Neuroprotective Activity of Some Marine Fungal Metabolites in the 6-Hydroxydopamin- and Paraquat-Induced Parkinson’s Disease Models
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Identification of Compounds
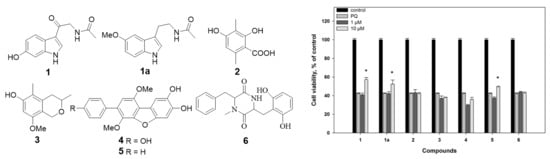
2.2. Biological Activities of the Studied Compounds
2.2.1. 6-Hydroxy-N-acetyl-β-oxotryptamine (1)
2.2.2. 3-O-Methylorsellinic acid (2) and 8-methoxy-3,5-dimethylisochroman-6-ol (3)
2.2.3. Candidusin A (4) and 4″-dehydroxycandidusin A (5)
2.2.4. Mactanamide (6)
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Strain
3.3. Cultivation of Fungus
3.4. Extraction and Isolation
3.5. Biological Activity of Compounds
3.5.1. Radical Scavenger Assay
3.5.2. Cell Line and Culture Condition
3.5.3. Cell Viability Assay
3.5.4. 6-Hydroxydopamine-Induced In Vitro Model of Parkinson’s Disease
3.5.5. Paraquat-Induced In Vitro Model of Parkinson’s Disease
3.5.6. Reactive Oxygen Species (ROS) Level Analysis in 6-OHDA- and PQ-Treated Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mhyre, T.R.; Boyd, J.T.; Hamill, R.W.; Maguire-Zeiss, K.A. Parkinson’s Disease. Sub-Cell. Biochem. 2012, 65, 389–455. [Google Scholar]
- Wang, Q.L.; Guo, C.; Qi, J.; Ma, J.H.; Liu, F.Y.; Lin, S.Q.; Zhang, C.Y.; Xie, W.D.; Zhuang, J.J.; Li, X. Protective effects of 3beta-angeloyloxy-8beta, 10beta-dihydroxyeremophila-7(11)-en-12, 8alpha-lactone on paraquat-induced oxidative injury in SH-SY5Y cells. J. Asian Nat. Prod. Res. 2018, 1–13. [Google Scholar]
- MacDonald-Wicks, L.K.; Wood, L.G.; Garg, M.L. Methodology for the determination of biological antioxidant capacity in vitro: A review. J. Sci. Food Agric. 2006, 86, 2046–2056. [Google Scholar] [CrossRef]
- Cheng, G.; Zielonka, M.; Dranka, B.; Kumar, S.N.; Myers, C.R.; Bennett, B.; Garces, A.M.; Dias Duarte Machado, L.G.; Thiebaut, D.; Ouari, O.; et al. Detection of mitochondria-generated reactive oxygen species in cells using multiple probes and methods: Potentials, pitfalls, and the future. J. Biol. Chem. 2018, 293, 10363–10380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastias-Candia, S.; Zolezzi, J.M.; Inestrosa, N.C. Revisiting the Paraquat-Induced Sporadic Parkinson’s Disease-Like Model. Mol. Neurobiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bove, J.; Prou, D.; Perier, C.; Przedborski, S. Toxin-induced models of Parkinson’s disease. NeuroRx 2005, 2, 484–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.W.; Ji, C.Z.; Gu, Q.Q.; Li, D.H.; Zhu, T.J. Three new polyketides from marine-derived fungus Aspergillus glaucus HB1-19. J. Asian Nat. Prod. Res. 2013, 15, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Li, X.M.; Liu, H.; Meng, L.H.; Wang, B.G. Two New Diphenylketones and a New Xanthone from Talaromyces islandicus EN-501, an Endophytic Fungus Derived from the Marine Red Alga Laurencia okamurai. Mar. Drugs 2016, 14, 223. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Lee, U.; Kang, J.S.; Choi, H.D.; Sona, B.W. A New Radical Scavenging Anthracene Glycoside, Asperflavin Ribofuranoside, and Polyketides from a Marine Isolate of the Fungus Microsporum. Chem. Pharm. Bull. 2006, 54, 882–883. [Google Scholar] [CrossRef]
- Chen, L.; Fang, Y.; Zhu, T.; Gu, Q.; Zhu, W. Gentisyl alcohol derivatives from the marine-derived fungus Penicillium terrestre. J. Nat. Prod. 2008, 71, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Kim, S.K.; Kang, J.S.; Choi, H.D.; Rho, J.R.; Son, B.W. Golmaenone, a New Diketopiperazine Alkaloid from the Marine-Derived Fungus Aspergillus sp. Chem. Pharm. Bull. 2004, 52, 375–376. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.M.; Wang, J.F.; Shi, X.F.; Wei, X.Y.; Chen, Y.C.; Zeng, Q.; Xiang, Y.; Chen, X.Y.; Tian, X.P.; Xiao, Z.H.; et al. Eurotiumins A(-)E, Five New Alkaloids from the Marine-Derived Fungus Eurotium sp. SCSIO F452. Mar. Drugs 2018, 16. [Google Scholar] [CrossRef] [PubMed]
- Li, D.L.; Li, X.M.; Li, T.G.; Dang, H.Y.; Wang, B.G. Dioxopiperazine alkaloids produced by the marine mangrove derived endophytic fungus Eurotium rubrum. Helv. Chim. Acta 2008, 91, 1888–1893. [Google Scholar] [CrossRef]
- Li, X.; Kim, S.K.; Nam, K.W.; Kang, J.S.; Choi, H.D.; Son, B.W. A new antibacterial dioxopiperazine alkaloid related to gliotoxin from a marine isolate of the fungus Pseudallescheria. J. Antibiot. (Tokyo) 2006, 59, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Akashi, S.; Kimura, T.; Takeuchi, T.; Kuramochi, K.; Kobayashi, S.; Sugawara, F.; Watanabe, N.; Arai, T. Neoechinulin A Impedes the Progression of Rotenone-Induced Cytotoxicity in PC12 Cells. Biol. Pharm. Bull. 2011, 34, 243–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, D.Y.; Choi, H. Natural products from marine organisms with neuroprotective activity in the experimental models of Alzheimer’s disease, Parkinson’s disease and ischemic brain stroke: Their molecular targets and action mechanisms. Arch. Pharm. Res. 2015, 38, 139–170. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.l.; Yao, X.l.; Liu, Z.; Zhang, H.; Li, W.; Li, Z.; Wang, G.L.; Pang, J.; Lin, Y.; Xu, Z.; et al. Protective effects of xyloketal B against MPP+-induced neurotoxicity in Caenorhabditis elegans and PC12 cells. Brain Res. 2010, 1332, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shen, C.; Guo, W.; Zhang, X.; Liu, S.; Liang, F.; Xu, Z.; Pei, Z.; Song, H.; Qiu, L.; et al. Synthesis and Neuroprotective Action of Xyloketal Derivatives in Parkinson’s Disease Models. Mar. Drugs 2013, 11, 5159–5189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, A.; Zhu, X.; Wang, X.; Chen, R.; Wang, H. Secalonic acid A protects dopaminergic neurons from 1-methyl-4-phenylpyridinium (MPP(+))-induced cell death via the mitochondrial apoptotic pathway. Eur. J. Pharmacol. 2013, 713, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Yurchenko, A.; Smetanina, O.; Ivanets, E.; Kalinovsky, A.; Khudyakova, Y.; Kirichuk, N.; Popov, R.; Bokemeyer, C.; von Amsberg, G.; Chingizova, E.; et al. Pretrichodermamides D–F from a Marine Algicolous Fungus Penicillium sp. KMM 4672. Mar. Drugs 2016, 14, 122. [Google Scholar] [CrossRef] [PubMed]
- Smetanina, O.F.; Yurchenko, A.N.; Ivanets, E.V.; Gerasimenko, A.V.; Trinh, P.T.H.; Ly, B.M.; Nhut, N.D.; Van, T.T.T.; Yurchenko, E.A.; Afiyatullov, S.S. Aromatic Metabolites of Marine Fungus Penicillium sp. KMM 4672 Associated with a Brown Alga Padina sp. Chem. Nat. Compd. 2017, 53, 600–602. [Google Scholar] [CrossRef]
- Martínez-Luis, S.; Gómez, J.F.; Spadafora, C.; Guzmán, H.M.; Gutiérrez, M. Antitrypanosomal alkaloids from the marine bacterium Bacillus pumilus. Molecules 2012, 17, 11146–11155. [Google Scholar] [CrossRef] [PubMed]
- Yongle, C.; Zeeck, A.; Zengxiang, C.; Zahner, H. Metabolic products of microorganisms. 222. β-Oxotryptamine derivatives isolated from Streptomyces ramulosus. J. Antibiot. 1983, 36, 913–915. [Google Scholar]
- Li, D.; Wang, F.; Xiao, X.; Zeng, X.; Gu, Q.Q.; Zhu, W. A new cytotoxic phenazine derivative from a deep sea bacterium Bacillus sp. Arch. Pharmacal Res. 2007, 30, 552–555. [Google Scholar] [CrossRef]
- Yang, J.; Wang, N.; Yuan, H.S.; Hu, J.C.; Dai, Y.C. A new sesquiterpene from the medicinal fungus Inonotus vaninii. Chem. Nat. Compd. 2013, 49, 261–263. [Google Scholar] [CrossRef]
- Elnaggar, M.S.; Ebada, S.S.; Ashour, M.L.; Ebrahim, W.; Singab, A.; Lin, W.; Liu, Z.; Proksch, P. Two new triterpenoids and a new naphthoquinone derivative isolated from a hard coral-derived fungus Scopulariopsis sp. Fitoterapia 2017, 116, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, N.; Nozawa, K.; Nakajima, S.; Kawai, K.I.; Udagawa, S.I. Studies on Fungal Products. XVI.: New Metabolites Related to 3-Methylorsellinate from Aspergillus silvaticus. Chem. Pharm. Bull. 1988, 36, 398–400. [Google Scholar] [CrossRef] [PubMed]
- Smetanina, O.F.; Yurchenko, A.N.; Pivkin, M.V.; Yurchenko, E.A.; Afiyatullov, S.S. Isochromene Metabolite from the Facultative Marine Fungus Penicillium Citrinum. Chem. Nat. Compd. 2011, 47, 118–119. [Google Scholar] [CrossRef]
- Yurchenko, A.N.; Ivanets, E.V.; Smetanina, O.F.; Pivkin, M.V.; Dyshlovoi, S.A.; von Amsberg, G.; Afiyatullov, S.S. Metabolites of the Marine Fungus Aspergillus candidus KMM 4676 Associated with a Kuril Colonial Ascidian. Chem. Nat. Compd. 2017, 53, 747–749. [Google Scholar] [CrossRef]
- Ivanets, E.V.; Yurchenko, A.N.; Smetanina, O.F.; Rasin, A.B.; Zhuravleva, O.I.; Pivkin, M.V.; Popov, R.S.; von Amsberg, G.; Afiyatullov, S.S.; Dyshlovoy, S.A. Asperindoles A–D and a p-terphenyl derivative from the ascidian-derived fungus Aspergillus sp. KMM 4676. Mar. Drugs 2018, 16. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, P.; Jensen, P.R.; Fenical, W. Mactanamide, a new fungistatic diketopiperazine produced by a marine Aspergillus sp. Nat. Prod. Lett. 1998, 12, 55–60. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Y.; Liu, D.; Proksch, P.; Yu, S.; Lin, W. Antioxidative phenolic compounds from a marine-derived fungus Aspergillus versicolor. Tetrahedron 2016, 72, 50–57. [Google Scholar] [CrossRef]
- Smetanina, O.F.; Yurchenko, A.N.; Ivanets, E.V.; Kirichuk, N.N.; Khudyakova, Y.V.; Yurchenko, E.A.; Afiyatullov, S.S. Metabolites of the Marine Fungus Penicillium citrinum Associated with a Brown Alga Padina sp. Chem. Nat. Compd. 2016, 52, 111–112. [Google Scholar] [CrossRef]
- Yen, G.C.; Chang, Y.C.; Sheu, F.; Chiang, H.C. Isolation and characterization of antioxidant compounds from Aspergillus candidus broth filtrate. J. Agric. Food Chem. 2001, 49, 1426–1431. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Sun, S.; Zhou, H.; Kong, X.; Zhu, T.; Li, D.; Gu, Q. Prenylated polyhydroxy-p-terphenyls from Aspergillus taichungensis ZHN-7-07. J. Nat. Prod. 2011, 74, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Guo, Z.K.; Jiang, R.; Wei, W.; Wang, T.; Guo, Y.; Song, Y.C.; Jiao, R.H.; Tan, R.X.; Ge, H.M. New Flavonol and Diterpenoids from the Endophytic Fungus Aspergillus sp. YXf3. Planta Med. 2013, 79, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Choi, B.K.; Trinh, P.T.H.; Lee, H.S.; Kang, J.S.; Van, T.T.T.; Lee, H.S.; Lee, J.S.; Lee, Y.J.; Lee, J. Suppression of RANKL-induced osteoclastogenesis by the metabolites from the marine fungus Aspergillus flocculosus isolated from a sponge Stylissa sp. Mar. Drugs 2018, 16. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Pallares, J.; Parga, J.A.; Munoz, A.; Rey, P.; Guerra, M.J.; Labandeira-Garcia, J.L. Mechanism of 6-hydroxydopamine neurotoxicity: The role of NADPH oxidase and microglial activation in 6-hydroxydopamine-induced degeneration of dopaminergic neurons. J. Neurochem. 2007, 103, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Villa, M.; Muñoz, P.; Ahumada-Castro, U.; Paris, I.; Jiménez, A.; Martínez, I.; Sevilla, F.; Segura-Aguilar, J. One-Electron Reduction of 6-Hydroxydopamine Quinone is Essential in 6-Hydroxydopamine Neurotoxicity. Neurotox. Res. 2013, 24, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Segura-Aguilar, J.; Kostrzewa, R.M. Neurotoxin Mechanisms and Processes Relevant to Parkinson’s Disease: An Update. Neurotox. Res. 2015, 27, 328–354. [Google Scholar] [CrossRef] [PubMed]
- Rappold, P.M.; Cui, M.; Chesser, A.S.; Tibbett, J.; Grima, J.C.; Duan, L.; Sen, N.; Javitch, J.A.; Tieu, K. Paraquat neurotoxicity is mediated by the dopamine transporter and organic cation transporter-3. Proc. Nat. Acad. Sci. USA 2011, 108, 20766–20771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dagda, R.K.; Das Banerjee, T.; Janda, E. How Parkinsonian toxins dysregulate the autophagy machinery. Internat. J. Mol. Sci. 2013, 14, 22163–22189. [Google Scholar] [CrossRef] [PubMed]
- Powers, R.; Lei, S.; Anandhan, A.; Marshall, D.D.; Worley, B.; Cerny, R.L.; Dodds, E.D.; Huang, Y.; Panayiotidis, M.I.; Pappa, A.; et al. Metabolic investigations of the molecular mechanisms associated with parkinson’s disease. Metabolites 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Zavala-Flores, L.; Garcia-Garcia, A.; Nandakumar, R.; Huang, Y.; Madayiputhiya, N.; Stanton, R.C.; Dodds, E.D.; Powers, R.; Franco, R. Alterations in Energy/Redox Metabolism Induced by Mitochondrial and Environmental Toxins: A Specific Role for Glucose-6-Phosphate-Dehydrogenase and the Pentose Phosphate Pathway in Paraquat Toxicity. ACS Chem. Boil. 2014, 9, 2032–2048. [Google Scholar] [CrossRef] [PubMed]
- Shirinzadeh, H.; Ince, E.; Westwell, A.D.; Gurer-Orhan, H.; Suzen, S. Novel indole-based melatonin analogues substituted with triazole, thiadiazole and carbothioamides: Studies on their antioxidant, chemopreventive and cytotoxic activities. J. Enzyme Inhib. Med. Chem. 2016, 31, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Chetsawang, J.; Govitrapong, P.; Chetsawang, B. Melatonin inhibits MPP+-induced caspase-mediated death pathway and DNA fragmentation factor-45 cleavage in SK-N-SH cultured cells. J. Pineal Res. 2007, 43, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Nopparat, C.; Chantadul, V.; Permpoonputtana, K.; Govitrapong, P. The anti-inflammatory effect of melatonin in SH-SY5Y neuroblastoma cells exposed to sublethal dose of hydrogen peroxide. Mech. Ageing Dev. 2017, 164, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Cheang, T.; Su, F.; Zheng, Y.; Chen, S.; Feng, J.; Pei, Z.; Chen, L. Melatonin inhibits rotenone-induced SH-SY5Y cell death via the downregulation of Dynamin-Related Protein 1 expression. Eur. J. Pharmacol. 2018, 819, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Mayo, J.C.; Sainz, R.M.; Uria, H.; Antolin, I.; Esteban, M.M.; Rodriguez, C. Melatonin prevents apoptosis induced by 6-hydroxydopamine in neuronal cells: Implications for Parkinson’s disease. J. Pineal Res. 1998, 24, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Mayo, J.C.; Sainz, R.M.; Antolı́n, I.; Rodriguez, C. Ultrastructural confirmation of neuronal protection by melatonin against the neurotoxin 6-hydroxydopamine cell damage. Brain Res. 1999, 818, 221–227. [Google Scholar] [CrossRef]
- Leutou, A.S.; Yun, K.; Son, B.W. Induced production of 6,9-dibromoflavasperone, a new radical scavenging naphthopyranone in the marine-mudflat-derived fungus Aspergillus niger. Arch. Pharmacal Res. 2016, 39, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Lyakhova, E.G.; Kolesnikova, S.A.; Kalinovsky, A.I.; Berdyshev, D.V.; Pislyagin, E.A.; Kuzmich, A.S.; Popov, R.S.; Dmitrenok, P.S.; Makarieva, T.N.; Stonik, V.A. Lissodendoric Acids A and B, Manzamine-Related Alkaloids from the Far Eastern Sponge Lissodendoryx florida. Org. Lett. 2017, 19, 5320–5323. [Google Scholar] [CrossRef] [PubMed]





| Position | δС, mult | δH (J in Hz) |
|---|---|---|
| 1(NH) | 11.55, d (2.9) | |
| 2 | 131.9, CH | 8.17, d (2.9) |
| 3 | 114.1, C | |
| 3a | 118.3, C | |
| 4 | 121.5, CH | 7.89, d 8.6) |
| 5 | 111.9, CH | 6.68, dd (8.6, 1.7) |
| 6 | 154.0, C | |
| 7 | 97.1, CH | 6.80, d (1.7) |
| 7a | 137.6, C | |
| 1′ | 189.9, C | |
| 2′ | 45.4, CH2 | 4.38, d (5.6) |
| 3′(NH) | 8.06, d (5.6) | |
| 4′ | 169.3, C | |
| 5′ | 22.4, CH3 | 1.90, s |
| 6-OH | 9.14, s |
| Compounds | DPPH Radical Scavenging | Cytotoxicity | |
|---|---|---|---|
| % at 100 µM | EC50, µM | IC50, µM | |
| 1 | 51.9 ± 1.3 | - | >100 |
| 2 | 88.9 ± 2.9 | - | >100 |
| 3 | 62.0 ± 4.6 [16] | - | >100 |
| 4 | 67.8 ± 1.1 | - | 75.7 ± 5.6 |
| 5 | 50.6 ± 1.3 | 101.3 ± 2.8 | 78.9 ± 1.9 |
| 6 | 83.5 ± 0.2 | - | >100 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yurchenko, E.A.; Menchinskaya, E.S.; Pislyagin, E.A.; Trinh, P.T.H.; Ivanets, E.V.; Smetanina, O.F.; Yurchenko, A.N. Neuroprotective Activity of Some Marine Fungal Metabolites in the 6-Hydroxydopamin- and Paraquat-Induced Parkinson’s Disease Models. Mar. Drugs 2018, 16, 457. https://0-doi-org.brum.beds.ac.uk/10.3390/md16110457
Yurchenko EA, Menchinskaya ES, Pislyagin EA, Trinh PTH, Ivanets EV, Smetanina OF, Yurchenko AN. Neuroprotective Activity of Some Marine Fungal Metabolites in the 6-Hydroxydopamin- and Paraquat-Induced Parkinson’s Disease Models. Marine Drugs. 2018; 16(11):457. https://0-doi-org.brum.beds.ac.uk/10.3390/md16110457
Chicago/Turabian StyleYurchenko, Ekaterina A., Ekaterina S. Menchinskaya, Evgeny A. Pislyagin, Phan Thi Hoai Trinh, Elena V. Ivanets, Olga F. Smetanina, and Anton N. Yurchenko. 2018. "Neuroprotective Activity of Some Marine Fungal Metabolites in the 6-Hydroxydopamin- and Paraquat-Induced Parkinson’s Disease Models" Marine Drugs 16, no. 11: 457. https://0-doi-org.brum.beds.ac.uk/10.3390/md16110457