Production and Characterization of Antioxidant Properties of Exopolysaccharide(s) from Peanibacillus mucilaginosus TKU032
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Identification of the TKU032 Strain
2.2. Culture Conditions for EPS Production
2.2.1. Effect of SPP Concentration
2.2.2. Effect of Culture Volume and Temperature
2.2.3. Effect of Initial pH
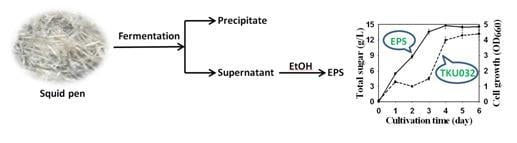
2.2.4. Time Course of EPS Production
2.3. Isolation and Molecular Weight Determination of TKU032 EPS
2.4. Analysis of EPS Hydrolysates
2.5. Antioxidant Activity Study
2.5.1. DPPH Radical Scavenging Activity Assay
2.5.2. Reducing Power
3. Materials and Methods
3.1. Materials
3.2. Screening and Identification of Microorganisms
3.3. Culture Conditions for EPS Production
3.3.1. Concentration of Carbon/Nitrogen Sources
3.3.2. Culture Volume
3.3.3. Culture Temperature and Medium pH
3.4. Total Sugar Measurement
3.5. Isolation of TKU032 EPS
3.6. Deproteinisation of TKU032 EPS
3.7. Purification of TKU032 EPS
3.8. MALDI-TOF MS Analysis
3.9. Analysis of EPS hydrolysates
3.10. Antioxidant Activity Assays
3.10.1. Measurement of DPPH Radical Scavenging Activity
3.10.2. Measurement of Reducing Power
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mahapatra, S.; Banerjee, D. Evaluation of in vitro antioxidant potency of exopolysaccharide from endophytic Fusarium solani SD5. Int. J. Biol. Macromol. 2013, 53, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Luo, J.; Ye, H.; Sun, Y.; Lu, Z.; Zeng, X. Production, characterization and antioxidant activities in vitro of exopolysaccharides from endophytic bacterium Paenibacillus polymyxa EJS-3. Carbohydr. Polym. 2009, 78, 275–281. [Google Scholar] [CrossRef]
- Wang, C.L.; Chen, C.J.; Nguyen, A.D.; Liang, T.W.; Twu, Y.K.; Huang, S.Y.; Wang, S.L. Environmental chitinous materials as adsorbents for the one-step purification of protease and chitosanase. Res. Chem. Intermed. 2014, 40, 2363–2370. [Google Scholar] [CrossRef]
- Ngan, L.T.K.; Wang, S.L.; Hiep, I.M.; Luong, P.M.; Vui, N.T.; Dinh, T.M.; Dzung, N.A. Preparation of chitosan nanoparticles by spray drying and their antibacterial activity. Res. Chem. Intermed. 2014, 40, 2165–2175. [Google Scholar] [CrossRef]
- Kumar, A.S.; Mody, K.; Jha, B. Bacterial exopolysaccharides—A perception. J. Basic Microbiol. 2007, 47, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Freitas, F.; Alves, V.D.; Pais, J.; Costa, N.; Oliveira, C.; Mafra, L.; Hilliou, L.; Oliveir, R.; Reis, M.A.M. Characterization of an extracellular polysaccharide produced by a Pseudomonas strain grown on glycerol. Bioresour. Technol. 2009, 100, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Liu, H.; Wu, S.; Pang, L.; Jia, M.; Fan, K.; Jia, S.; Jia, L. Production and in vitro antioxidant activity of exopolysaccharide by a mutant, Cordyceps militaris SU5–08. Int. J. Biol. Macromol. 2012, 51, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Confortin, F.G.; Marchetto, R.; Bettin, F.; Camassola, M.; Salvado, M.; Dillon, A.J. Production of Pleurotus sajor-caju strain PS-2001 biomass in submerged culture. J. Ind. Microbiol. Biotechnol. 2008, 35, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhao, Z.; Chen, S.F.; Li, Y.Q. Optimization for the production of exopolysaccharide from Fomes fomentarius in submerged culture and its antitumor effect in vitro. Bioresour. Technol. 2008, 99, 3187–3194. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.J.; Oh, J.Y.; Chang, H.Y.; Yun, J.W. Production of exopolysaccharides by submerged mycelial culture of a mushroom Tremella fuciformis. J. Biotechnol. 2006, 127, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.O.; Lim, J.M.; Joo, J.H.; Kim, S.W.; Hwang, H.J.; Choi, J.W.; Yun, J.W. Optimization of submerged culture condition for the production of mycelial biomass and exopolysaccharides by Agrocybe cylindracea. Bioresour. Technology 2005, 96, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.M.; Kim, S.W.; Hwang, H.J.; Joo, J.H.; Kim, H.O.; Choi, J.W.; Yun, J.W. Optimization of medium by orthogonal matrix method for submerged mycelial culture and exopolysaccharide production in Collybia maculata. Appl. Biochem. Biotechnol. 2004, 119, 159–170. [Google Scholar] [CrossRef]
- Xiao, J.H.; Chen, D.X.; Liu, J.W.; Liu, Z.L.; Wan, W.H.; Fang, N.; Xiao, Y.; Qi, Y.; Liang, Z.Q. Optimization of submerged culture requirements for the production of mycelial growth and exopolysaccharide by Cordyceps jiangxiensis JXPJ 0109. J. Appl. Microbiol. 2004, 96, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- De Baets, S.; Du Laing, S.; Francois, C.; Vandamme, E.J. Optimization of exopolysaccharide production by Tremella mesenterica NRRL Y-6158 through implementation of fed-batch fermentation. J. Ind. Microbiol. Biotechnol. 2002, 29, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, S.; Palaninathan, V.; Chauhan, N.; Sakamoto, Y.; Yoshida, Y.; Maekawa, T.; Mohanan, P.V.; Kumar, D.S. In vitro evaluation of antioxidant defense mechanism and hemocompatibility of mauran. Carbohydr. Polym. 2013, 98, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Jin, M.M.; Meng, J.; Gao, S.M.; Lu, R.R. Exopolysaccharide from Lactobacillus planterum LP6: Antioxidation and the effect on oxidative stress. Carbohydr. Polym. 2013, 98, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Satish kumar, R.; Yuvaraj, N.; Paari, K.A.; Pattukumar, V.; Arul, V. Production and purification of a novel exopolysaccharide from lactic acid bacterium Streptococcus phocae PI80 and its functional characteristics activity in vitro. Bioresour. Technol. 2011, 102, 4827–4833. [Google Scholar] [CrossRef] [PubMed]
- Park, N.H.; Choi, H.J.; Oh, D.K. Lactosucrose production by various microorganisms harboring levansucrase activity. Biotechnol. Lett. 2005, 27, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.W.; Clarke, M.A. Production and characterization of microbial levan. J. Agric. Food Chem. 1990, 38, 393–396. [Google Scholar] [CrossRef]
- Lee, I.Y.; Seo, W.T.; Kim, G.J.; Kim, M.K.; Ahn, S.G.; Kwon, G.S.; Park, Y.H. Optimization of fermentation conditions for production of exopolysaccharide by Bacillus polymyxa. Bioprocess Eng. 1997, 16, 71–75. [Google Scholar] [CrossRef]
- Freitas, F.D.; Alves, V.; Pais, J.; Carvalheira, M.; Costa, N.; Oliveira, R.; A.M. Reis, M. Production of a new exopolysaccharide (EPS) by Pseudomonas oleovorans NRRL B-14682 grown on glycerol. Process Biochem. 2010, 45, 297–305. [Google Scholar] [CrossRef]
- Xu, C.P.; Yun, J.W. Influence of aeration on the production and the quality of the exopolysaccharides from Paecilomyces tenuipes C240 in a stirred-tank fermenter. Enzyme Microb. Technol. 2004, 35, 33–39. [Google Scholar] [CrossRef]
- Pavlova, K.; Grigorova, D. Production and properties of exopolysaccharide by Rhodotorula acheniorum MC. Food Res. Int. 1999, 32, 473–477. [Google Scholar] [CrossRef]
- Béjar, V.; Llamas, I.; Calvo, C.; Quesada, E. Characterization of exopolysaccharides produced by 19 halophilic strains of the species Halomonas eurihalina. J. Biotechnol. 1998, 61, 135–141. [Google Scholar] [CrossRef]
- Wang, C.L.; Huang, T.H.; Liang, T.W.; Fang, C.Y.; Wang, S.L. Production and characterization of exopolysaccharides and antioxidantfrom Paenibacillus sp. TKU023. N. Biotechnol. 2011, 28, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Liu, K.C.; Liang, T.W.; Kuo, Y.H.; Wang, C.Y. In vitro antioxidant activity of liquor and semi-purified fractions from squid pen biowaste by Serratia ureilytica TKU013. Food Chem. 2010, 119, 1380–1385. [Google Scholar] [CrossRef]
- Zheng, J.Q.; Wang, J.Z.; Shi, C.W.; Mao, D.B.; He, P.X.; Xu, C.P. Characterization and antioxidant activity for exopolysaccharide from submerged culture of Boletus aereus. Process Biochem. 2014, 49, 1047–1053. [Google Scholar] [CrossRef]
- Fan, Y.; He, X.; Zhou, S.; Luo, A.; He, T.; Chun, Z. Composition analysis and antioxidant activity of polysaccharide from Dendrobium denneanum. Int. J. Biol. Macromol. 2009, 45, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, L.; Cheung, P.C.K.; Ooi, V.E.C. Molecular weight and anti-tumor activity of the water-soluble polysaccharides isolated by hot water and ultrasonic treatment from the sclerotia and mycelia of Pleurotus tuber-regium. Carbohydr. Polym. 2004, 56, 123–128. [Google Scholar] [CrossRef]
- Wang, S.L.; Wu, P.C.; Liang, T.W. Utilization of squid pen for the efficient production of chitosanase and antioxidants through prolonged autoclave treatment. Carbohydr. Res. 2009, 344, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Staub, A.M. Removal of protein-Sevag method. Methods Carbohydr. Chem. 1965, 5, 5–6. [Google Scholar]
- Oyaizu, M. Studies on products of browning reactions: Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]






© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, T.-W.; Tseng, S.-C.; Wang, S.-L. Production and Characterization of Antioxidant Properties of Exopolysaccharide(s) from Peanibacillus mucilaginosus TKU032. Mar. Drugs 2016, 14, 40. https://0-doi-org.brum.beds.ac.uk/10.3390/md14020040
Liang T-W, Tseng S-C, Wang S-L. Production and Characterization of Antioxidant Properties of Exopolysaccharide(s) from Peanibacillus mucilaginosus TKU032. Marine Drugs. 2016; 14(2):40. https://0-doi-org.brum.beds.ac.uk/10.3390/md14020040
Chicago/Turabian StyleLiang, Tzu-Wen, Shih-Chun Tseng, and San-Lang Wang. 2016. "Production and Characterization of Antioxidant Properties of Exopolysaccharide(s) from Peanibacillus mucilaginosus TKU032" Marine Drugs 14, no. 2: 40. https://0-doi-org.brum.beds.ac.uk/10.3390/md14020040