Phosphatidylinositol 5 Phosphate (PI5P): From Behind the Scenes to the Front (Nuclear) Stage
Abstract
:1. Introduction
1.1. Phosphatidylinositol Signaling
1.2. Nuclear Lipid Signalling: Focus on Nuclear Phosphoinositides
2. Phosphatidylinositol 5 Phosphate: A Rare But Essential Lipid
2.1. PI5P Discovery
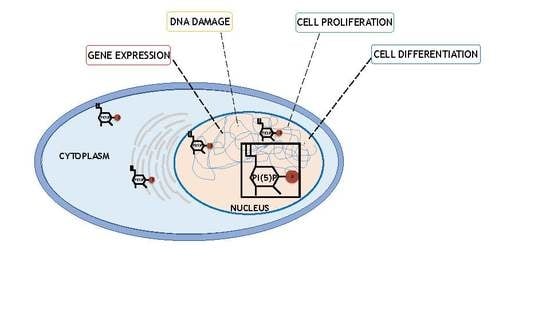
2.2. Changes in PI5P Levels Regulate Many Cellular Functions
3. Enzymes Involved in the Turnover of PI5P
3.1. PIKfyve/Phosphatidylinositol-3-Phosphate 5-Kinase
3.2. MTM-MTMR/Myotubularins
3.3. Phosphatidylinositol 5 Phosphate 4 Kinases (PI5P4K/PIP4K)
3.4. Type I/II PI(4,5)P2 4-Phosphatases
4. PI5P and Nuclear Outputs
4.1. Nuclear PI5P Regulates ING2-Mediated p53 Acetylation and Apoptosis During Stress Response
4.2. PIP4K2B and Type I PI(4,5)P2 4-Phosphatase Signaling Pathways Control Nuclear PI5P Levels and ING2 Functions During Cellular Stress Response
4.3. PI5P Involvement in ING2 DNA Binding and Stability
4.4. Pin1-Dependent PI5P Modulation Affects Oxidative Stress Cellular Response and Reactive Oxygen Species (ROS) Production
4.5. Cul3 E3 Ligase Complex Activity Is Inhibited by PI5P through Inhibition of Substrate-Specific Adaptor Protein (SPOP)
4.6. ATX1 Nuclear Export and Function Is Negatively Associated with Nuclear PI5P Pool Changes
4.7. SAP30/SAP30L-Sin3A-HDAC-Mediated DNA De-Acetylation Is Negatively Correlated with PI5P Nuclear Levels
4.8. PI5P Drives Uhrf1 to Specifically Bind Either H3K9me0 or H3K9me3
4.9. PI5P Regulates Myogenic Differentiation Modulating TAF3 DNA Binding to Specific DNA Regions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Berridge, M.J.; Irvine, R.F. Inositol phosphates and cell signalling. Nature 1989, 341, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, G.; De Camilli, P. Phosphoinositides in cell regulation and membrane dynamics. Nature 2006, 443, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Wymann, M.P.; Schneiter, R. Lipid signalling in disease. Nat. Rev. Mol. Cell. Biol. 2008, 9, 162–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poli, A.; Billi, A.M.; Mongiorgi, S.; Ratti, S.; McCubrey, J.A.; Suh, P.G.; Cocco, L.; Ramazzotti, G. Nuclear Phosphatidylinositol Signaling: Focus on Phosphatidylinositol Phosphate Kinases and Phospholipases, C. J. Cell. Physiol. 2016, 231, 1645–1655. [Google Scholar] [CrossRef]
- Dickson, E.J.; Hille, B. Understanding phosphoinositides: Rare, dynamic, and essential membrane phospholipids. Biochem. J. 2019, 476, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Epand, R.M. Features of the Phosphatidylinositol Cycle and its Role in Signal Transduction. J. Membr. Biol. 2017, 250, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.C. Protein kinase C: Structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem. Rev. 2001, 101, 2353–2364. [Google Scholar] [CrossRef]
- Burke, J.E. Structural Basis for Regulation of Phosphoinositide Kinases and Their Involvement in Human Disease. Mol. Cell 2018, 71, 653–673. [Google Scholar] [CrossRef]
- Simpson, L.; Parsons, R. PTEN: Life as a tumor suppressor. Exp. Cell Res. 2001, 264, 29–41. [Google Scholar] [CrossRef]
- Erneux, C.; Edimo, W.E.; Deneubourg, L.; Pirson, I. SHIP2 multiple functions: A balance between a negative control of PtdIns(3,4,5)P(3) level, a positive control of PtdIns(3,4)P(2) production, and intrinsic docking properties. J. Cell. Biochem. 2011, 112, 2203–2209. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.D.; Wells, W.W. Phosphorylation of rat liver nuclear envelopes. II. Characterization of in vitro lipid phosphorylation. J. Biol. Chem. 1983, 258, 9368–9373. [Google Scholar]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell. Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Cocco, L.; Gilmour, R.S.; Ognibene, A.; Letcher, A.J.; Manzoli, F.A.; Irvine, R.F. Synthesis of polyphosphoinositides in nuclei of Friend cells. Evidence for polyphosphoinositide metabolism inside the nucleus which changes with cell differentiation. Biochem. J. 1987, 248, 765–770. [Google Scholar] [CrossRef] [Green Version]
- Divecha, N.; Banfic, H.; Irvine, R.F. The polyphosphoinositide cycle exists in the nuclei of Swiss 3T3 cells under the control of a receptor (for IGF-I) in the plasma membrane, and stimulation of the cycle increases nuclear diacylglycerol and apparently induces translocation of protein kinase C to the nucleus. EMBO J. 1991, 10, 3207–3214. [Google Scholar]
- Payrastre, B.; Nievers, M.; Boonstra, J.; Breton, M.; Verkleij, A.J.; Van Bergen en Henegouwen, P.M. A differential location of phosphoinositide kinases, diacylglycerol kinase, and phospholipase C in the nuclear matrix. J. Biol. Chem. 1992, 267, 5078–5084. [Google Scholar]
- Vann, L.R.; Wooding, F.B.; Irvine, R.F.; Divecha, N. Metabolism and possible compartmentalization of inositol lipids in isolated rat-liver nuclei. Biochem. J. 1997, 327, 569–576. [Google Scholar] [CrossRef] [Green Version]
- Irvine, R.F. Nuclear lipid signalling. Nat. Rev. Mol. Cell. Biol. 2003, 4, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Merida, I.; Avila-Flores, A.; Merino, E. Diacylglycerol kinases: At the hub of cell signalling. Biochem. J. 2008, 409, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Suh, P.G.; Park, J.I.; Manzoli, L.; Cocco, L.; Peak, J.C.; Katan, M.; Fukami, K.; Kataoka, T.; Yun, S.; Ryu, S.H. Multiple roles of phosphoinositide-specific phospholipase C isozymes. BMB Rep. 2008, 41, 415–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capitani, S.; Cocco, L.; Maraldi, N.M.; Mazzotti, G.; Barnabei, O.; Manzoli, F.A. Inositol lipid phosphorylation in the cell nucleus. Adv. Enzym. Regul. 1991, 31, 399–416. [Google Scholar] [CrossRef]
- Divecha, N.; Letcher, A.J.; Banfic, H.H.; Rhee, S.G.; Irvine, R.F. Changes in the components of a nuclear inositide cycle during differentiation in murine erythroleukaemia cells. Biochem. J. 1995, 312, 63–67. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, J.; Strunk, B.S.; Weisman, L.S. PI5P and PI(3,5)P2: Minor, but Essential Phosphoinositides. Cell Struct. Funct. 2017, 42, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Rameh, L.E.; Tolias, K.F.; Duckworth, B.C.; Cantley, L.C. A new pathway for synthesis of phosphatidylinositol-4,5-bisphosphate. Nature 1997, 390, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Nunes, J.A.; Guittard, G. An Emerging Role for PI5P in T Cell Biology. Front. Immunol. 2013, 4, 80. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.B.; Hinchliffe, K.A.; Ciruela, A.; Letcher, A.J.; Irvine, R.F. Thrombin stimulation of platelets causes an increase in phosphatidylinositol 5-phosphate revealed by mass assay. FEBS Lett. 2000, 475, 57–60. [Google Scholar] [CrossRef] [Green Version]
- Coronas, S.; Ramel, D.; Pendaries, C.; Gaits-Iacovoni, F.; Tronchere, H.; Payrastre, B. PtdIns5P: A little phosphoinositide with big functions? Biochem. Soc. Symp. 2007, 74, 117–128. [Google Scholar] [CrossRef]
- Grainger, D.L.; Tavelis, C.; Ryan, A.J.; Hinchliffe, K.A. Involvement of phosphatidylinositol 5-phosphate in insulin-stimulated glucose uptake in the L6 myotube model of skeletal muscle. Pflug. Arch. Eur. J. Physiol. 2011, 462, 723–732. [Google Scholar] [CrossRef]
- Viaud, J.; Lagarrigue, F.; Ramel, D.; Allart, S.; Chicanne, G.; Ceccato, L.; Courilleau, D.; Xuereb, J.M.; Pertz, O.; Payrastre, B.; et al. Phosphatidylinositol 5-phosphate regulates invasion through binding and activation of Tiam1. Nat. Commun. 2014, 5, 4080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boal, F.; Puhar, A.; Xuereb, J.M.; Kunduzova, O.; Sansonetti, P.J.; Payrastre, B.; Tronchere, H. PI5P Triggers ICAM-1 Degradation in Shigella Infected Cells, Thus Dampening Immune Cell Recruitment. Cell Rep. 2016, 14, 750–759. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, T.; Takemura, N.; Standley, D.M.; Akira, S.; Kawai, T. The second messenger phosphatidylinositol-5-phosphate facilitates antiviral innate immune signaling. Cell Host Microbe 2013, 14, 148–158. [Google Scholar] [CrossRef]
- Sbrissa, D.; Ikonomov, O.C.; Shisheva, A. PIKfyve, a mammalian ortholog of yeast Fab1p lipid kinase, synthesizes 5-phosphoinositides. Effect of insulin. J. Biol. Chem. 1999, 274, 21589–21597. [Google Scholar] [CrossRef]
- Shisheva, A.; Sbrissa, D.; Ikonomov, O. Cloning, characterization, and expression of a novel Zn2+-binding FYVE finger-containing phosphoinositide kinase in insulin-sensitive cells. Mol. Cell. Biol. 1999, 19, 623–634. [Google Scholar] [CrossRef]
- Sbrissa, D.; Ikonomov, O.C.; Deeb, R.; Shisheva, A. Phosphatidylinositol 5-phosphate biosynthesis is linked to PIKfyve and is involved in osmotic response pathway in mammalian cells. J. Biol. Chem. 2002, 277, 47276–47284. [Google Scholar] [CrossRef] [PubMed]
- Shisheva, A. PIKfyve and its Lipid products in health and in sickness. Curr. Top. Microbiol. Immunol. 2012, 362, 127–162. [Google Scholar]
- Sbrissa, D.; Ikonomov, O.C.; Filios, C.; Delvecchio, K.; Shisheva, A. Functional dissociation between PIKfyve-synthesized PtdIns5P and PtdIns(3,5)P2 by means of the PIKfyve inhibitor YM201636. Am. J. Physiol. Cell Physiol. 2012, 303, C436–C446. [Google Scholar] [CrossRef] [Green Version]
- Sbrissa, D.; Ikonomov, O.C.; Shisheva, A. PIKfyve lipid kinase is a protein kinase: Downregulation of 5’-phosphoinositide product formation by autophosphorylation. Biochemistry 2000, 39, 15980–15989. [Google Scholar] [CrossRef]
- Shisheva, A.; Sbrissa, D.; Ikonomov, O. Plentiful PtdIns5P from scanty PtdIns(3,5)P2 or from ample PtdIns? PIKfyve-dependent models: Evidence and speculation (response to: DOI 10.1002/bies.201300012). Bioessays 2015, 37, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Vaccari, I.; Dina, G.; Tronchere, H.; Kaufman, E.; Chicanne, G.; Cerri, F.; Wrabetz, L.; Payrastre, B.; Quattrini, A.; Weisman, L.S.; et al. Genetic interaction between MTMR2 and FIG4 phospholipid phosphatases involved in Charcot-Marie-Tooth neuropathies. PLoS Genet. 2011, 7, e1002319. [Google Scholar] [CrossRef]
- Oppelt, A.; Lobert, V.H.; Haglund, K.; Mackey, A.M.; Rameh, L.E.; Liestol, K.; Schink, K.O.; Pedersen, N.M.; Wenzel, E.M.; Haugsten, E.M.; et al. Production of phosphatidylinositol 5-phosphate via PIKfyve and MTMR3 regulates cell migration. EMBO Rep. 2013, 14, 57–64. [Google Scholar] [CrossRef]
- Blondeau, F.; Laporte, J.; Bodin, S.; Superti-Furga, G.; Payrastre, B.; Mandel, J.L. Myotubularin, a phosphatase deficient in myotubular myopathy, acts on phosphatidylinositol 3-kinase and phosphatidylinositol 3-phosphate pathway. Hum. Mol. Genet. 2000, 9, 2223–2229. [Google Scholar] [CrossRef] [Green Version]
- Taylor, G.S.; Maehama, T.; Dixon, J.E. Myotubularin, a protein tyrosine phosphatase mutated in myotubular myopathy, dephosphorylates the lipid second messenger, phosphatidylinositol 3-phosphate. Proc. Natl. Acad. Sci. USA 2000, 97, 8910–8915. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.A.; Taylor, G.S.; Torgersen, K.M.; Dixon, J.E. Myotubularin and MTMR2, phosphatidylinositol 3-phosphatases mutated in myotubular myopathy and type 4B Charcot-Marie-Tooth disease. J. Biol. Chem. 2002, 277, 4526–4531. [Google Scholar] [CrossRef]
- Schaletzky, J.; Dove, S.K.; Short, B.; Lorenzo, O.; Clague, M.J.; Barr, F.A. Phosphatidylinositol-5-phosphate activation and conserved substrate specificity of the myotubularin phosphatidylinositol 3-phosphatases. Curr. Biol. 2003, 13, 504–509. [Google Scholar] [CrossRef]
- Doerks, T.; Strauss, M.; Brendel, M.; Bork, P. GRAM, a novel domain in glucosyltransferases, myotubularins and other putative membrane-associated proteins. Trends. Biochem. Sci. 2000, 25, 483–485. [Google Scholar] [CrossRef]
- Begley, M.J.; Dixon, J.E. The structure and regulation of myotubularin phosphatases. Curr. Opin. Struct. Biol. 2005, 15, 614–620. [Google Scholar] [CrossRef]
- Begley, M.J.; Taylor, G.S.; Kim, S.A.; Veine, D.M.; Dixon, J.E.; Stuckey, J.A. Crystal structure of a phosphoinositide phosphatase, MTMR2: Insights into myotubular myopathy and Charcot-Marie-Tooth syndrome. Mol. Cell 2003, 12, 1391–1402. [Google Scholar] [CrossRef]
- Begley, M.J.; Taylor, G.S.; Brock, M.A.; Ghosh, P.; Woods, V.L.; Dixon, J.E. Molecular basis for substrate recognition by MTMR2, a myotubularin family phosphoinositide phosphatase. Proc. Natl. Acad. Sci. USA 2006, 103, 927–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, J.H.; Irvine, R.F. Evolutionarily conserved structural changes in phosphatidylinositol 5-phosphate 4-kinase (PI5P4K) isoforms are responsible for differences in enzyme activity and localization. Biochem. J. 2013, 454, 49–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulley, S.J.; Clarke, J.H.; Droubi, A.; Giudici, M.L.; Irvine, R.F. Exploring phosphatidylinositol 5-phosphate 4-kinase function. Adv. Biol. Regul. 2015, 57, 193–202. [Google Scholar] [CrossRef] [Green Version]
- Fiume, R.; Stijf-Bultsma, Y.; Shah, Z.H.; Keune, W.J.; Jones, D.R.; Jude, J.G.; Divecha, N. PIP4K and the role of nuclear phosphoinositides in tumour suppression. Biochim. Biophys. Acta 2015, 1851, 898–910. [Google Scholar] [CrossRef]
- Rao, V.D.; Misra, S.; Boronenkov, I.V.; Anderson, R.A.; Hurley, J.H. Structure of type IIbeta phosphatidylinositol phosphate kinase: A protein kinase fold flattened for interfacial phosphorylation. Cell 1998, 94, 829–839. [Google Scholar] [CrossRef]
- Fiume, R.; Jones, D.R.; Divecha, N. PIP4K2B: Coupling GTP Sensing to PtdIns5P Levels to Regulate Tumorigenesis. Trends Biochem. Sci. 2016, 41, 473–475. [Google Scholar] [CrossRef] [PubMed]
- Sumita, K.; Lo, Y.H.; Takeuchi, K.; Senda, M.; Kofuji, S.; Ikeda, Y.; Terakawa, J.; Sasaki, M.; Yoshino, H.; Majd, N.; et al. The Lipid Kinase PI5P4Kbeta Is an Intracellular GTP Sensor for Metabolism and Tumorigenesis. Mol. Cell 2016, 61, 187–198. [Google Scholar] [CrossRef] [PubMed]
- van den Bout, I.; Divecha, N. PIP5K-driven PtdIns(4,5)P2 synthesis: Regulation and cellular functions. J. Cell Sci. 2009, 122, 3837–3850. [Google Scholar] [CrossRef] [PubMed]
- Al-Ramahi, I.; Giridharan, S.S.P.; Chen, Y.C.; Patnaik, S.; Safren, N.; Hasegawa, J.; de Haro, M.; Wagner Gee, A.K.; Titus, S.A.; Jeong, H.; et al. Inhibition of PIP4Kgamma ameliorates the pathological effects of mutant huntingtin protein. Elife 2017, 6, e29123. [Google Scholar] [CrossRef] [PubMed]
- Lundquist, M.R.; Goncalves, M.D.; Loughran, R.M.; Possik, E.; Vijayaraghavan, T.; Yang, A.; Pauli, C.; Ravi, A.; Verma, A.; Yang, Z.; et al. Phosphatidylinositol-5-Phosphate 4-Kinases Regulate Cellular Lipid Metabolism By Facilitating Autophagy. Mol. Cell 2018, 70, 531–544. [Google Scholar] [CrossRef]
- Emerling, B.M.; Hurov, J.B.; Poulogiannis, G.; Tsukazawa, K.S.; Choo-Wing, R.; Wulf, G.M.; Bell, E.L.; Shim, H.S.; Lamia, K.A.; Rameh, L.E.; et al. Depletion of a putatively druggable class of phosphatidylinositol kinases inhibits growth of p53-null tumors. Cell 2013, 155, 844–857. [Google Scholar] [CrossRef]
- Keune, W.J.; Sims, A.H.; Jones, D.R.; Bultsma, Y.; Lynch, J.T.; Jirstrom, K.; Landberg, G.; Divecha, N. Low PIP4K2B expression in human breast tumors correlates with reduced patient survival: A role for PIP4K2B in the regulation of E-cadherin expression. Cancer Res. 2013, 73, 6913–6925. [Google Scholar] [CrossRef]
- Jude, J.G.; Spencer, G.J.; Huang, X.; Somerville, T.D.D.; Jones, D.R.; Divecha, N.; Somervaille, T.C.P. A targeted knockdown screen of genes coding for phosphoinositide modulators identifies PIP4K2A as required for acute myeloid leukemia cell proliferation and survival. Oncogene 2015, 34, 1253–1262. [Google Scholar] [CrossRef]
- Shim, H.; Wu, C.; Ramsamooj, S.; Bosch, K.N.; Chen, Z.; Emerling, B.M.; Yun, J.; Liu, H.; Choo-Wing, R.; Yang, Z.; et al. Deletion of the gene Pip4k2c, a novel phosphatidylinositol kinase, results in hyperactivation of the immune system. Proc. Natl. Acad. Sci. USA 2016, 113, 7596–7601. [Google Scholar] [CrossRef] [Green Version]
- Niebuhr, K.; Giuriato, S.; Pedron, T.; Philpott, D.J.; Gaits, F.; Sable, J.; Sheetz, M.P.; Parsot, C.; Sansonetti, P.J.; Payrastre, B. Conversion of PtdIns(4,5)P(2) into PtdIns(5)P by the S.flexneri effector IpgD reorganizes host cell morphology. EMBO J. 2002, 21, 5069–5078. [Google Scholar] [CrossRef] [PubMed]
- Ungewickell, A.; Hugge, C.; Kisseleva, M.; Chang, S.C.; Zou, J.; Feng, Y.; Galyov, E.E.; Wilson, M.; Majerus, P.W. The identification and characterization of two phosphatidylinositol-4,5-bisphosphate 4-phosphatases. Proc. Natl. Acad. Sci. USA 2005, 102, 18854–18859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mason, D.; Mallo, G.V.; Terebiznik, M.R.; Payrastre, B.; Finlay, B.B.; Brumell, J.H.; Rameh, L.; Grinstein, S. Alteration of epithelial structure and function associated with PtdIns(4,5)P2 degradation by a bacterial phosphatase. J. Gen. Physiol. 2007, 129, 267–283. [Google Scholar] [CrossRef]
- Zou, J.; Marjanovic, J.; Kisseleva, M.V.; Wilson, M.; Majerus, P.W. Type I phosphatidylinositol-4,5-bisphosphate 4-phosphatase regulates stress-induced apoptosis. Proc. Natl. Acad. Sci. USA 2007, 104, 16834–16839. [Google Scholar] [CrossRef] [PubMed]
- Divecha, N.; Banfic, H.; Irvine, R.F. Inositides and the nucleus and inositides in the nucleus. Cell 1993, 74, 405–407. [Google Scholar] [CrossRef]
- Gong, W.; Suzuki, K.; Russell, M.; Riabowol, K. Function of the ING family of PHD proteins in cancer. Int. J. Biochem. Cell Biol. 2005, 37, 1054–1065. [Google Scholar] [CrossRef] [PubMed]
- Gozani, O.; Karuman, P.; Jones, D.R.; Ivanov, D.; Cha, J.; Lugovskoy, A.A.; Baird, C.L.; Zhu, H.; Field, S.J.; Lessnick, S.L.; et al. The PHD finger of the chromatin-associated protein ING2 functions as a nuclear phosphoinositide receptor. Cell 2003, 114, 99–111. [Google Scholar] [CrossRef]
- Shi, X.; Hong, T.; Walter, K.L.; Ewalt, M.; Michishita, E.; Hung, T.; Carney, D.; Pena, P.; Lan, F.; Kaadige, M.R.; et al. ING2 PHD domain links histone H3 lysine 4 methylation to active gene repression. Nature 2006, 442, 96–99. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Kikuchi, K.; Takano, Y. ING Genes Work as Tumor Suppressor Genes in the Carcinogenesis of Head and Neck Squamous Cell Carcinoma. J. Oncol. 2011, 2011, 963614. [Google Scholar] [CrossRef]
- Zeng, S.; Baillargeat, D.; Ho, H.P.; Yong, K.T. Nanomaterials enhanced surface plasmon resonance for biological and chemical sensing applications. Chem. Soc. Rev. 2014, 43, 3426–3452. [Google Scholar] [CrossRef]
- Cho, Y.E.; Lomeda, R.A.; Ryu, S.H.; Lee, J.H.; Beattie, J.H.; Kwun, I.S. Cellular Zn depletion by metal ion chelators (TPEN, DTPA and chelex resin) and its application to osteoblastic MC3T3-E1 cells. Nutr. Res. Pract. 2007, 1, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.R.; Bultsma, Y.; Keune, W.J.; Halstead, J.R.; Elouarrat, D.; Mohammed, S.; Heck, A.J.; D’Santos, C.S.; Divecha, N. Nuclear PtdIns5P as a transducer of stress signaling: An in vivo role for PIP4Kbeta. Mol. Cell 2006, 23, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef] [PubMed]
- Bua, D.J.; Martin, G.M.; Binda, O.; Gozani, O. Nuclear phosphatidylinositol-5-phosphate regulates ING2 stability at discrete chromatin targets in response to DNA damage. Sci. Rep. 2013, 3, 2137. [Google Scholar] [CrossRef]
- Keune, W.J.; Jones, D.R.; Bultsma, Y.; Sommer, L.; Zhou, X.Z.; Lu, K.P.; Divecha, N. Regulation of phosphatidylinositol-5-phosphate signaling by Pin1 determines sensitivity to oxidative stress. Sci. Signal 2012, 5, ra86. [Google Scholar] [CrossRef] [PubMed]
- Keune, W.J.; Jones, D.R.; Divecha, N. PtdIns5P and Pin1 in oxidative stress signaling. Adv. Biol. Regul. 2013, 53, 179–189. [Google Scholar] [CrossRef]
- Rostam, M.A.; Piva, T.J.; Rezaei, H.B.; Kamato, D.; Little, P.J.; Zheng, W.; Osman, N. Peptidyl-prolyl isomerases: Functionality and potential therapeutic targets in cardiovascular disease. Clin. Exp. Pharmacol. Physiol. 2015, 42, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide Ion: Generation and Chemical Implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef]
- Nagai, Y.; Kojima, T.; Muro, Y.; Hachiya, T.; Nishizawa, Y.; Wakabayashi, T.; Hagiwara, M. Identification of a novel nuclear speckle-type protein, SPOP. FEBS Lett. 1997, 418, 23–26. [Google Scholar] [CrossRef] [Green Version]
- Mazzucotelli, E.; Belloni, S.; Marone, D.; De Leonardis, A.; Guerra, D.; Di Fonzo, N.; Cattivelli, L.; Mastrangelo, A. The e3 ubiquitin ligase gene family in plants: Regulation by degradation. Curr. Genom. 2006, 7, 509–522. [Google Scholar] [CrossRef]
- Bunce, M.W.; Boronenkov, I.V.; Anderson, R.A. Coordinated activation of the nuclear ubiquitin ligase Cul3-SPOP by the generation of phosphatidylinositol 5-phosphate. J. Biol. Chem. 2008, 283, 8678–8686. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Venegas, R.; Sadder, M.; Hlavacka, A.; Baluska, F.; Xia, Y.; Lu, G.; Firsov, A.; Sarath, G.; Moriyama, H.; Dubrovsky, J.G.; et al. The Arabidopsis homolog of trithorax, ATX1, binds phosphatidylinositol 5-phosphate, and the two regulate a common set of target genes. Proc. Natl. Acad. Sci. USA 2006, 103, 6049–6054. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Venegas, R.; Pien, S.; Sadder, M.; Witmer, X.; Grossniklaus, U.; Avramova, Z. ATX-1, an Arabidopsis homolog of trithorax, activates flower homeotic genes. Curr. Biol. 2003, 13, 627–637. [Google Scholar] [CrossRef]
- Wortman, J.R.; Haas, B.J.; Hannick, L.I.; Smith, R.K., Jr.; Maiti, R.; Ronning, C.M.; Chan, A.P.; Yu, C.; Ayele, M.; Whitelaw, C.A.; et al. Annotation of the Arabidopsis genome. Plant Physiol. 2003, 132, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Ndamukong, I.; Zhao, Y.; Xia, Y.; Riethoven, J.J.; Jones, D.R.; Divecha, N.; Avramova, Z. Divergent functions of the myotubularin (MTM) homologs AtMTM1 and AtMTM2 in Arabidopsis thaliana: Evolution of the plant MTM family. Plant J. 2012, 70, 866–878. [Google Scholar] [CrossRef] [PubMed]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef] [Green Version]
- Greer, E.L.; Shi, Y. Histone methylation: A dynamic mark in health, disease and inheritance. Nat. Rev. Genet. 2012, 13, 343–357. [Google Scholar] [CrossRef]
- Viiri, K.M.; Korkeamaki, H.; Kukkonen, M.K.; Nieminen, L.K.; Lindfors, K.; Peterson, P.; Maki, M.; Kainulainen, H.; Lohi, O. SAP30L interacts with members of the Sin3A corepressor complex and targets Sin3A to the nucleolus. Nucl. Acids Res. 2006, 34, 3288–3298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagelkruys, A.; Sawicka, A.; Rennmayr, M.; Seiser, C. The biology of HDAC in cancer: The nuclear and epigenetic components. Handb. Exp. Pharmacol. 2011, 206, 13–37. [Google Scholar]
- Saunders, A.; Huang, X.; Fidalgo, M.; Reimer, M.H., Jr.; Faiola, F.; Ding, J.; Sanchez-Priego, C.; Guallar, D.; Saenz, C.; Li, D.; et al. The SIN3A/HDAC Corepressor Complex Functionally Cooperates with NANOG to Promote Pluripotency. Cell Rep. 2017, 18, 1713–1726. [Google Scholar] [CrossRef] [PubMed]
- Viiri, K.M.; Janis, J.; Siggers, T.; Heinonen, T.Y.; Valjakka, J.; Bulyk, M.L.; Maki, M.; Lohi, O. DNA-binding and -bending activities of SAP30L and SAP30 are mediated by a zinc-dependent module and monophosphoinositides. Mol. Cell. Biol. 2009, 29, 342–356. [Google Scholar] [CrossRef]
- Hermann, A.; Goyal, R.; Jeltsch, A. The Dnmt1 DNA-(cytosine-C5)-methyltransferase methylates DNA processively with high preference for hemimethylated target sites. J. Biol. Chem. 2004, 279, 48350–48359. [Google Scholar] [CrossRef]
- Fang, J.; Cheng, J.; Wang, J.; Zhang, Q.; Liu, M.; Gong, R.; Wang, P.; Zhang, X.; Feng, Y.; Lan, W.; et al. Hemi-methylated DNA opens a closed conformation of UHRF1 to facilitate its histone recognition. Nat. Commun. 2016, 7, 11197. [Google Scholar] [CrossRef] [Green Version]
- Sidhu, H.; Capalash, N. UHRF1: The key regulator of epigenetics and molecular target for cancer therapeutics. Tumor Biol. 2017, 39, 1010428317692205. [Google Scholar] [CrossRef]
- Reynoird, N.; Gozani, O. Nuclear PI5P, Uhrf1, and the road not taken. Mol. Cell 2014, 54, 901–903. [Google Scholar] [CrossRef]
- Gelato, K.A.; Tauber, M.; Ong, M.S.; Winter, S.; Hiragami-Hamada, K.; Sindlinger, J.; Lemak, A.; Bultsma, Y.; Houliston, S.; Schwarzer, D.; et al. Accessibility of different histone H3-binding domains of UHRF1 is allosterically regulated by phosphatidylinositol 5-phosphate. Mol. Cell 2014, 54, 905–919. [Google Scholar] [CrossRef]
- Burley, S.K.; Roeder, R.G. Biochemistry and structural biology of transcription factor IID (TFIID). Annu. Rev. Biochem. 1996, 65, 769–799. [Google Scholar] [CrossRef]
- Deato, M.D.; Marr, M.T.; Sottero, T.; Inouye, C.; Hu, P.; Tjian, R. MyoD targets TAF3/TRF3 to activate myogenin transcription. Mol. Cell 2008, 32, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.C.; Chiang, C.M. The general transcription machinery and general cofactors. Crit. Rev. Biochem. Mol. Biol. 2006, 41, 105–178. [Google Scholar] [CrossRef] [PubMed]
- Stijf-Bultsma, Y.; Sommer, L.; Tauber, M.; Baalbaki, M.; Giardoglou, P.; Jones, D.R.; Gelato, K.A.; van Pelt, J.; Shah, Z.; Rahnamoun, H.; et al. The basal transcription complex component TAF3 transduces changes in nuclear phosphoinositides into transcriptional output. Mol. Cell 2015, 58, 453–467. [Google Scholar] [CrossRef]
- Blais, A.; Tsikitis, M.; Acosta-Alvear, D.; Sharan, R.; Kluger, Y.; Dynlacht, B.D. An initial blueprint for myogenic differentiation. Genes Dev. 2005, 19, 553–569. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poli, A.; Zaurito, A.E.; Abdul-Hamid, S.; Fiume, R.; Faenza, I.; Divecha, N. Phosphatidylinositol 5 Phosphate (PI5P): From Behind the Scenes to the Front (Nuclear) Stage. Int. J. Mol. Sci. 2019, 20, 2080. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20092080
Poli A, Zaurito AE, Abdul-Hamid S, Fiume R, Faenza I, Divecha N. Phosphatidylinositol 5 Phosphate (PI5P): From Behind the Scenes to the Front (Nuclear) Stage. International Journal of Molecular Sciences. 2019; 20(9):2080. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20092080
Chicago/Turabian StylePoli, Alessandro, Antonio Enrico Zaurito, Shidqiyyah Abdul-Hamid, Roberta Fiume, Irene Faenza, and Nullin Divecha. 2019. "Phosphatidylinositol 5 Phosphate (PI5P): From Behind the Scenes to the Front (Nuclear) Stage" International Journal of Molecular Sciences 20, no. 9: 2080. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20092080