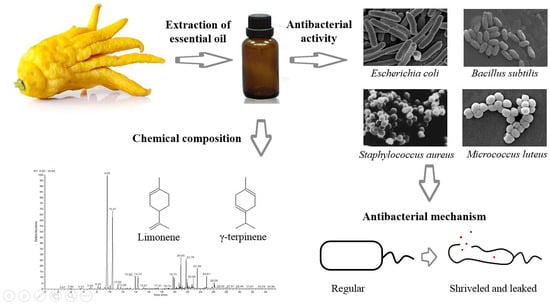
Antibacterial Activity and Mechanisms of Essential Oil from Citrus medica L. var. sarcodactylis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Characterization of FCEO
2.2. Antibacterial Activity of FCEO
2.2.1. Effects of FCEO on Bacteria
2.2.2. Minimal Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.3. Antibacterial Mechanisms of FCEO Against E. coli and S. aureus
2.3.1. The Effect of FCEO on Morphological Change
2.3.2. Effect of FCEO on the Viability
2.3.3. The Effect of FCEO on Cell Membrane Permeability
2.3.4. The Effect of FCEO on Integrity of the Cell Membrane
3. Materials and Methods
3.1. Materials
3.2. Chemicals and Microorganisms
3.3. GC-MS Analysis
3.4. Evaluation of Antibacterial Activity of FCEO
3.4.1. Antibacterial Activity of FCEO
3.4.2. Determination of MIC and MBC
3.5. Demonstration of Antibacterial Mechanisms of FCEO
3.5.1. SEM Analysis of Morphological Changes
3.5.2. Time-Kill Analysis
3.5.3. Cell Membrane Permeability
3.5.4. The Integrity of Cell Membrane
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hennekinne, J.A.; Buyser, M.L.D.; Dragacci, S. S taphylococcus aureus and its food poisoning toxins: Characterization and outbreak investigation. FEMS Microbiol. Rev. 2012, 36, 815–836. [Google Scholar] [CrossRef] [PubMed]
- Böhme, K.; Barros-Velázquez, J.; Calo-Mata, P.; Aubourg, S.P. Antibacterial, Antiviral and Antifungal Activity of Essential Oils: Mechanisms and Applications. In Antimicrobial Compounds; Springer: Berlin, Germany, 2014; pp. 51–81. [Google Scholar] [CrossRef]
- Prakash, B.; Shukla, R.; Singh, P.; Mishra, P.K.; Dubey, N.K.; Kharwar, R.N. Efficacy of chemically characterized Ocimum gratissimum L. essential oil as an antioxidant and a safe plant based antimicrobial against fungal and aflatoxin B 1 contamination of spices. Food Res. Int. 2011, 44, 385–390. [Google Scholar] [CrossRef]
- Sakurada, T.; Kuwahata, H.; Katsuyama, S.; Komatsu, T.; Morrone, L.A.; Corasaniti, M.T.; Bagetta, G.; Sakurada, S. Intraplantar injection of bergamot essential oil into the mouse hindpaw: Effects on capsaicin-induced nociceptive behaviors. Int. Rev. Neurobiol. 2009, 85, 237–248. [Google Scholar]
- Saiyudthong, S.; Marsden, C.A. Acute effects of bergamot oil on anxiety-related behaviour and corticosterone level in rats. Phytother. Res. 2010, 25, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.N.; Ko, Y.J.; Yang, H.M.; Ham, Y.M.; Roh, S.W.; Jeon, Y.J.; Ahn, G.; Kang, M.C.; Yoon, W.J.; Kim, D. Anti-inflammatory effect of essential oil and its constituents from fingered citron (Citrus medica L. var. sarcodactylis) through blocking JNK, ERK and NF-κB signaling pathways in LPS-activated RAW 264.7 cells. Food Chem. Toxicol. 2013, 57, 126–131. [Google Scholar] [CrossRef]
- Russo, R.; Ciociaro, A.; Berliocchi, L.; Cassiano, M.G.V.; Rombolà, L.; Ragusa, S.; Bagetta, G.; Blandini, F.; Corasaniti, M.T. Implication of limonene and linalyl acetate in cytotoxicity induced by bergamot essential oil in human neuroblastoma cells. Fitoterapia 2013, 89, 48–57. [Google Scholar] [CrossRef]
- Djabou, N.; Lorenzi, V.; Guinoiseau, E.; Andreani, S.; Giuliani, M.-C.; Desjobert, J.-M.; Bolla, J.-M.; Costa, J.; Berti, L.; Luciani, A.; et al. Phytochemical composition of Corsican Teucrium essential oils and antibacterial activity against foodborne or toxi-infectious pathogens. Food Control 2013, 30, 354–363. [Google Scholar] [CrossRef]
- Van Vuuren, S.F.; Viljoen, A.M. Antimicrobial activity of limonene enantiomers and 1,8-cineole alone and in combination. Flavour. Fragr. J. 2007, 22, 540–544. [Google Scholar] [CrossRef]
- Shakeri, A.; Khakdan, F.; Soheili, V.; Sahebkar, A.; Rassam, G.; Asili, J. Chemical composition, antibacterial activity, and cytotoxicity of essential oil from Nepeta ucrainica L. spp. kopetdaghensis. Ind. Crop. Prod. 2014, 58, 315–321. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Liu, C.S.; Liu, C.G.; Meng, X.H.; Yu, L.J. Antibacterial mechanism of chitosan microspheres in a solid dispersing system against E. coli. Colloid Surf. B-Biointerfaces 2008, 65, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-J.; Gao, Z.-P.; Xia, J.-P.; Ritenour, M.A.; Li, G.-Y.; Shan, Y. Comparative analysis of chemical composition, antimicrobial and antioxidant activity of citrus essential oils from the main cultivated varieties in China. LWT-Food Sci. Technol. 2018, 97, 825–839. [Google Scholar] [CrossRef]
- Ghabraie, M.; Vu, K.D.; Tata, L.; Salmieri, S.; Lacroix, M. Antimicrobial effect of essential oils in combinations against five bacteria and their effect on sensorial quality of ground meat. LWT-Food Sci. Technol. 2016, 66, 332–339. [Google Scholar] [CrossRef] [Green Version]
- Diao, W.-R.; Hu, Q.-P.; Zhang, H.; Xu, J.-G. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.). Food Control 2014, 35, 109–116. [Google Scholar] [CrossRef]
- Cox, S.D.; Mann, C.M.; Markham, J.L.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. Determining the Antimicrobial Actions of Tea Tree Oil. Molecules 2001, 6, 87–91. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Bajpai, V.K.; Baek, K. Determination of Antibacterial Mode of Action of Allium sativum Essential Oil against Foodborne Pathogens Using Membrane Permeability and Surface Characteristic Parameters. J. Food Saf. 2013, 33, 197–208. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Baek, K.H.; Sun, C.K. Control of Salmonella in foods by using essential oils: A review. Food Res. Int. 2012, 45, 722–734. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Sharma, A.; Baek, K.H. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control 2013, 32, 582–590. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils-A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Wu, Z.; Li, H.; Yang, Y.; Zhan, Y.; Tu, D. Variation in the components and antioxidant activity of Citrus medica L. var. sarcodactylis essential oils at different stages of maturity. Ind. Crop. Prod. 2013, 46, 311–316. [Google Scholar] [CrossRef]
- Liu, X.; Zhong, S.; Wu, K.; Yu, H.; Chai, X. Antimicrobial activity in the vapour phase of a combination of cinnamon and clove essential oils. Food Chem. 2009, 116, 982–989. [Google Scholar]
- De Silveira, S.M.; Luciano, F.B.; Fronza, N.; Cunha, A., Jr.; Scheuermann, G.N.; Vieira, C.R.W. Chemical composition and antibacterial activity of Laurus nobilis essential oil towards foodborne pathogens and its application in fresh Tuscan sausage stored at 7 °C. LWT-Food Sci. Technol. 2014, 59, 86–93. [Google Scholar] [CrossRef]
- Joray, M.B.; del Rollán, M.R.; Ruiz, G.M.; Palacios, S.M.; Carpinella, M.C. Antibacterial activity of extracts from plants of central Argentina—isolation of an active principle from Achyrocline satureioides. Planta Med. 2011, 77, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Sun, C.; Liang, Z.; Han, Y.; Yu, J. Antibacterial activity of hypocrellin A against Staphylococcus aureus. World J. Microbiol. Biotechnol. 2012, 28, 3151–3157. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the essential oils are available from the authors. |




| No. | Compound | RI a | RI (lab) b | Area% | CAS No. |
|---|---|---|---|---|---|
| 1 | α-Pinene | 944 | 948 | 1.42 | 80-56-8 |
| 2 | 3-Carene | 951 | 950 | 0.69 | 13466-78-9 |
| 3 | β-Pinene | 953 | 956 | 1.15 | 127-91-3 |
| 4 | β-Myrcene | 980 | 979 | 0.81 | 123-35-3 |
| 5 | α-phellandrene | 983 | 983 | 0.63 | 99-83-2 |
| 6 | Allo-ocimene | 998 | 993 | 0.24 | 673-84-7 |
| 7 | α-Terpinene | 1011 | 1008 | 0.56 | 99-86-5 |
| 8 | Limonene | 1021 | 1020 | 45.36 | 138-86-3 |
| 9 | cis-β-Ocimene | 1024 | 1024 | 0.38 | 3338-55-4 |
| 10 | γ-Terpinene | 1045 | 1047 | 21.23 | 99-85-4 |
| 11 | Linalool | 1080 | 1081 | 0.47 | 78-70-6 |
| 12 | Terpinen-4-ol | 1134 | 1137 | 2.35 | 562-74-3 |
| 13 | α-Terpineol | 1168 | 1172 | 2.51 | 98-55-5 |
| 14 | Geranial | 1173 | 1174 | 0.05 | 141-27-5 |
| 15 | Carveol | 1203 | 1206 | 0.35 | 99-48-9 |
| 16 | Neral | 1212 | 1214 | 0.04 | 106-26-3 |
| 17 | Geranyl acetate | 1353 | 1352 | 0.15 | 105-87-3 |
| 18 | α-Bergamotene | 1427 | 1427 | 1.42 | 17699-05-7 |
| 19 | δ-Cadinene | 1439 | 1440 | 0.35 | 483-76-1 |
| 20 | Germacrene D | 1480 | 1480 | 0.7 | 23986-74-5 |
| 21 | Caryophyllene | 1500 | 1494 | 1.6 | 87-44-5 |
| 22 | γ-Muurolene | 1496 | 1494 | 0.19 | 30021-74-0 |
| 23 | β-Bisabolene | 1500 | 1500 | 3.23 | 495-61-4 |
| 24 | Dodecanoic acid | 1571 | 1570 | 7.52 | 143-07-7 |
| 25 | Humulene | 1581 | 1579 | 0.3 | 6753-98-6 |
| 26 | α-Bisabolol | 1686 | 1683 | 1.37 | 515-69-5 |
| 27 | Tetradecanoic acid | 1769 | 1769 | 2.85 | 544-63-8 |
| 28 | Hexadecanoic acid | 1970 | 1968 | 1.46 | 57-10-3 |
| Total | 99.38 | ||||
| Microorganisms | DIZ * (mm) | MIC (mg/mL) | MBC (mg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| FCEO | Limonene | Ciprofloxacin | FCEO | Limonene | Ciprofloxacin | FCEO | Limonene | Ciprofloxacin | |
| Gram-positive | |||||||||
| B. subtilis | 16.3 ± 1.3 b | 10.8 ± 1.2 ab | 23.7 ± 0.6 a | 0.625 | 1.25 | 0.001 | 2.5 | 1.25 | 0.001 |
| S. aureus | 19.2 ± 2.1 a | 11.2 ± 1.0 a | 25.0 ± 0.3 a | 0.625 | 0.625 | 0.001 | 1.25 | 1.25 | 0.001 |
| M. luteus | 16.1 ± 0.4 b | 9.3 ± 0.6 bc | 21.7 ± 1.4 b | 1.25 | 2.5 | 0.001 | 1.25 | 5 | 0.001 |
| Gram-negative | |||||||||
| E. coli | 11.2 ± 0.9 c | 8.6 ± 0.6 c | 19.3 ± 0.9 c | 2.5 | 1.25 | 0.001 | 2.5 | 1.25 | 0.002 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.-H.; Cai, M.; Liu, Y.-S.; Sun, P.-L.; Luo, S.-L. Antibacterial Activity and Mechanisms of Essential Oil from Citrus medica L. var. sarcodactylis. Molecules 2019, 24, 1577. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24081577
Li Z-H, Cai M, Liu Y-S, Sun P-L, Luo S-L. Antibacterial Activity and Mechanisms of Essential Oil from Citrus medica L. var. sarcodactylis. Molecules. 2019; 24(8):1577. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24081577
Chicago/Turabian StyleLi, Ze-Hua, Ming Cai, Yuan-Shuai Liu, Pei-Long Sun, and Shao-Lei Luo. 2019. "Antibacterial Activity and Mechanisms of Essential Oil from Citrus medica L. var. sarcodactylis" Molecules 24, no. 8: 1577. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24081577