Synthesis of Novel 1-(4-Substituted pyridine-3-sulfonyl)-3-phenylureas with Potential Anticancer Activity
Abstract
:1. Introduction


2. Results and Discussion
2.1. Chemistry


2.2. Anticancer Activity
| Panel | Cell Line | IGP [%] of Compound | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 11 | 12 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 24 | 25 | 26 | ||
| Leukemia | CCRF-CEM | 1 | 4 | 27 | 30 | 7 | – | 2 | 11 | * | 85 | 27 | 19 | – |
| K-562 | 6 | 67 | – | 45 | – | 7 | 12 | 70 | 20 | 95 | 42 | 72 | * | |
| MOLT-4 | 7 | 21 | – | 27 | – | 6 | 5 | 18 | 9 | 85 | 9 | 20 | 8 | |
| RPMI-8226 | * | * | – | 70 | – | * | 1 | 7 | * | 93 | 72 | 10 | * | |
| SR | – | 15 | – | 16 | – | 12 | – | – | – | 95 | 1 | 29 | 19 | |
| NSCLC | HOP-92 | * | * | – | 53 | – | * | * | 2 | * | 131 | 28 | * | – |
| NCI-H522 | 7 | 25 | * | 43 | * | 8 | 9 | 19 | 5 | 110 | 28 | 12 | 8 | |
| Colon cancer | HCC-2998 | 11 | 1 | * | 1 | * | * | 11 | 9 | 2 | 73 | * | 2 | * |
| HCT-15 | * | 39 | 37 | * | 31 | 9 | * | 28 | * | 41 | * | 53 | 4 | |
| KM12 | * | 43 | * | 25 | 4 | 7 | – | 24 | 14 | 89 | 27 | 49 | * | |
| SW-620 | * | 24 | 30 | 10 | 21 | * | * | 30 | * | 82 | 12 | 40 | * | |
| CNS cancer | SF-268 | * | 18 | 3 | 1 | 6 | * | * | 19 | 6 | 71 | * | 26 | * |
| SF-295 | 11 | 13 | 6 | 43 | 13 | 10 | * | 10 | – | 147 | 48 | * | 1 | |
| Melanoma | LOX IMVI | 7 | - | 59 | 10 | 56 | – | 3 | 47 | 4 | 96 | 10 | 59 | – |
| MALME-3M | 9 | 1 | * | 58 | * | 3 | 6 | 6 | 5 | 101 | 44 | 2 | * | |
| M14 | * | 17 | 16 | 27 | 17 | * | * | 9 | 0 | 77 | 25 | 29 | * | |
| MDA-MB-435 | * | 9 | 16 | 11 | 12 | * | * | * | * | 93 | 15 | 23 | * | |
| UACC-62 | * | 57 | 51 | 33 | 38 | * | * | 32 | * | 73 | 31 | 42 | 10 | |
| Ovarian cancer | OVCAR-3 | * | * | * | 42 | * | * | * | * | * | 75 | 50 | * | * |
| Renal cancer | A498 | 15 | 19 | 33 | 4 | 42 | 5 | 17 | 13 | 12 | 80 | 7 | 6 | 0 |
| ACHN | * | 31 | 22 | 25 | 21 | * | * | 24 | * | 93 | 26 | 25 | * | |
| CAKI-1 | 2 | 33 | 29 | * | 28 | 11 | * | 23 | * | 65 | 5 | 17 | 2 | |
| UO-31 | 12 | 18 | 17 | 27 | 19 | 9 | 5 | 21 | 10 | 110 | 20 | 29 | 16 | |
| Prostate cancer | PC-3 | 11 | 6 | 14 | 54 | 17 | * | 6 | 21 | 16 | 101 | 51 | 12 | * |
| DU-145 | * | 16 | 5 | * | 7 | * | * | 18 | * | 67 | 1 | 32 | * | |
| Breast cancer | MDA-MB-468 | * | 7 | – | 59 | – | 3 | * | * | * | 109 | 57 | * | * |
| T-47D | 2 | 20 | 21 | 33 | 17 | 6 | 10 | 27 | 9 | 92 | 26 | 18 | 7 | |
- (a)
- All compounds with 4-chlorophenylcarbamoyl (R2 = 4-Cl) moieties (12, 14, 16, 19, 21 and 25) are characterized by moderate to high activity with IGP range from 17 to 96% against the common cancer cell lines: leukemia (K-562, MOLT-4), colon cancer (HCT-15 and SW-620,) melanoma (LOX IMVI and UACC-62), and renal cancer (ACHN, CAKI-1) (Table 1), as well exhibit high overall activity with the average IGP for the whole panel in the range from 7 to 90%.
- (b)
- Apparently the introduction of a second chlorine atom in position 3 of the 4-chloro-phenylcarbamoyl moiety (R2 = 3,4-diCl) in compounds 17 and 26 definitely causes a loss of activity. In this case the highest IGP values of 12 and 19% are observed only for the leukemia SR cell line.
- (c)
- From among of the compounds with unsubstituted phenylcarbamoyl moieties (R2 = H; i.e., 11, 15, 18, 20 and 24) only compounds 15 and 24 exhibit high antiproliferative activity against certain cell lines: leukemia RPMI-8226 (IGP = 70 and 72%), NSCLC HOP-92 (IGP = 53 and 28%), CNS cancer (IGP = SF-295 43 and 48%), melanoma MALME-3M (IGP = 58 and 44%), ovarian cancer OVCAR-3 (IGP = 42 and 50%), prostate cancer PC-3 (IGP = 54 and 51%) or breast cancer MDA-MB-468 (IGP = 59 and 57%), respectively (Table 1). It should be noted that the mentioned cell lines, which are highly susceptible for compounds 15 and 24, do not exhibit significant sensitivity for their 4-chlorophenylurea (R2 = 4-Cl) analogs 16 and 25.
| Panel | Cell line | 21 | Sulofenur b | ||||
|---|---|---|---|---|---|---|---|
| GI50 c | TGI d | LC50 e | GI50 c | TGI d | LC50 e | ||
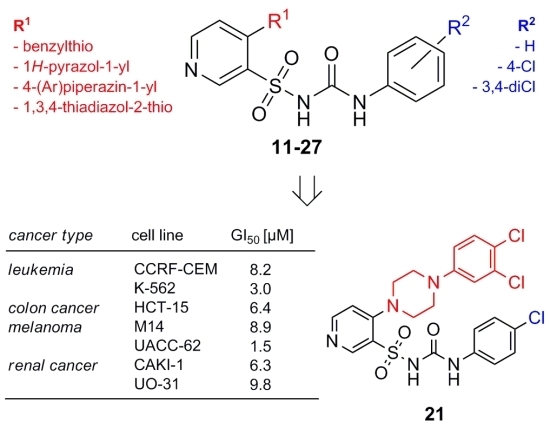
| Leukemia | CCRF-CEM | 8.2 | 43.6 | >100 | 29.8 | >100 | >100 |
| K-562 | 3.0 | >100 | >100 | 10.2 | 77.1 | >100 | |
| MOLT-4 | 19.9 | 68.1 | >100 | 26.4 | 98.2 | >100 | |
| RPMI-8226 | 14.7 | 54.2 | >100 | 24.4 | 85.7 | >100 | |
| SR | 11.8 | 51.6 | >100 | 57.0 | >100 | >100 | |
| NSCLC | A-549/ATCC | 14.7 | 62.8 | >100 | 32.7 | 97.7 | >100 |
| HOP-62 | 18.8 | 43.5 | >100 | 32.2 | >100 | >100 | |
| NCI-H226 | 13.4 | 57.9 | >100 | 37.9 | 77.8 | >100 | |
| NCI-H23 | 19.9 | 51.9 | >100 | 28.1 | 56.0 | 90.8 | |
| NCI-H322M | 18.3 | >100 | >100 | 34.2 | 95.5 | >100 | |
| NCI-H460 | 17.2 | 69.4 | >100 | 38.3 | 100 | >100 | |
| NCI-H522 | 14.1 | 49.4 | >100 | 27.9 | 94.2 | >100 | |
| Colon cancer | COLO 205 | 16.8 | 33.0 | 65.0 | 29.8 | 60.8 | 90.2 |
| HCC-2998 | 15.5 | 31.6 | 64.3 | 31.6 | 77.6 | 98.4 | |
| HCT-116 | 15.1 | 34.4 | 78.2 | 26.2 | 67.3 | 85.1 | |
| HCT-15 | 6.4 | 68.3 | >100 | 35.6 | 90.2 | >100 | |
| HT29 | 15.9 | 45.7 | >100 | 61.1 | >100 | >100 | |
| KM12 | 13.0 | >100 | >100 | 36.1 | 98.9 | >100 | |
| SW-620 | 14.4 | >100 | >100 | 35.3 | >100 | >100 | |
| CNS cancer | SF-295 | 12.2 | 37.9 | >100 | 46.7 | >100 | >100 |
| SF-539 | 13.1 | 29.0 | 64.4 | 28.3 | 69.2 | >100 | |
| SNB-19 | 19.1 | 57.6 | >100 | 40.9 | >100 | >100 | |
| U251 | 13.0 | 36.5 | >100 | 30.7 | 77.4 | >100 | |
| Melanoma | M14 | 8.9 | 53.4 | >100 | 37.8 | 97.5 | >100 |
| MDA-MB-435 | 18.9 | >100 | >100 | 17.6 | 48.3 | >100 | |
| SK-MEL-5 | 10.9 | 36.7 | >100 | 26.8 | 74.0 | 98.2 | |
| UACC-62 | 1.5 | 15.5 | >100 | 23.9 | 84.7 | >100 | |
| Ovarian cancer | IGROV1 | 16.3 | 68.1 | >100 | 12.3 | 94.4 | >100 |
| OVCAR-3 | 16.6 | 36.7 | 81.1 | 24.3 | 73.8 | >100 | |
| OVCAR-8 | 18.6 | >100 | >100 | 40.8 | >100 | >100 | |
| NCI/ADR-RES | 18.4 | >100 | >100 | – | – | – | |
| Renal cancer | 786-0 | 16.1 | 43.7 | >100 | 35.3 | >100 | >100 |
| A498 | 15.2 | 63.1 | >100 | 45.0 | >100 | >100 | |
| ACHN | 11.6 | >100 | >100 | 29.4 | >100 | >100 | |
| CAKI-1 | 6.3 | >100 | >100 | 42.8 | >100 | >100 | |
| SN12C | 18.4 | >100 | >100 | 39.2 | >100 | >100 | |
| UO-31 | 9.8 | 27.3 | 75.0 | 25.1 | 76.4 | >100 | |
| Prostate cancer | PC-3 | 14.4 | 71.1 | >100 | 19.0 | 47.2 | >100 |
| DU-145 | 17.3 | 55.7 | >100 | 24.0 | 92.0 | >100 | |
| Breast cancer | MCF7 | 19.7 | 97.4 | >100 | 24.1 | 81.8 | >100 |
| MDA-MB-231/ATCC | 14.6 | 63.3 | >100 | – | – | – | |
| BT-549 | 16.1 | 44.7 | >100 | 32.6 | >100 | >100 | |
| T-47D | 13.9 | 87.6 | >100 | 16.4 | 74.6 | >100 | |
| MDA-MB-468 | 18.9 | >100 | >100 | – | – | – | |
3. Experimental Section
3.1. General Procedures
3.2. Synthesis
3.2.1. Procedure for the Preparation of 4-[(5-Methyl-1,3,4-thiadiazol-2-yl)thio]-3-pyridinesulfonamide (3)
3.2.2. General Procedure for the Preparation of 4-Substituted-N-(R2-phenylcarbamoyl)-3-pyridine-sulfonamides
3.3. In Vitro Anticancer Screening
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ceras, J.; Cirauqui, N.; Pérez-Silanes, S.; Aldana, I.; Monge, A.; Galiano, S. Novel Sulfonylurea Derivatives as H3 Receptor Antagonists. Preliminary SAR Studies. Eur. J. Med. Chem. 2012, 52, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Khatri, N.; Madan, K. Models for H3 Receptor Antagonist Activity of Sulfonylurea Derivatives. J. Mol. Graph. Model. 2014, 48, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Michaux, C.; Dogné, J.M.; Rolin, S.; Masereel, B.; Wouters, J.; Durant, F. A Pharmacophore Model for Sulphonyl-Urea (-Cyanoguanidine) Compounds with Dual Action, Thromboxane Receptor Antagonists and Thromboxane Synthase Inhibitors. Eur. J. Med. Chem. 2003, 38, 703–710. [Google Scholar] [CrossRef]
- Bambi-Nyanguile, S.M.; Hanson, J.; Ooms, A.; Alpan, L.; Kolh, P.; Dogné, J.M.; Pirotte, B. Synthesis and Pharmacological Evaluation of 2-Aryloxy/arylamino-5-Cyanobenzenesulfonylureas as Novel Thromboxane A2 Receptor Antagonists. Eur. J. Med. Chem. 2013, 65, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Jiang, Y.; Liu, Z.; Liu, X.H.; Liu, Z.; Wang, G.; Li, Z.M.; Wang, D. Synthesis and Evaluation of Novel Monosubstituted Sulfonylurea Derivatives as Antituberculosis Agents. Eur. J. Med. Chem. 2012, 50, 18–26. [Google Scholar] [CrossRef] [PubMed]
- León, C.; Rodrigues, J.; Gamboa de Domínguez, N.; Charris, J.; Gut, J.; Rosenthal, P.J.; Domínguez, J.N. Synthesis and Evaluation of Sulfonylurea Derivatives as Novel Antimalarials. Eur. J. Med. Chem. 2007, 42, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Cui, C.J.; Chow, E.W.L.; Pue, N.; Lonhienne, T.; Wang, J.G.; Fraser, J.A.; Guddat, L.W. Sulfonylureas Have Antifungal Activity and Are Potent Inhibitors of Candida Albicans Acetohydroxyacid Synthase. J. Med. Chem. 2013, 56, 210–219. [Google Scholar] [CrossRef] [PubMed]
- El-Deeb, I.M.; Bayoumi, S.M.; El-Sherbeny, M.A.; Abdel-Aziz, A.A.M. Synthesis and Antitumor Evaluation of Novel Cyclic Arylsulfonylureas: ADME-T and Pharmacophore Prediction. Eur. J. Med. Chem. 2010, 45, 2516–2530. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbeny, M.; Abdel-Aziz, A.M.; Ahmed, M. Synthesis and Antitumor Evaluation of Novel Diarylsulfonylurea Derivatives: Molecular Modeling Applications. Eur. J. Med. Chem. 2010, 45, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Avupati, V.R.; Yejella, R.P.; Guntuku, G.; Gunta, P. Synthesis, Characterization and in Vitro Biological Evaluation of Some Novel Diarylsulfonylureas as Potential Cytotoxic and Antimicrobial Agents. Bioorg. Med. Chem. Lett. 2012, 22, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Rathore, P.; Yaseen, S.; Ovais, S.; Bashir, R.; Yaseen, R.; Hameed, A.D.; Samim, M.; Gupta, R.; Hussain, F.; Javed, K. Synthesis and Evaluation of Some New Pyrazoline Substituted Benzenesulfonylureas as Potential Antiproliferative Agents. Bioorg. Med. Chem. Lett. 2014, 24, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Tian, J.; Wang, L.T.; Wang, M.J.; Nan, X.; Yang, L.; Liu, Y.Q.; Morris-Natschke, S.L.; Lee, K.H. Design, Synthesis and Cytotoxic Activity of Novel Sulfonylurea Derivatives of Podophyllotoxin. Bioorg. Med. Chem. 2014, 22, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Howbert, J.; Grossman, C.S.; Crowell, T.; Rieder, B.J.; Harper, R.W.; Kramer, K.E.; Tao, E.V.; Aikins, J.; Poore, G.; Rinzel, S.M. Novel Agents Effective against Solid Tumors: The Diarylsulfonylureas. Synthesis, Activities, and Analysis of Quantitative Structure-Activity Relationships. J. Med. Chem. 1990, 33, 2393–2407. [Google Scholar] [CrossRef] [PubMed]
- Scozzafava, A.; Owa, T.; Mastrolorenzo, A.; Supuran, C.T. Anticancer and Antiviral Sulfonamides. Curr. Med. Chem. 2003, 10, 925–953. [Google Scholar] [CrossRef] [PubMed]
- Pasello, G.; Urso, L.; Conte, P.; Favaretto, A. Effects of Sulfonylureas on Tumor Growth: A Review of the Literature. Oncologist 2013, 18, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, Z.; Sławiński, J.; Sączewski, F.; Innocenti, A.; Supuran, C.T. Carbonic anhydrase inhibitors: Synthesis and inhibition of the human cytosolic isozymes I and II and transmembrane isozymes IX, XII (cancer-associated) and XIV with 4-substituted 3-pyridinesulfonamides. Eur. J. Med. Chem. 2010, 45, 2396–2404. [Google Scholar] [CrossRef] [PubMed]
- Sławiński, J.; Szafrański, K.; Vullo, D.; Supuran, C.T. Carbonic Anhydrase Inhibitors. Synthesis of Heterocyclic 4-Substituted Pyridine-3-Sulfonamide Derivatives and Their Inhibition of the Human Cytosolic Isozymes I and II and Transmembrane Tumor-Associated Isozymes IX and XII. Eur. J. Med. Chem. 2013, 69, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Pacchiano, F.; Carta, F.; McDonald, P.C.; Lou, Y.; Vullo, D.; Scozzafava, A.; Dedhar, S.; Supuran, C.T. Ureido-Substituted Benzenesulfonamides Potently Inhibit Carbonic Anhydrase IX and Show Antimetastatic Activity in a Model of Breast Cancer Metastasis. J. Med. Chem. 2011, 54, 1896–1902. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; McDonald, P.C.; Oloumi, A.; Chia, S.; Ostlund, C.; Ahmadi, A.; Kyle, A.; Auf Dem Keller, U.; Leung, S.; Huntsman, D.; et al. Targeting Tumor Hypoxia: Suppression of Breast Tumor Growth and Metastasis by Novel Carbonic Anhydrase IX Inhibitors. Cancer Res. 2011, 71, 3364–3376. [Google Scholar] [CrossRef] [PubMed]
- Pastorekova, S.; Zatovicova, M.; Pastorek, J. Cancer-Associated Carbonic Anhydrases and Their Inhibition. Curr. Pharm. Des. 2008, 14, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic Anhydrases: Novel Therapeutic Applications for Inhibitors and Activators. Nat. Rev. Drug Discov. 2008, 7, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Neri, D.; Supuran, C.T. Interfering with pH Regulation in Tumours as a Therapeutic Strategy. Nat. Rev. Drug Discov. 2011, 10, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Inhibition of Carbonic Anhydrase IX as a Novel Anticancer Mechanism. World J. Clin. Oncol. 2012, 3, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Gieling, R.G.; Williams, K.J. Carbonic Anhydrase IX as a Target for Metastatic Disease. Bioorg. Med. Chem. 2012, 21, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Alley, M.C.; Scudiero, D.A.; Monks, P.A.; Hursey, M.L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.; Boyd, M.R. Feasibility of Drug Screening with Panels of Human Tumor Cell Lines Using a Microculture Tetrazolium Assay. Cancer Res. 1988, 48, 589–601. [Google Scholar] [PubMed]
- Grever, M.R.; Schepartz, S.A.; Chabner, B.A. The National Cancer Institute: Cancer Drug Discovery and Development Program. Semin. Oncol. 1992, 19, 622–638. [Google Scholar] [PubMed]
- Boyd, M.R.; Paull, K.D. Some Practical Considerations and Applications of the National Cancer Institute in Vitro Anticancer Drug Discovery Screen. Drug Dev. Res. 1995, 34, 91–109. [Google Scholar] [CrossRef]
- Shoemaker, R.H. The NCI60 Human Tumour Cell line Anticancer Drug Screen. Nat. Rev. 2006, 6, 813–823. [Google Scholar] [CrossRef] [PubMed]
- NCI-60 DTP Human Tumor Cell Line Screen. Available online: http://dtp.nci.nih.gov/branches/btb/ivclsp.html (accessed on 7 May 2015).
- Cancer Screening Data. Available online: http://dtp.nci.nih.gov/dtpstandard/cancerscreeningdata/index.jsp (accessed on 15 June 2015).
- Sample Availability: Samples of the compounds 2–27 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szafrański, K.; Sławiński, J. Synthesis of Novel 1-(4-Substituted pyridine-3-sulfonyl)-3-phenylureas with Potential Anticancer Activity. Molecules 2015, 20, 12029-12044. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules200712029
Szafrański K, Sławiński J. Synthesis of Novel 1-(4-Substituted pyridine-3-sulfonyl)-3-phenylureas with Potential Anticancer Activity. Molecules. 2015; 20(7):12029-12044. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules200712029
Chicago/Turabian StyleSzafrański, Krzysztof, and Jarosław Sławiński. 2015. "Synthesis of Novel 1-(4-Substituted pyridine-3-sulfonyl)-3-phenylureas with Potential Anticancer Activity" Molecules 20, no. 7: 12029-12044. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules200712029