Resistance. In Biomarkers of the Tumor Microenvironment: Basic Studies and Practical Applications; Akslen, L.A., Watnick, R.S., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 351–376. [Google Scholar]
- Holden, R.M.; Morton, A.R.; Garland, J.S.; Pavlov, A.; Day, A.G.; Booth, S.L. Vitamins K and D status in stages 3-5 chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 590–597. [Google Scholar] [CrossRef]
- Cranenburg, E.C.M.; Schurgers, L.J.; Uiterwijk, H.H.; Beulens, J.W.J.; Dalmeijer, G.W.; Westerhuis, R.; Magdeleyns, E.J.; Herfs, M.; Vermeer, C.; Laverman, G.D. Vitamin K intake and status are low in hemodialysis patients. Kidney Int. 2012, 82, 605–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levey, A.S.; Eckardt, K.-U.; Tsukamoto, Y.; Levin, A.; Coresh, J.; Rossert, J.; De Zeeuw, D.; Hostetter, T.H.; Lameire, N.; Eknoyan, G. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005, 67, 2089–2100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlieper, G.; Westenfeld, R.; Krüger, T.; Cranenburg, E.C.; Magdeleyns, E.J.; Brandenburg, V.M.; Djuric, Z.; Damjanovic, T.; Ketteler, M.; Vermeer, C.; et al. Circulating nonphosphorylated carboxylated matrix gla protein predicts survival in ESRD. J. Am. Soc. Nephrol. 2011, 22, 387–395. [Google Scholar] [CrossRef]
- Riphagen, I.J.; Keyzer, C.A.; Drummen, N.E.A.; de Borst, M.H.; Beulens, J.W.J.; Gansevoort, R.T.; Geleijnse, J.M.; Muskiet, F.A.J.; Navis, G.; Visser, S.T.; et al. Prevalence and Effects of Functional Vitamin K Insufficiency: The PREVEND Study. Nutrients 2017, 9, 1334. [Google Scholar] [CrossRef]
- Thamratnopkoon, S.; Susantitaphong, P.; Tumkosit, M.; Katavetin, P.; Tiranathanagul, K.; Praditpornsilpa, K.; Eiam-Ong, S. Correlations of Plasma Desphosphorylated Uncarboxylated Matrix Gla Protein with Vascular Calcification and Vascular Stiffness in Chronic Kidney Disease. Nephron 2017, 135, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Kurnatowska, I.; Grzelak, P.; Masajtis-Zagajewska, A.; Kaczmarska, M.; Stefańczyk, L.; Vermeer, C.; Maresz, K.; Nowicki, M. Plasma Desphospho-Uncarboxylated Matrix Gla Protein as a Marker of Kidney Damage and Cardiovascular Risk in Advanced Stage of Chronic Kidney Disease. Kidney Blood Press. Res. 2016, 41, 231–239. [Google Scholar] [CrossRef]
- Meuwese, C.L.; Olauson, H.; Qureshi, A.R.; Ripsweden, J.; Barany, P.; Vermeer, C.; Drummen, N.; Stenvinkel, P. Associations between Thyroid Hormones, Calcification Inhibitor Levels and Vascular Calcification in End-Stage Renal Disease. PLoS ONE 2015, 10, e0132353. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Barreto, D.V.; Barreto, F.C.; Liabeuf, S.; Renard, C.; Magdeleyns, E.J.; Vermeer, C.; Choukroun, G.; Massy, Z.A. The circulating inactive form of matrix gla protein is a surrogate marker for vascular calcification in chronic kidney disease: A preliminary report. Clin. J. Am. Soc. Nephrol. 2010, 5, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Puzantian, H.; Akers, S.R.; Oldland, G.; Javaid, K.; Miller, R.; Ge, Y.; Ansari, B.; Lee, J.; Suri, A.; Hasmath, Z.; et al. Circulating Dephospho-Uncarboxylated Matrix Gla-Protein Is Associated with Kidney Dysfunction and Arterial Stiffness. Am. J. Hypertens. 2018, 31, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Fain, M.E.; Kapuku, G.K.; Paulson, W.D.; Williams, C.F.; Raed, A.; Dong, Y.; Knapen, M.H.J.; Vermeer, C.; Pollock, N.K. Inactive Matrix Gla Protein, Arterial Stiffness, and Endothelial Function in African American Hemodialysis Patients. Am. J. Hypertens. 2018, 31, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Westenfeld, R.; Krueger, T.; Schlieper, G.; Cranenburg, E.C.M.; Magdeleyns, E.J.; Heidenreich, S.; Holzmann, S.; Vermeer, C.; Jahnen-Dechent, W.; Ketteler, M.; et al. Effect of vitamin K2 supplementation on functional vitamin K deficiency in hemodialysis patients: A randomized trial. Am. J. Kidney Dis. 2012, 59, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.G.; Hariri, E.; Daaboul, Y.; Korjian, S.; El Alam, A.; Protogerou, A.D.; Kilany, H.; Karam, A.; Stephan, A.; Bahous, S.A. Vitamin K2 supplementation and arterial stiffness among renal transplant recipients-a single-arm, single-center clinical trial. J. Am. Soc. Hypertens. 2017, 11, 589–597. [Google Scholar] [CrossRef]
- Jansz, T.T.; Neradova, A.; van Ballegooijen, A.J.; Verhaar, M.C.; Vervloet, M.G.; Schurgers, L.J.; van Jaarsveld, B.C. The role of kidney transplantation and phosphate binder use in vitamin K status. PLoS ONE 2018, 13, e0203157. [Google Scholar] [CrossRef]
- Boxma, P.Y.; van den Berg, E.; Geleijnse, J.M.; Laverman, G.D.; Schurgers, L.J.; Vermeer, C.; Kema, I.P.; Muskiet, F.A.; Navis, G.; Bakker, S.J.L.; et al. Vitamin k intake and plasma desphospho-uncarboxylated matrix Gla-protein levels in kidney transplant recipients. PLoS ONE 2012, 7, e47991. [Google Scholar] [CrossRef]
- Keyzer, C.A.; Vermeer, C.; Joosten, M.M.; Knapen, M.H.J.; Drummen, N.E.A.; Navis, G.; Bakker, S.J.L.; de Borst, M.H. Vitamin K status and mortality after kidney transplantation: A cohort study. Am. J. Kidney Dis. 2015, 65, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Cranenburg, E.C.M.; Brandenburg, V.M.; Vermeer, C.; Stenger, M.; Mühlenbruch, G.; Mahnken, A.H.; Gladziwa, U.; Ketteler, M.; Schurgers, L.J. Uncarboxylated matrix Gla protein (ucMGP) is associated with coronary artery calcification in haemodialysis patients. Thromb. Haemost. 2009, 101, 359–366. [Google Scholar]
- Shroff, R.C.; Shah, V.; Hiorns, M.P.; Schoppet, M.; Hofbauer, L.C.; Hawa, G.; Schurgers, L.J.; Singhal, A.; Merryweather, I.; Brogan, P.; et al. The circulating calcification inhibitors, fetuin-A and osteoprotegerin, but not matrix Gla protein, are associated with vascular stiffness and calcification in children on dialysis. Nephrol. Dial. Transplant. 2008, 23, 3263–3271. [Google Scholar] [CrossRef] [PubMed]
- Roumeliotis, S.; Roumeliotis, A.; Panagoutsos, S.; Giannakopoulou, E.; Papanas, N.; Manolopoulos, V.G.; Passadakis, P.; Tavridou, A. Matrix Gla protein T-138C polymorphism is associated with carotid intima media thickness and predicts mortality in patients with diabetic nephropathy. J. Diabetes Complic. 2017, 31, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Schlieper, G.; Brandenburg, V.; Djuric, Z.; Damjanovic, T.; Markovic, N.; Schurgers, L.; Kruger, T.; Westenfeld, R.; Ackermann, D.; Haselhuhn, A.; et al. Risk factors for cardiovascular calcifications in non-diabetic Caucasian haemodialysis patients. Kidney Blood Press. Res. 2009, 32, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Willy, K.; Girndt, M.; Voelkl, J.; Fiedler, R.; Martus, P.; Storr, M.; Schindler, R.; Zickler, D. Expanded Haemodialysis Therapy of Chronic Haemodialysis Patients Prevents Calcification and Apoptosis of Vascular Smooth Muscle Cells in vitro. Blood Purif. 2018, 45, 131–138. [Google Scholar] [CrossRef]
- Willy, K.; Hulko, M.; Storr, M.; Speidel, R.; Gauss, J.; Schindler, R.; Zickler, D. In Vitro Dialysis of Cytokine-Rich Plasma with High and Medium Cut-Off Membranes Reduces Its Procalcific Activity. Artif. Organs 2017, 41, 803–809. [Google Scholar] [CrossRef]
- Khan, A.; Wang, W.; Khan, S.R. Calcium oxalate nephrolithiasis and expression of matrix GLA protein in the kidneys. World J. Urol. 2014, 32, 123–130. [Google Scholar] [CrossRef]
- Lu, X.; Gao, B.; Yasui, T.; Li, Y.; Liu, T.; Mao, X.; Hirose, M.; Wu, Y.; Yu, D.; Zhu, Q.; et al. Matrix Gla protein is involved in crystal formation in kidney of hyperoxaluric rats. Kidney Blood Press. Res. 2013, 37, 15–23. [Google Scholar] [CrossRef]
- Lomashvili, K.A.; Wang, X.; Wallin, R.; O’Neill, W.C. Matrix Gla protein metabolism in vascular smooth muscle and role in uremic vascular calcification. J. Biol. Chem. 2011, 286, 28715–28722. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, J.M.; Martino, F.; Scheffner, I.; Bröcker, V.; Leitolf, H.; Haller, H.; Gwinner, W. Fetuin, matrix-Gla protein and osteopontin in calcification of renal allografts. PLoS ONE 2012, 7, e52039. [Google Scholar] [CrossRef]
- Kramann, R.; Brandenburg, V.M.; Schurgers, L.J.; Ketteler, M.; Westphal, S.; Leisten, I.; Bovi, M.; Jahnen-Dechent, W.; Knüchel, R.; Floege, J.; et al. Novel insights into osteogenesis and matrix remodelling associated with calcific uraemic arteriolopathy. Nephrol. Dial. Transplant. 2013, 28, 856–868. [Google Scholar] [CrossRef]
- Shroff, R.C.; McNair, R.; Figg, N.; Skepper, J.N.; Schurgers, L.; Gupta, A.; Hiorns, M.; Donald, A.E.; Deanfield, J.; Rees, L.; et al. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation 2008, 118, 1748–1757. [Google Scholar] [CrossRef]
- Wei, F.-F.; Drummen, N.E.A.; Thijs, L.; Jacobs, L.; Herfs, M.; Van’t Hoofd, C.; Vermeer, C.; Staessen, J.A. Vitamin-K-Dependent Protection of the Renal Microvasculature: Histopathological Studies in Normal and Diseased Kidneys. Pulse Basel Switz. 2016, 4, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Kurnatowska, I.; Grzelak, P.; Masajtis-Zagajewska, A.; Kaczmarska, M.; Stefańczyk, L.; Vermeer, C.; Maresz, K.; Nowicki, M. Effect of vitamin K2 on progression of atherosclerosis and vascular calcification in nondialyzed patients with chronic kidney disease stages 3-5. Pol. Arch. Med. Wewn. 2015, 125, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Wyskida, K.; Żak-Gołąb, A.; Wajda, J.; Klein, D.; Witkowicz, J.; Ficek, R.; Rotkegel, S.; Spiechowicz, U.; Kocemba Dyczek, J.; Ciepał, J.; et al. Functional deficiency of vitamin K in hemodialysis patients in Upper Silesia in Poland. Int. Urol. Nephrol. 2016, 48, 765–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Wang, S.; Zhao, S.; Zhang, X. Effect of lanthanum carbonate on coronary artery calcification and bone mineral density in maintenance hemodialysis patients with diabetes complicated with adynamic bone disease: A prospective pilot study. Medicine (Baltimore) 2017, 96, e8664. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.D.; Ix, J.H.; Cranenburg, E.C.M.; Vermeer, C.; Whooley, M.A.; Schurgers, L.J. Association of kidney function and uncarboxylated matrix Gla protein: Data from the Heart and Soul Study. Nephrol. Dial. Transplant. 2009, 24, 2095–2101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Ballegooijen, A.J.; Beulens, J.W. The Role of Vitamin K Status in Cardiovascular Health: Evidence from Observational and Clinical Studies. Curr. Nutr. Rep. 2017, 6, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, T.; Okano, K.; Tsuruta, Y.; Akiba, T.; Nitta, K. Serum osteocalcin levels are useful as a predictor of cardiovascular events in maintenance hemodialysis patients. Int. Urol. Nephrol. 2013, 45, 207–214. [Google Scholar] [CrossRef]
- Bervoets, A.R.J.; Spasovski, G.B.; Behets, G.J.; Dams, G.; Polenakovic, M.H.; Zafirovska, K.; Van Hoof, V.O.; De Broe, M.E.; D’Haese, P.C. Useful biochemical markers for diagnosing renal osteodystrophy in predialysis end-stage renal failure patients. Am. J. Kidney Dis. 2003, 41, 997–1007. [Google Scholar] [CrossRef]
- Gluba-Brzózka, A.; Michalska-Kasiczak, M.; Franczyk, B.; Nocuń, M.; Toth, P.; Banach, M.; Rysz, J. Markers of increased atherosclerotic risk in patients with chronic kidney disease: A preliminary study. Lipids Health Dis. 2016, 15, 22. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P.; Molnar, M.Z.; Czira, M.E.; Rudas, A.; Ujszaszi, A.; Sarvary, E.; Ambrus, C.; Szathmari, M.; Remport, A.; Mucsi, I. Diagnostic accuracy of serum parathyroid hormone levels in kidney transplant recipients with moderate-to-advanced CKD. Nephron Clin. Pract. 2011, 118, c78–c85. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, A.; Hayashi, Y.; Miyazaki, H. Relationships among bone turnover, renal function and periodontal disease in elderly Japanese. J. Periodontal Res. 2011, 46, 491–496. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Molnar, M.Z.; Czira, M.E.; Rudas, A.; Ujszaszi, A.; Rosivall, L.; Szathmari, M.; Covic, A.; Keszei, A.; Beko, G.; et al. Associations between serum leptin level and bone turnover in kidney transplant recipients. Clin. J. Am. Soc. Nephrol. 2010, 5, 2297–2304. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, J.; Boutroy, S.; Guebre-Egziabher, F.; Juillard, L.; Drai, J.; Pelletier, S.; Richard, M.; Charrié, A.; Carlier, M.C.; Chapurlat, R.; et al. The relationship between adipokines, osteocalcin and bone quality in chronic kidney disease. Nephrol. Dial. Transplant. 2009, 24, 3120–3125. [Google Scholar] [CrossRef] [Green Version]
- Falkiewicz, K.; Boratyńska, M.; Zmonarski, S.C.; Milewicz, A.; Patrzałek, D.; Biecek, P.; Klinger, M. Evolution of bone disease at 2 years after transplantation: A single-center study. Transplant. Proc. 2009, 41, 3063–3066. [Google Scholar] [CrossRef] [PubMed]
- Krause, R.; Stange, R.; Roth, H.J.; Kaase, H.; Michalsen, A.; Holick, M.F. Partial Body UV Exposure in Chronic Kidney Disease and Extrarenal Vitamin D Metabolism. Anticancer Res. 2018, 38, 1217–1219. [Google Scholar] [PubMed]
- Ma, L.; Zhao, S.; Li, Z. Effects of parathyroidectomy on bone metabolism in haemodialysis patients with secondary hyperparathyroidism. Scand. J. Clin. Lab. Investig. 2017, 77, 527–534. [Google Scholar] [CrossRef]
- Keronen, S.; Martola, L.; Finne, P.; Burton, I.S.; Kauppila, L.; Kröger, H.; Larsson, T.E.; Honkanen, E. Bone histomorphometry and indicators of bone and mineral metabolism in wait-listed dialysis patients. Clin. Nephrol. 2016, 85, 127–134. [Google Scholar] [CrossRef]
- Fedak, D.; Kuźniewski, M.; Dumnicka, P.; Kapusta, M.; Chmiel, G.; Solnica, B.; Sułowicz, W. Relationship between fetuin-A, bone turnover and inflammatory markers concentrations in serum of maintenance hemodialyzed patients. Przegl. Lek. 2016, 73, 799–804. [Google Scholar]
- Okuno, S.; Ishimura, E.; Tsuboniwa, N.; Norimine, K.; Yamakawa, K.; Yamakawa, T.; Shoji, S.; Mori, K.; Nishizawa, Y.; Inaba, M. Significant inverse relationship between serum undercarboxylated osteocalcin and glycemic control in maintenance hemodialysis patients. Osteoporos. Int. 2013, 24, 605–612. [Google Scholar] [CrossRef]
- Jeong, J.U.; Lee, H.K.; Kim, Y.J.; Kim, J.S.; Kang, S.S.; Kim, S.B. Nutritional markers, not markers of bone turnover, are related predictors of bone mineral density in chronic peritoneal dialysis patients. Clin. Nephrol. 2010, 74, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Avila, M.; Mora, C.; Prado, M.D.C.; Zavala, M.; Paniagua, R.; Mexican Collaborative Group. Osteoprotegerin Is the Strongest Predictor for Progression of Arterial Calcification in Peritoneal Dialysis Patients. Am. J. Nephrol. 2017, 46, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Krzanowski, M.; Janda, K.; Dumnicka, P.; Dubiel, M.; Stompór, M.; Kuśnierz-Cabala, B.; Grodzicki, T.; Sułowicz, W. Relationship between aortic pulse wave velocity, selected proinflammatory cytokines, and vascular calcification parameters in peritoneal dialysis patients. J. Hypertens. 2014, 32, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Ketteler, M.; Sprague, S.M.; Covic, A.C.; Rastogi, A.; Spinowitz, B.; Rakov, V.; Walpen, S.; Floege, J. Effects of sucroferric oxyhydroxide and sevelamer carbonate on chronic kidney disease-mineral bone disorder parameters in dialysis patients. Nephrol. Dial. Transplant. 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mirfatahi, M.; Imani, H.; Tabibi, H.; Nasrollahi, A.; Hedayati, M. Effects of Flaxseed Oil on Serum Bone Turnover Markers in Hemodialysis Patients: A Randomized Controlled Trial. Iran. J. Kidney Dis. 2018, 12, 215–222. [Google Scholar]
- Greeviroj, P.; Kitrungphaiboon, T.; Katavetin, P.; Praditpornsilpa, K.; Eiam-Ong, S.; Jaber, B.L.; Susantitaphong, P. Cinacalcet for Treatment of Chronic Kidney Disease-Mineral and Bone Disorder: A Meta-Analysis of Randomized Controlled Trials. Nephron 2018, 139, 197–210. [Google Scholar] [CrossRef]
- Schwarz, A.; Merkel, S.; Leitolf, H.; Haller, H. The effect of cinacalcet on bone remodeling and renal function in transplant patients with persistent hyperparathyroidism. Transplantation 2011, 91, 560–565. [Google Scholar] [CrossRef]
- Hirai, T.; Nakashima, A.; Takasugi, N.; Yorioka, N. Association of nodular hyperplasia with resistance to cinacalcet therapy for secondary hyperparathyroidism in hemodialysis patients. Ther. Apher. Dial. 2010, 14, 577–582. [Google Scholar] [CrossRef]
- Shigematsu, T.; Lanthanum Carbonate Research Group. Three-year extension study of lanthanum carbonate therapy in Japanese hemodialysis patients. Clin. Exp. Nephrol. 2010, 14, 589–597. [Google Scholar] [CrossRef]
- Malluche, H.H.; Siami, G.A.; Swanepoel, C.; Wang, G.H.; Mawad, H.; Confer, S.; Smith, M.; Pratt, R.D.; Monier-Faugere, M.-C.; SPD405-307 Lanthanum Carbonate Study Group. Improvements in renal osteodystrophy in patients treated with lanthanum carbonate for two years. Clin. Nephrol. 2008, 70, 284–295. [Google Scholar]
- Gomes, T.S.; Aoike, D.T.; Baria, F.; Graciolli, F.G.; Moyses, R.M.A.; Cuppari, L. Effect of Aerobic Exercise on Markers of Bone Metabolism of Overweight and Obese Patients with Chronic Kidney Disease. J. Ren. Nutr. 2017, 27, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Tominari, T.; Hirata, M.; Matsumoto, C.; Hirata, J.; Murphy, G.; Nagase, H.; Miyaura, C.; Inada, M. Indoxyl sulfate, a uremic toxin in chronic kidney disease, suppresses both bone formation and bone resorption. FEBS Open Bio 2017, 7, 1178–1185. [Google Scholar] [CrossRef] [Green Version]
- Gauthier-Bastien, A.; Ung, R.-V.; Larivière, R.; Mac-Way, F.; Lebel, M.; Agharazii, M. Vascular remodeling and media calcification increases arterial stiffness in chronic kidney disease. Clin. Exp. Hypertens. 2014, 36, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Troib, A.; Guterman, M.; Rabkin, R.; Landau, D.; Segev, Y. Endurance exercise and growth hormone improve bone formation in young and growth-retarded chronic kidney disease rats. Nephrol. Dial. Transplant. 2016, 31, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.J.; Hilliard, B.; Swami, A.; Madara, J.C.; Rao, S.; Patel, T.; Gaughan, J.P.; Lee, J.; Gadegbeku, C.A.; Choi, E.T.; et al. Growth arrest-specific gene 6 (Gas6) levels are elevated in patients with chronic renal failure. Nephrol. Dial. Transplant. 2012, 27, 4166–4172. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Tang, K.; Zhang, Y.; Ma, Y.; Zhuang, R.; Zheng, X.; Jin, B.; Zhang, Y. Elevated Plasma Growth Arrest-Specific 6 Protein Levels Are Associated with the Severity of Disease During Hantaan Virus Infection in Humans. Viral Immunol. 2017, 30, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, J.; Ge, L.; Shan, J.; Zhang, C.; Liu, J. Growth arrest-specific protein 6 (Gas6) as a noninvasive biomarker for early detection of diabetic nephropathy. Clin. Exp. Hypertens. 2017, 39, 382–387. [Google Scholar] [CrossRef]
- Rankin, E.B.; Fuh, K.C.; Castellini, L.; Viswanathan, K.; Finger, E.C.; Diep, A.N.; LaGory, E.L.; Kariolis, M.S.; Chan, A.; Lindgren, D.; et al. Direct regulation of GAS6/AXL signaling by HIF promotes renal metastasis through SRC and MET. Proc. Natl. Acad. Sci. USA 2014, 111, 13373–13378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gustafsson, A.; Boström, A.-K.; Ljungberg, B.; Axelson, H.; Dahlbäck, B. Gas6 and the Receptor Tyrosine Kinase Axl in Clear Cell Renal Cell Carcinoma. PLoS ONE 2009, 4, e7575. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, A.; Martuszewska, D.; Johansson, M.; Ekman, C.; Hafizi, S.; Ljungberg, B.; Dahlbäck, B. Differential expression of Axl and Gas6 in renal cell carcinoma reflecting tumor advancement and survival. Clin. Cancer Res. 2009, 15, 4742–4749. [Google Scholar] [CrossRef]
- Gustafsson, A.; Fritz, H.K.M.; Dahlbäck, B. Gas6-Axl signaling in presence of Sunitinib is enhanced, diversified and sustained in renal tumor cells, resulting in tumor-progressive advantages. Exp. Cell Res. 2017, 355, 47–56. [Google Scholar] [CrossRef]
- Ciceri, P.; Elli, F.; Braidotti, P.; Falleni, M.; Tosi, D.; Bulfamante, G.; Block, G.A.; Cozzolino, M. Iron citrate reduces high phosphate-induced vascular calcification by inhibiting apoptosis. Atherosclerosis 2016, 254, 93–101. [Google Scholar] [CrossRef]
- Park, J.-K.; Theuer, S.; Kirsch, T.; Lindschau, C.; Klinge, U.; Heuser, A.; Plehm, R.; Todiras, M.; Carmeliet, P.; Haller, H.; et al. Growth arrest specific protein 6 participates in DOCA-induced target-organ damage. Hypertension 2009, 54, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Shuvy, M.; Abedat, S.; Beeri, R.; Valitsky, M.; Daher, S.; Kott-Gutkowski, M.; Gal-Moscovici, A.; Sosna, J.; Rajamannan, N.M.; Lotan, C. Raloxifene attenuates Gas6 and apoptosis in experimental aortic valve disease in renal failure. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H1829–H1840. [Google Scholar] [CrossRef] [Green Version]
- Qiu, C.; Zheng, H.; Tao, H.; Yu, W.; Jiang, X.; Li, A.; Jin, H.; Lv, A.; Li, H. Vitamin K2 inhibits rat vascular smooth muscle cell calcification by restoring the Gas6/Axl/Akt anti-apoptotic pathway. Mol. Cell. Biochem. 2017, 433, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, W.; Hu, Z.; Bian, J.; Ying, L.; Hong, G.; Qiu, Q.; Zhao, G.; Lu, Z. Protective Effects of Growth Arrest-Specific Protein 6 (Gas6) on Sepsis-Induced Acute Kidney Injury. Inflammation 2016, 39, 575–582. [Google Scholar] [CrossRef]
- Giangola, M.D.; Yang, W.-L.; Rajayer, S.R.; Kuncewitch, M.; Molmenti, E.; Nicastro, J.; Coppa, G.F.; Wang, P. Growth arrest-specific protein 6 protects against renal ischemia-reperfusion injury. J. Surg. Res. 2015, 199, 572–579. [Google Scholar] [CrossRef]
- Guo, J.-K.; Marlier, A.; Shi, H.; Shan, A.; Ardito, T.A.; Du, Z.-P.; Kashgarian, M.; Krause, D.S.; Biemesderfer, D.; Cantley, L.G. Increased tubular proliferation as an adaptive response to glomerular albuminuria. J. Am. Soc. Nephrol. 2012, 23, 429–437. [Google Scholar] [CrossRef]
- Eng, P.C.; Chua, W.C.-N.; Suk Peng Chew, V.; Wong, P.T.H.; Yin, J.L.; Hambly, B.; McLachlan, C.S. Chronic angiotensin-converting enzyme inhibition up-regulates mouse kidney growth arrest specific-6 protein and the AXL subfamily of receptor tyrosine kinases. J. Renin-Angiotensin-Aldosterone Syst. 2008, 9, 238–241. [Google Scholar] [PubMed] [Green Version]
- Batchu, S.N.; Hughson, A.; Gerloff, J.; Fowell, D.J.; Korshunov, V.A. Role of Axl in early kidney inflammation and progression of salt-dependent hypertension. Hypertension 2013, 62, 302–309. [Google Scholar] [CrossRef]
- Viegas, C.S.B.; Santos, L.; Macedo, A.L.; Matos, A.A.; Silva, A.P.; Neves, P.L.; Staes, A.; Gevaert, K.; Morais, R.; Vermeer, C.; et al. Chronic Kidney Disease Circulating Calciprotein Particles and Extracellular Vesicles Promote Vascular Calcification: A Role for GRP (Gla-Rich Protein). Arterioscler. Thromb. Vasc. Biol. 2018, 38, 575–587. [Google Scholar] [CrossRef]
- Hackeng, T.M.; Rosing, J.; Spronk, H.M.; Vermeer, C. Total chemical synthesis of human matrix Gla protein. Protein Sci. Publ. Protein Soc. 2001, 10, 864–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, P.A.; Otsuka, A.A.; Poser, J.W.; Kristaponis, J.; Raman, N. Characterization of a gamma-carboxyglutamic acid-containing protein from bone. Proc. Natl. Acad. Sci. USA 1976, 73, 1447–1451. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Spronk, H.M.H.; Skepper, J.N.; Hackeng, T.M.; Shanahan, C.M.; Vermeer, C.; Weissberg, P.L.; Proudfoot, D. Post-translational modifications regulate matrix Gla protein function: Importance for inhibition of vascular smooth muscle cell calcification. J. Thromb. Haemost. 2007, 5, 2503–2511. [Google Scholar] [CrossRef]
- Furie, B.; Furie, B.C. Molecular basis of vitamin K-dependent gamma-carboxylation. Blood 1990, 75, 1753–1762. [Google Scholar] [PubMed]
- Price, P.A.; Rice, J.S.; Williamson, M.K. Conserved phosphorylation of serines in the Ser-X-Glu/Ser(P) sequences of the vitamin K-dependent matrix Gla protein from shark, lamb, rat, cow, and human. Protein Sci. Publ. Protein Soc. 1994, 3, 822–830. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Teunissen, K.J.F.; Knapen, M.H.J.; Kwaijtaal, M.; van Diest, R.; Appels, A.; Reutelingsperger, C.P.; Cleutjens, J.P.M.; Vermeer, C. Novel conformation-specific antibodies against matrix gamma-carboxyglutamic acid (Gla) protein: Undercarboxylated matrix Gla protein as marker for vascular calcification. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1629–1633. [Google Scholar] [CrossRef]
- Price, P.A.; Faus, S.A.; Williamson, M.K. Warfarin causes rapid calcification of the elastic lamellae in rat arteries and heart valves. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1400–1407. [Google Scholar] [CrossRef] [PubMed]
- Silaghi, C.N.; Fodor, D.; Gheorghe, S.R.; Crăciun, A.M. Serum total matrix Gla protein: Reference interval in healthy adults and variations in patients with vascular and osteoarticular diseases. Clin. Chim. Acta 2019, 490, 128–134. [Google Scholar] [CrossRef]
- Price, P.A.; Thomas, G.R.; Pardini, A.W.; Figueira, W.F.; Caputo, J.M.; Williamson, M.K. Discovery of a high molecular weight complex of calcium, phosphate, fetuin, and matrix gamma-carboxyglutamic acid protein in the serum of etidronate-treated rats. J. Biol. Chem. 2002, 277, 3926–3934. [Google Scholar] [CrossRef] [PubMed]
- Roumeliotis, S.; Dounousi, E.; Eleftheriadis, T.; Liakopoulos, V. Association of the Inactive Circulating Matrix Gla Protein with Vitamin K Intake, Calcification, Mortality, and Cardiovascular Disease: A Review. Int. J. Mol. Sci. 2019, 20, 628. [Google Scholar] [CrossRef]
- Zoch, M.L.; Clemens, T.L.; Riddle, R.C. New insights into the biology of osteocalcin. Bone 2016, 82, 42–49. [Google Scholar] [CrossRef]
- Millar, S.A.; Patel, H.; Anderson, S.I.; England, T.J.; O’Sullivan, S.E. Osteocalcin, Vascular Calcification, and Atherosclerosis: A Systematic Review and Meta-analysis. Front. Endocrinol. 2017, 8, 183. [Google Scholar] [CrossRef]
- Plantalech, L.; Guillaumont, M.; Vergnaud, P.; Leclercq, M.; Delmas, P.D. Impairment of gamma carboxylation of circulating osteocalcin (bone gla protein) in elderly women. J. Bone Miner. Res. 1991, 6, 1211–1216. [Google Scholar] [CrossRef]
- Kanazawa, I. Osteocalcin as a hormone regulating glucose metabolism. World J. Diabetes 2015, 6, 1345–1354. [Google Scholar] [CrossRef]
- Ducy, P. The role of osteocalcin in the endocrine cross-talk between bone remodelling and energy metabolism. Diabetologia 2011, 54, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Cremers, S.; Garnero, P.; Seibel, J.M. Biochemical Markers of Bone Metabolism. In Principles of Bone Biology; Bilezikian, J.P., Raisz, L.G., Martin, T.J., Eds.; Elsevier: San Diego, CA, USA, 2008; Volume 2, pp. 1857–1881. [Google Scholar]
- Zhang, M.; Ni, Z.; Zhou, W.; Qian, J. Undercarboxylated osteocalcin as a biomarker of subclinical atherosclerosis in non-dialysis patients with chronic kidney disease. J. Biomed. Sci. 2015, 22, 75. [Google Scholar] [CrossRef]
- Csiky, B.; Sági, B.; Peti, A.; Lakatos, O.; Prémusz, V.; Sulyok, E. The Impact of Osteocalcin, Osteoprotegerin and Osteopontin on Arterial Stiffness in Chronic Renal Failure Patients on Hemodialysis. Kidney Blood Press. Res. 2017, 42, 1312–1321. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.K.; Sowa, H.; Hinoi, E.; Ferron, M.; Ahn, J.D.; Confavreux, C.; Dacquin, R.; Mee, P.J.; McKee, M.D.; Jung, D.Y.; et al. Endocrine regulation of energy metabolism by the skeleton. Cell 2007, 130, 456–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ducy, P.; Desbois, C.; Boyce, B.; Pinero, G.; Story, B.; Dunstan, C.; Smith, E.; Bonadio, J.; Goldstein, S.; Gundberg, C.; et al. Increased bone formation in osteocalcin-deficient mice. Nature 1996, 382, 448–452. [Google Scholar] [CrossRef]
- Hwang, Y.-C.; Jeong, I.-K.; Ahn, K.J.; Chung, H.Y. The uncarboxylated form of osteocalcin is associated with improved glucose tolerance and enhanced beta-cell function in middle-aged male subjects. Diabetes Metab. Res. Rev. 2009, 25, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.D.; Choudhury, A.B. Relationships between serum osteocalcin levels versus blood glucose, insulin resistance and markers of systemic inflammation in central Indian type 2 diabetic patients. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1631–1635. [Google Scholar] [PubMed]
- Laurance, S.; Lemarié, C.A.; Blostein, M.D. Growth arrest-specific gene 6 (gas6) and vascular hemostasis. Adv. Nutr. 2012, 3, 196–203. [Google Scholar] [CrossRef]
- Goruppi, S.; Ruaro, E.; Schneider, C. Gas6, the ligand of Axl tyrosine kinase receptor, has mitogenic and survival activities for serum starved NIH3T3 fibroblasts. Oncogene 1996, 12, 471–480. [Google Scholar]
- Kaesler, N.; Immendorf, S.; Ouyang, C.; Herfs, M.; Drummen, N.; Carmeliet, P.; Vermeer, C.; Floege, J.; Krüger, T.; Schlieper, G. Gas6 protein: Its role in cardiovascular calcification. BMC Nephrol. 2016, 17, 52. [Google Scholar] [CrossRef]
- Nakano, T.; Higashino, K.; Kikuchi, N.; Kishino, J.; Nomura, K.; Fujita, H.; Ohara, O.; Arita, H. Vascular smooth muscle cell-derived, Gla-containing growth-potentiating factor for Ca2+-mobilizing growth factors. J. Biol. Chem. 1995, 270, 5702–5705. [Google Scholar] [CrossRef] [PubMed]
- Hafizi, S.; Dahlbäck, B. Gas6 and protein S. Vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily. FEBS J. 2006, 273, 5231–5244. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Ma, Z.; Hu, W.; Wang, D.; Gong, B.; Fan, C.; Jiang, S.; Li, T.; Gao, J.; Yang, Y. Molecular insights of Gas6/TAM in cancer development and therapy. Cell Death Dis. 2017, 8, e2700. [Google Scholar] [CrossRef] [Green Version]
- Ekman, C.; Stenhoff, J.; Dahlbäck, B. Gas6 is complexed to the soluble tyrosine kinase receptor Axl in human blood. J. Thromb. Haemost. 2010, 8, 838–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shea, M.K.; Kritchevsky, S.B.; Hsu, F.-C.; Nevitt, M.; Booth, S.L.; Kwoh, C.K.; McAlindon, T.E.; Vermeer, C.; Drummen, N.; Harris, T.B.; et al. The association between vitamin K status and knee osteoarthritis features in older adults: The Health, Aging and Body Composition Study. Osteoarthr. Cartil. 2015, 23, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.S.B.; Simes, D.C.; Laizé, V.; Williamson, M.K.; Price, P.A.; Cancela, M.L. Gla-rich protein (GRP), a new vitamin K-dependent protein identified from sturgeon cartilage and highly conserved in vertebrates. J. Biol. Chem. 2008, 283, 36655–36664. [Google Scholar] [CrossRef] [PubMed]
- Cavaco, S.; Viegas, C.S.B.; Rafael, M.S.; Ramos, A.; Magalhães, J.; Blanco, F.J.; Vermeer, C.; Simes, D.C. Gla-rich protein is involved in the cross-talk between calcification and inflammation in osteoarthritis. Cell. Mol. Life Sci. 2016, 73, 1051–1065. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.S.B.; Rafael, M.S.; Enriquez, J.L.; Teixeira, A.; Vitorino, R.; Luís, I.M.; Costa, R.M.; Santos, S.; Cavaco, S.; Neves, J.; et al. Gla-rich protein acts as a calcification inhibitor in the human cardiovascular system. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 399–408. [Google Scholar] [CrossRef]
- Tagariello, A.; Luther, J.; Streiter, M.; Didt-Koziel, L.; Wuelling, M.; Surmann-Schmitt, C.; Stock, M.; Adam, N.; Vortkamp, A.; Winterpacht, A. Ucma—A novel secreted factor represents a highly specific marker for distal chondrocytes. Matrix Biol. 2008, 27, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.S.B.; Herfs, M.; Rafael, M.S.; Enriquez, J.L.; Teixeira, A.; Luís, I.M.; van ’t Hoofd, C.M.R.; João, A.; Maria, V.L.; Cavaco, S.; et al. Gla-rich protein is a potential new vitamin K target in cancer: Evidences for a direct GRP-mineral interaction. BioMed Res. Int. 2014, 2014, 340216. [Google Scholar] [CrossRef]
- Viegas, C.S.B.; Cavaco, S.; Neves, P.L.; Ferreira, A.; João, A.; Williamson, M.K.; Price, P.A.; Cancela, M.L.; Simes, D.C. Gla-rich protein is a novel vitamin K-dependent protein present in serum that accumulates at sites of pathological calcifications. Am. J. Pathol. 2009, 175, 2288–2298. [Google Scholar] [CrossRef]
- Sandor, R.; Leucuta, D.; Dronca, E.; Niculae, A.; Cret, V.; Silaghi, C.; Șoimița, S. Low Serum Paraoxonase-1 Lactonase and Arylesterase Activities in Obese Children and Adolescents. Rev. Romana Med. Lab. 2015, 23, 385–395. [Google Scholar] [CrossRef]
- Tjwa, M.; Bellido-Martin, L.; Lin, Y.; Lutgens, E.; Plaisance, S.; Bono, F.; Delesque-Touchard, N.; Hervé, C.; Moura, R.; Billiau, A.D.; et al. Gas6 promotes inflammation by enhancing interactions between endothelial cells, platelets, and leukocytes. Blood 2008, 111, 4096–4105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiner, D.E.; Tabatabai, S.; Tighiouart, H.; Elsayed, E.; Bansal, N.; Griffith, J.; Salem, D.N.; Levey, A.S.; Sarnak, M.J. Cardiovascular outcomes and all-cause mortality: Exploring the interaction between CKD and cardiovascular disease. Am. J. Kidney Dis. 2006, 48, 392–401. [Google Scholar] [CrossRef]
- Yamada, H.; Kuro-O., M.; Ishikawa, S.-E.; Funazaki, S.; Kusaka, I.; Kakei, M.; Hara, K. Daily variability in serum levels of calciprotein particles and their association with mineral metabolism parameters: A cross-sectional pilot study. Nephrology (Carlton) 2018, 23, 226–230. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Lond. Engl. 2016, 388, 1545–1602. [Google Scholar]
- Miner, J.H. The glomerular basement membrane. Exp. Cell Res. 2012, 318, 973–978. [Google Scholar] [CrossRef]
- Lee, P.; Wu, X. Review: Modifications of human serum albumin and their binding effect. Curr. Pharm. Des. 2015, 21, 1862–1865. [Google Scholar] [CrossRef] [PubMed]
- Soichiro, U.; Katsuhiro, G.; Hiroshi, H.; Yoshiyuki, M. Plasma Levels of Growth Arrest Specific Protein (Gas6) and the Soluble Form of Its Tyrosine Kinase Receptor Axl (sAxl) in Patients with Hepatocellular Carcinoma. J. Cancer Ther. 2013, 4, 632–639. [Google Scholar]
- Rathore, B.; Singh, M.; Kumar, V.; Misra, A. Osteocalcin: An emerging biomarker for bone turnover. Int. J. Res. Med. Sci. 2016, 4, 3670–3674. [Google Scholar] [CrossRef]





| Protein | Search Term | Date | Number of Results | Number of Studies Included | |
|---|---|---|---|---|---|
| By Search Term | Total | ||||
| MGP | “Matrix Gla protein AND kidney disease” | 15 August 2018 | 132 | 224 | 31 |
| “MGP AND kidney disease” | 2 September 2018 | 92 | |||
| OC | “Osteocalcin AND kidney disease” | 19 September 2018 | 235 | 470 | 29 |
| “Osteocalcine AND kidney disease” | 19 September 2018 | 235 | |||
| Gas6 | “Gas6 AND kidney disease” | 2 September 2018 | 34 | 63 | 16 |
| “Growth arrest specific protein 6 AND kidney disease” | 2 September 2018 | 29 | |||
| GRP | “GRP AND kidney disease” | 2 September 2018 | 38 | 45 | 1 |
| “Gla rich protein AND kidney disease” | 2 September 2018 | 5 | |||
| “Gla-rich protein AND kidney disease” | 2 September 2018 | 2 | |||
| Reference | Study Type | Number of Patients and Disease State | MGP Conformations | MGP Variation vs. Controls |
|---|---|---|---|---|
| Schlieper et al. 2011 [12] | cross-sectional | 188, HD | dp-ucMGP dp-cMGP | Both higher |
| Meuwese et al. 2015 [16] | cross-sectional | 97, HD | t-ucMGP | Lower |
| Schurgers et al. 2010 [17] | prospective cohort | 107, CKD stages II–V and HD | dp-ucMGP | Higher |
| Puzantian et al. 2018 [18] | prospective cohort | 137, CKD stages II–V | dp-ucMGP | Higher |
| Fain et al. 2018 [19] | cross-sectional | 37, HD | dp-ucMGP | Higher |
| Westenfeld et al. 2012 [20] | interventional | 53, HD | dp-ucMGP | Higher |
| Mansour et al. 2017 [21] | interventional | 60, Renal transplant | dp-ucMGP | Higher |
| Jansz et al. 2018 [22] | cross-sectional | 82, HD; 31, peritoneal dialysis; 36, Renal transplant | dp-ucMGP | Lower than HD |
| Boxma et al. 2012 [23] | prospective cohort | 60, Renal transplant | dp-ucMGP | Higher |
| Keyzer et al. 2015 [24] | prospective cohort | 518, Renal transplant | dp-ucMGP | Higher |
| Cranenburg et al. 2009 [25] | cross-sectional | 40, HD | ucMGP | Lower |
| Shroff et al. 2008 [26] | cross-sectional | 61, HD | ucMGP | Lower |
| Reference | Type of Cells | Findings |
|---|---|---|
| Willy et al. 2018 [29] | Supernatants from calcifying VSMCs incubated in serum of HD patients | Lower in HRO group than HF group |
| Willy et al. 2017 [30] | Supernatants from calcifying VSMCs incubated in serum of HD patients | Lower in MCO group than HF group Lower in HCO group than HF group |
| Khan et al. 2014 [31] | Induced nephrolithiasis on MDCK cells culture | Increased MGP expression |
| Lu et al. 2013 [32] | Kidneys of hyperoxaluric rats | Increased MGP expression |
| Reference | Pathology | MGP Conformations | Findings |
|---|---|---|---|
| Lomashvili et al. 2011 [33] | Induced renal failure with VC (rats) | cMGP, ucMGP | Both had increased expression in calcified aortic VSMCs |
| Lorenzen et al. 2012 [34] | Renal allograft calcification (humans) | MGP | Increased expression versus non-calcified allografts |
| Kramann et al. 2013 [35] | Calcific uremic arteriolopathy (humans) | ucMGP | Increased expression in skin |
| Shroff et al. 2008 [36] | HD (humans) | cMGP, ucMGP | Increased expression in calcified blood vessels |
| Wei et al. 2016 [37] | Renal tissue from CKD patients vs. healthy donors (humans) | cMGP, ucMGP | Both were present in calcified renal tissue |
| Reference | Type of Study | Number of Patients and Disease State | OC Conformation | Findings |
|---|---|---|---|---|
| Holden et al. 2010 [9] | cross-sectional | 172, CKD stages III–V | %ucOC | Higher as CKD progresses, associated with CKD stage |
| Gluba- Brzózka et al. 2016 [45] | cross-sectional | 80, CKD stages I–V | Intact OC | Non-significant decreasing trend as CKD advance |
| Kovesdy et al. 2011 [46] | prospective cohort | 639, Post renal transplant with CKD stages III–IV | Intact OC | Higher in post renal transplant patients with CKD stage IV than CKD stage III |
| Reference | Number of Subjects and Pathology | Drug/Treatment | Findings |
|---|---|---|---|
| Krause et al. 2018 [51] | 22 patients, HD | Partial body cutaneous exposure to UVB radiation | Reduced in serum |
| Ma et al. 2017 [52] | 31 patients with HD | Partial parathyroidectomy | Reduced in serum |
| Kettler et al. 2018 [59] | 1059 patients with hyperphosphatemic CKD | Sucroferric oxyhydroxide, Sevelamer carbonate (phosphate binders) | Increased in serum |
| Mirfatahi et al. 2018 [60] | 34 patients with HD | Flaxseed oil (omega-3 fatty acid and alpha-linolenic acid) | No significant change in serum |
| Greeviroj et al. 2018 [61] | 10,031 patients with HD (meta-analysis) | Cinacalcet (calcimimetic) | No significant change in serum |
| Schwarz et al. 2011 [62] | 58 patients with hyperparathyroidism after renal transplant | Cinacalcet | No significant change in serum |
| Hirai et al. 2010 [63] | 47 patients with HD | Cinacalcet | No significant change in serum |
| Shigematsu et al. 2010 [64] | 145 patients with HD | Lanthanum carbonate (phosphate binder) | No significant change in serum |
| Malluche et al. 2008 [65] | 65 patients with HD | Lanthanum carbonate | No significant change in serum |
| Gomes et al. 2017 [66] | 39 patients with non-dialysis dependent CKD | Aerobic exercise | No significant change in serum for cOC and ucOC |
| Watanabe et al. 2017 [67] | Osteoclast cell culture in mice | Indoxyl sulfate (uremic toxin) | Suppress expression |
| Gauthier-Bastien et al. 2014 [68] | Induced CKD by subtotal nephrectomy in mice | Calcium and phosphate diet, with vitamin D supplementation | De novo expression in VSMCs |
| Troib et al. 2016 [69] | Induced CKD by subtotal nephrectomy in rats | Endurance exercise | Improved expression in epiphyseal growth plate |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silaghi, C.N.; Ilyés, T.; Filip, V.P.; Farcaș, M.; van Ballegooijen, A.J.; Crăciun, A.M. Vitamin K Dependent Proteins in Kidney Disease. Int. J. Mol. Sci. 2019, 20, 1571. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20071571
Silaghi CN, Ilyés T, Filip VP, Farcaș M, van Ballegooijen AJ, Crăciun AM. Vitamin K Dependent Proteins in Kidney Disease. International Journal of Molecular Sciences. 2019; 20(7):1571. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20071571
Chicago/Turabian StyleSilaghi, Ciprian N., Tamás Ilyés, Vladimir P. Filip, Marius Farcaș, Adriana J. van Ballegooijen, and Alexandra M. Crăciun. 2019. "Vitamin K Dependent Proteins in Kidney Disease" International Journal of Molecular Sciences 20, no. 7: 1571. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20071571