Synthesis and Evaluation of Aminothiazole-Paeonol Derivatives as Potential Anticancer Agents
Abstract
:1. Introduction


2. Results and Discussion
2.1. Chemistry

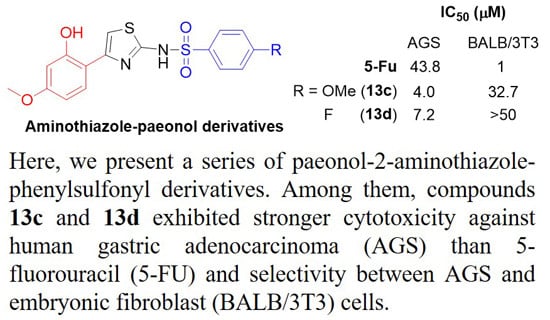
2.2. Anticancer Activity and Structure Activity Relationship Analysis
| Compounds | IC50 (μM) | |||||||
|---|---|---|---|---|---|---|---|---|
| BALB/3T3 | AGS | HeLa | PaTu8988t | HT-29 | U87-MG | A549 | CT26.WT | |
| 13a | >50 | >50 | >50 | 31.1 | 20.7 | >50 | >50 | >50 |
| 13b | >50 | 22.0 | 27.4 | 38.7 | 14.1 | >50 | >50 | >50 |
| 13c | 32.7 | 4.0 | 5.8 | 15.8 | 4.4 | 22.5 | >50 | 10.0 |
| 13d | >50 | 7.2 | 13.8 | 31.4 | 11.2 | >50 | >50 | >50 |
| 13e | >50 | > 50 | >50 | >50 | 13.4 | >50 | >50 | >50 |
| 13f | >50 | > 50 | 16.4 | 22.8 | 11.0 | >50 | >50 | >50 |
| 13g | 32.2 | > 50 | >50 | >50 | 47.8 | >50 | >50 | 30.0 |
| 5-Fu * | 1.0 | 43.8 | 2.2 | 12.5 | 7.2 | >50 | >50 | 9.2 |
| 5-FU ** [25,26,27,28,29,30,31,32] | 13.0 | 79.5 | 0.232 | 11.3 | 19.3 | 4.9 | 10.3 | 61.0 |
| 72 h | 24 h | 48 h | 48 h | 48 h | 48 h | 48 h | 72 h | |
| Compounds | Molecular Weight, Lipophilicity and Water Solubility | |||
|---|---|---|---|---|
| M.W. | clogP | clogS | S (mmol/L) | |
| 13a | 362.418 | 3.26 | −4.14 | 2.625486 |
| 13b | 376.445 | 3.57 | −4.31 | 1.843748 |
| 13c | 392.444 | 3.27 | −4.11 | 3.046335 |
| 13d | 380.4804 | 3.76 | −4.39 | 1.550002 |
| 13e | 396.86 | 3.83 | −4.43 | 1.474475 |
| 13f | 441.314 | 4.07 | −4.57 | 1.187812 |
| 13g | 407.026 | 3.21 | −4.33 | 1.903804 |
3. Experimental Section
3.1. General Experimental Procedures
3.2. Procedure for the Preparation of Aminothiazole-Paeonol (11)
3.3. Standard Procedure for the Preparation of Aminothiazole-Paeonol Derivatives (13)
3.4. Cell Culture
3.5. Drug Treatment and Cell Viability Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fu, P.K.; Wu, C.L.; Tsai, T.H.; Hsieh, C.L. Anti-inflammatory and anticoagulative effects of paeonol on lps-induced acute lung injury in rats. Evid. Based Complement. Altern. Med. 2012, 2012, 837513. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Anti-inflammatory and analgesic effects of paeonol in carrageenan-evoked thermal hyperalgesia. Br. J. Pharmacol. 2003, 139, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Ning, M.; Yang, G. Effect of paeonol on antioxidant and immune regulatory activity in hepatocellular carcinoma rats. Molecules 2012, 17, 4672–4683. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.H.; Chan, C.M.; Chan, Y.W.; Lau, K.M.; Lau, T.W.; Lam, F.C.; Law, W.T.; Che, C.T.; Leung, P.C.; Fung, K.P.; et al. Pharmacological investigations of the anti-diabetic effect of cortex moutan and its active component paeonol. Phytomedicine 2007, 14, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wu, N.; Zeng, F.; Cheng, C.; Kang, K.; Yang, H. Paeonol induces apoptosis in human ovarian cancer cells. Acta Histochem. 2013, 115, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.F.; Jim Leu, S.J.; Shyue, S.K.; Su, K.H.; Wei, J.; Lee, T.S. Novel effect of paeonol on the formation of foam cells: Promotion of LXRα-ABCA1-dependent cholesterol efflux in macrophages. Am. J. Chin. Med. 2013, 41, 1079–1096. [Google Scholar] [CrossRef] [PubMed]
- Su, S.Y.; Cheng, C.Y.; Tsai, T.H.; Hsieh, C.L. Paeonol protects memory after ischemic stroke via inhibiting β-secretase and apoptosis. Evid. Based Complement. Altern. Med. 2012, 2012, 932823. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Wu, H.; Pan, J.; Wang, X.; Li, J.; Wu, Z.; Hui, A. Synthesis and evaluation of paeonol derivatives as potential multifunctional agents for the treatment of alzheimer's disease. Molecules 2015, 20, 1304–1318. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.D.; Yang, Z.Y.; Zhang, F.H.; Du, B.; Wang, P.; Li, T.R. Evaluation of the antioxidant, DNA interaction and tumor cell cytotoxicity activities of copper(II) complexes with paeonol schiff-base. Inorg. Chem. Commun. 2010, 13, 727–729. [Google Scholar] [CrossRef]
- Zhu, T.H.; Cao, S.W.; Yu, Y.Y. Synthesis, characterization and biological evaluation of paeonol thiosemicarbazone analogues as mushroom tyrosinase inhibitors. Int. J. Biol. Macromol. 2013, 62, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.J.; Chuang, H.; Liang, Y.C.; Lin, H.H.; Horng, J.C.; Kuo, Y.C.; Chen, C.W.; Tsai, F.Y.; Yen, S.C.; Chou, S.C.; et al. Design, synthesis, and bioevaluation of paeonol derivatives as potential anti-HBV agents. Eur. J. Med. Chem. 2015, 90, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Pao, K.C.; Zhao, J.F.; Lee, T.S.; Huang, Y.P.; Han, C.C.; Huang, L.C.S.; Wu, K.H.; Hsu, M.H. Low-dose paeonol derivatives alleviate lipid accumulation. Rsc. Adv. 2015, 5, 5652–5656. [Google Scholar] [CrossRef]
- Nevagi, R.J. Biological and medicinal significance of 2-aminothiazoles. Der Pharm. Lett. 2014, 6, 134–150. [Google Scholar]
- Jaen, J.C.; Wise, L.D.; Caprathe, B.W.; Tecle, H.; Bergmeier, S.; Humblet, C.C.; Heffner, T.G.; Meltzer, L.T.; Pugsley, T.A. 4-(1,2,5,6-tetrahydro-1-alkyl-3-pyridinyl)-2-thiazolamines: A novel class of compounds with central dopamine agonist properties. J. Med. Chem. 1990, 33, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, L.A.; Rodenhuis, N.; Wikstrom, H.; Pugsley, T.A.; Serpa, K.A.; Meltzer, L.T.; Heffner, T.G.; Wise, L.D.; Lajiness, M.E.; Huff, R.M.; et al. Thiazoloindans and thiazolobenzopyrans: A novel class of orally active central dopamine (partial) agonists. J. Med. Chem. 2000, 43, 3549–3557. [Google Scholar] [CrossRef] [PubMed]
- Pieroni, M.; Wan, B.; Cho, S.; Franzblau, S.G.; Costantino, G. Design, synthesis and investigation on the structure-activity relationships of n-substituted 2-aminothiazole derivatives as antitubercular agents. Eur. J. Med. Chem. 2014, 72, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Niwata, S.; Fukami, H.; Sumida, M.; Ito, A.; Kakutani, S.; Saitoh, M.; Suzuki, K.; Imoto, M.; Shibata, H.; Imajo, S.; et al. Substituted 3-(phenylsulfonyl)-1-phenylimidazolidine-2,4-dione derivatives as novel nonpeptide inhibitors of human heart chymase. J. Med. Chem. 1997, 40, 2156–2163. [Google Scholar] [CrossRef] [PubMed]
- Schroder, J.; Henke, A.; Wenzel, H.; Brandstetter, H.; Stammler, H.G.; Stammler, A.; Pfeiffer, W.D.; Tschesche, H. Structure-based design and synthesis of potent matrix metalloproteinase inhibitors derived from a 6H-1,3,4-thiadiazine scaffold. J. Med. Chem. 2001, 44, 3231–3243. [Google Scholar] [CrossRef] [PubMed]
- Bachovchin, D.A.; Zuhl, A.M.; Speers, A.E.; Wolfe, M.R.; Weerapana, E.; Brown, S.J.; Rosen, H.; Cravatt, B.F. Discovery and optimization of sulfonyl acrylonitriles as selective, covalent inhibitors of protein phosphatase methylesterase-1. J. Med. Chem. 2011, 54, 5229–5236. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.L.D.; Lima, L.M.; Tesch, R.; Sant’Anna, C.M.R.; Totzke, F.; Kubbutat, M.H.G.; Schachtele, C.; Laufer, S.A.; Barreiro, E.J. Novel 2-chloro-4-anilino-quinazoline derivatives as EGFR and VEGFR-2 dual inhibitors. Eur. J. Med. Chem. 2014, 71, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Abouzid, K.; Shouman, S. Design, synthesis and in vitro antitumor activity of 4-aminoquinoline and 4-aminoquinazoline derivatives targeting EGFR tyrosine kinase. Bioorg. Med. Chem. 2008, 16, 7543–7551. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.C.; Duan, Y.T.; Qiu, H.Y.; Huang, W.Y.; Wang, P.F.; Yan, X.Q.; Zhang, S.F.; Zhu, H.L. Novel metronidazole-sulfonamide derivatives as potent and selective carbonic anhydrase inhibitors: Design, synthesis and biology analysis. Rsc. Adv. 2014, 4, 33029–33038. [Google Scholar] [CrossRef]
- Tetko, I.V.; Gasteiger, J.; Todeschini, R.; Mauri, A.; Livingstone, D.; Ertl, P.; Palyulin, V.; Radchenko, E.; Zefirov, N.S.; Makarenko, A.S.; et al. Virtual computational chemistry laboratory—Design and description. J. Comput. Aided Mol. Des. 2005, 19, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Alagille, D.; Baldwin, R.M.; Tamagnan, G.D. One-step synthesis of 2-arylbenzothiazole (“BTA”) and -benzoxazole precursors for in vivo imaging of β-amyloid plaques. Tetrahedron Lett. 2005, 46, 1349–1351. [Google Scholar] [CrossRef]
- Flis, S.; Splwinski, J. Inhibitory effects of 5-fluorouracil and oxaliplatin on human colorectal cancer cell survival are synergistically enhanced by sulindac sulfide. Anticancer Res. 2009, 29, 435–441. [Google Scholar] [PubMed]
- Bhattacharya, B.; Akram, M.; Balasubramanian, I.; Tam, K.K.; Koh, K.X.; Yee, M.Q.; Soong, R. Pharmacologic synergy between dual phosphoinositide-3-kinase and mammalian target of rapamycin inhibition and 5-fluorouracil in PIK3CA mutant gastric cancer cells. Cancer Biol. Therapy 2012, 13, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.Y.; Huang, M.; Xu, C.H.; Yang, W.Y.; Hu, C.X.; Lin, L.P.; Tong, L.J.; Li, M.H.; Lu, W.; Zhang, X.W.; et al. Anti-proliferative effects, cell cycle G2/M phase arrest and blocking of chromosome segregation by probimane and MST-16 in human tumor cell lines. BMC Pharmacol. 2005, 5, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nambaru, P.K.; Hubner, T.; Kock, K.; Mews, S.; Grube, M.; Payen, L.; Guitton, J.; Sendler, M.; Jedlitschky, G.; Rimmbach, C.; et al. Drug efflux transporter multidrug resistance-associated protein 5 affects sensitivity of pancreatic cancer cell lines to the nucleoside anticancer drug 5-fluorouracil. Drug Metabol. Dispos. Biol. Fate Chem. 2011, 39, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Nita, M.E.; Nagawa, H.; Tominaga, O.; Tsuno, N.; Fujii, S.; Sasaki, S.; Fu, C.G.; Takenoue, T.; Tsuruo, T.; Muto, T. 5-fluorouracil induces apoptosis in human colon cancer cell lines with modulation of BCL-2 family proteins. Br. J. Cancer 1998, 78, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Blois, J.; Smith, A.; Josephson, L. The slow cell death response when screening chemotherapeutic agents. Cancer Chemother. Pharmacol. 2011, 68, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zhang, X.; Sun, H.; Zhang, J.; Yan, M.; Zhang, H. Autophagy inhibition promotes 5-fluorouraci-induced apoptosis by stimulating ros formation in human non-small cell lung cancer a549 cells. PLoS ONE 2013, 8, e56679. [Google Scholar] [CrossRef] [PubMed]
- Da, S.; Gomide, M.; de O. Lemos, F.; Lopes, M.T.P.; de Alves, T.M.; Viccini, L.F.; Coelho, C.M. The effect of the essential oils from five different lippia species on the viability of tumor cell lines. Braz. J. Pharm. 2013, 23, 895–902. [Google Scholar]
- Li, M.; Tan, S.Y.; Wang, X.F. Paeonol exerts an anticancer effect on human colorectal cancer cells through inhibition of PGE2 synthesis and COX-2 expression. Oncol. Rep. 2014, 32, 2845–2853. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, C.-Y.; Kapoor, M.; Huang, Y.-P.; Lin, H.-H.; Liang, Y.-C.; Lin, Y.-L.; Huang, S.-C.; Liao, W.-N.; Chen, J.-K.; Huang, J.-S.; et al. Synthesis and Evaluation of Aminothiazole-Paeonol Derivatives as Potential Anticancer Agents. Molecules 2016, 21, 145. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules21020145
Tsai C-Y, Kapoor M, Huang Y-P, Lin H-H, Liang Y-C, Lin Y-L, Huang S-C, Liao W-N, Chen J-K, Huang J-S, et al. Synthesis and Evaluation of Aminothiazole-Paeonol Derivatives as Potential Anticancer Agents. Molecules. 2016; 21(2):145. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules21020145
Chicago/Turabian StyleTsai, Chia-Ying, Mohit Kapoor, Ying-Pei Huang, Hui-Hsien Lin, Yu-Chuan Liang, Yu-Ling Lin, Su-Chin Huang, Wei-Neng Liao, Jen-Kun Chen, Jer-Shing Huang, and et al. 2016. "Synthesis and Evaluation of Aminothiazole-Paeonol Derivatives as Potential Anticancer Agents" Molecules 21, no. 2: 145. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules21020145