A Multi-Species Phenotypic Screening Assay for Leishmaniasis Drug Discovery Shows That Active Compounds Display a High Degree of Species-Specificity
Abstract
:1. Introduction
2. Results
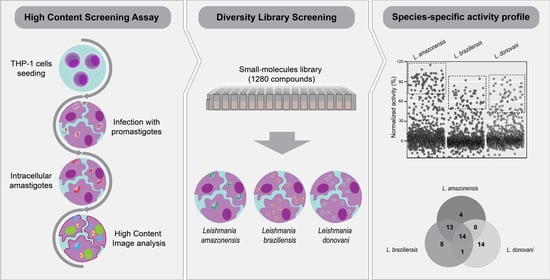
2.1. Development of a Multi-Species High Content Screening Assay
2.2. Diversity Library Screening against Leishmania Species
2.3. Identification of Pan-Active Compounds
3. Discussion
4. Methods
4.1. Reference Compounds and Library
4.2. Host Cell and Parasite Cultures
4.3. Intracellular Amastigotes Assay and Library Screening
4.4. High Content Image Acquisition and Analysis
4.5. Data Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M.; WHO Leishmaniasis Control Team. Leishmaniasis Control Team Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- WHO. Weekly Epidemiological Record Relevé Épidémiologique Hebdomadaire; WHO: Geneva, Switzerland, 2016; Volume 91, pp. 285–296. [Google Scholar]
- WHO. WHO Technical Report Series Control of the Leishmaniases; World Health Organization: Geneva, Switzerland, 2010; Volume 978. [Google Scholar]
- Kevric, I.; Cappel, M.A.; Keeling, J.H. New World and Old World Leishmania Infections: A Practical Review. Dermatol. Clin. 2015, 33, 579–593. [Google Scholar] [CrossRef]
- Akhoundi, M.; Kuhls, K.; Cannet, A.; Votýpka, J.; Marty, P.; Delaunay, P.; Sereno, D. A Historical Overview of the Classification, Evolution, and Dispersion of Leishmania Parasites and Sandflies. PLoS Negl. Trop. Dis. 2016, 10, e0004349. [Google Scholar] [CrossRef]
- Llanes, A.; Restrepo, C.M.; Del Vecchio, G.; Anguizola, F.J.; Lleonart, R. The genome of Leishmania panamensis: Insights into genomics of the L. (Viannia) subgenus. Sci. Rep. 2015, 5, 8550. [Google Scholar] [CrossRef] [Green Version]
- Zijlstra, E.E. The immunology of post-kala-azar dermal leishmaniasis (PKDL). Parasit. Vectors 2016, 9, 464. [Google Scholar] [CrossRef] [Green Version]
- Uliana, S.R.B.; Trinconi, C.T.; Coelho, A.C. Chemotherapy of leishmaniasis: Present challenges. Parasitology 2018, 145, 464–480. [Google Scholar] [CrossRef]
- Croft, S.L.; Seifert, K.; Yardley, V. Current scenario of drug development for leishmaniasis. Indian J. Med. Res. 2006, 123, 399–410. [Google Scholar]
- Matoussi, N.; Ameur, H.B.; Amor, S.B.; Fitouri, Z.; Becher, S.B. Cardiotoxicity of n-methyl-glucamine antimoniate (Glucantime). A case report. Méd. Mal. Infect. 2007, 37, S257–S259. [Google Scholar] [CrossRef]
- Glasser, J.S.; Murray, C.K. Central Nervous System Toxicity Associated with Liposomal Amphotericin B Therapy for Cutaneous Leishmaniasis. Am. J. Trop. Med. Hyg. 2011, 84, 566–568. [Google Scholar] [CrossRef] [Green Version]
- Gasser, R.A.; Magill, A.J.; Oster, C.N.; Franke, E.D.; Grögl, M.; Berman, J.D. Pancreatitis induced by pentavalent antimonial agents during treatment of leishmaniasis. Clin. Infect. Dis. 1994, 18, 83–90. [Google Scholar] [CrossRef]
- Meyerhoff, A. U.S. Food and Drug Administration Approval of AmBisome (Liposomal Amphotericin B) for Treatment of Visceral Leishmaniasis. Clin. Infect. Dis. 1999, 28, 42–48. [Google Scholar] [CrossRef]
- Sundar, S.; Jha, T.K.; Thakur, C.P.; Engel, J.; Sindermann, H.; Fischer, C.; Junge, K.; Bryceson, A.; Berman, J. Oral Miltefosine for Indian Visceral Leishmaniasis. N. Engl. J. Med. 2002, 347, 1739–1746. [Google Scholar] [CrossRef] [Green Version]
- Rijal, S.; Ostyn, B.; Uranw, S.; Rai, K.; Bhattarai, N.R.; Dorlo, T.P.C.; Beijnen, J.H.; Vanaerschot, M.; Decuypere, S.; Dhakal, S.S.; et al. Increasing Failure of Miltefosine in the Treatment of Kala-azar in Nepal and the Potential Role of Parasite Drug Resistance, Reinfection, or Noncompliance. Clin. Infect. Dis. 2013, 56, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Decuypere, S.; Vanaerschot, M.; Brunker, K.; Imamura, H.; Müller, S.; Khanal, B.; Rijal, S.; Dujardin, J.-C.; Coombs, G.H.; Murray, H.; et al. Molecular Mechanisms of Drug Resistance in Natural Leishmania Populations Vary with Genetic Background. PLoS Negl. Trop. Dis. 2012, 6, e1514. [Google Scholar] [CrossRef]
- Rojas, R.; Valderrama, L.; Valderrama, M.; Varona, M.X.; Ouellette, M.; Saravia, N.G. Resistance to antimony and treatment failure in human Leishmania (Viannia) infection. J. Infect. Dis. 2006, 193, 1375–1383. [Google Scholar] [CrossRef] [Green Version]
- Purkait, B.; Kumar, A.; Nandi, N.; Sardar, A.H.; Das, S.; Kumar, S.; Pandey, K.; Ravidas, V.; Kumar, M.; De, T.; et al. Mechanism of amphotericin B resistance in clinical isolates of Leishmania donovani. Antimicrob. Agents Chemother. 2012, 56, 1031–1041. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, S.; Mishra, J.; Gupta, A.K.; Singh, A.; Shankar, P.; Singh, S. Laboratory confirmed miltefosine resistant cases of visceral leishmaniasis from India. Parasit. Vectors 2017, 10, 49. [Google Scholar] [CrossRef] [Green Version]
- Hefnawy, A.; Berg, M.; Dujardin, J.-C.; De Muylder, G. Exploiting Knowledge on Leishmania Drug Resistance to Support the Quest for New Drugs. Trends Parasitol. 2017, 33, 162–174. [Google Scholar] [CrossRef] [Green Version]
- Croft, S.L.; Yardley, V.; Kendrick, H. Field epidemiology drug sensitivity of Leishmania species: Some unresolved problems. Trans. R. Soc. Trop. Med. Hyg. 2002, 96, 127–130. [Google Scholar] [CrossRef]
- DNDi. DNDi Portlofio December 2018; DNDi: Geneva, Switzerland, 2018. [Google Scholar]
- Bush, J.T.; Wasunna, M.; Alves, F.; Alvar, J.; Olliaro, P.L.; Otieno, M.; Sibley, C.H.; Strub Wourgaft, N.; Guerin, P.J. Systematic review of clinical trials assessing the therapeutic efficacy of visceral leishmaniasis treatments: A first step to assess the feasibility of establishing an individual patient data sharing platform. PLoS Negl. Trop. Dis. 2017, 11, e0005781. [Google Scholar] [CrossRef] [Green Version]
- Peña, I.; Pilar Manzano, M.; Cantizani, J.; Kessler, A.; Alonso-Padilla, J.; Bardera, A.I.; Alvarez, E.; Colmenarejo, G.; Cotillo, I.; Roquero, I.; et al. New compound sets identified from high throughput phenotypic screening against three kinetoplastid parasites: An open resource. Sci. Rep. 2015, 5, 8771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khare, S.; Nagle, A.S.; Biggart, A.; Lai, Y.H.; Liang, F.; Davis, L.C.; Barnes, S.W.; Mathison, C.J.N.; Myburgh, E.; Gao, M.-Y.; et al. Proteasome inhibition for treatment of leishmaniasis, Chagas disease and sleeping sickness. Nature 2016, 537, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Wyllie, S.; Brand, S.; Thomas, M.; De Rycker, M.; Chung, C.-W.; Pena, I.; Bingham, R.P.; Bueren-Calabuig, J.A.; Cantizani, J.; Cebrian, D.; et al. Preclinical candidate for the treatment of visceral leishmaniasis that acts through proteasome inhibition. Proc. Natl. Acad. Sci. USA 2019, 116, 9318–9323. [Google Scholar] [CrossRef] [Green Version]
- Torrie, L.S.; Brand, S.; Robinson, D.A.; Ko, E.J.; Stojanovski, L.; Simeons, F.R.C.; Wyllie, S.; Thomas, J.; Ellis, L.; Osuna-Cabello, M.; et al. Chemical Validation of Methionyl-tRNA Synthetase as a Druggable Target in Leishmania donovani. ACS Infect. Dis. 2017, 3, 718–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyllie, S.; Thomas, M.; Patterson, S.; Crouch, S.; De Rycker, M.; Lowe, R.; Gresham, S.; Urbaniak, M.D.; Otto, T.D.; Stojanovski, L.; et al. Cyclin-dependent kinase 12 is a drug target for visceral leishmaniasis. Nature 2018, 560, 192–197. [Google Scholar] [CrossRef]
- Siqueira-Neto, J.L.; Moon, S.; Jang, J.; Yang, G.; Lee, C.; Moon, H.K.; Chatelain, E.; Genovesio, A.; Cechetto, J.; Freitas-Junior, L.H. An image-based high-content screening assay for compounds targeting intracellular Leishmania donovani amastigotes in human macrophages. PLoS Negl. Trop. Dis. 2012, 6, e1671. [Google Scholar] [CrossRef] [Green Version]
- De Rycker, M.; Hallyburton, I.; Thomas, J.; Campbell, L.; Wyllie, S.; Joshi, D.; Cameron, S.; Gilbert, I.H.; Wyatt, P.G.; Frearson, J.A.; et al. Comparison of a high-throughput high-content intracellular Leishmania donovani assay with an axenic amastigote assay. Antimicrob. Agents Chemother. 2013, 57, 2913–2922. [Google Scholar] [CrossRef] [Green Version]
- Tegazzini, D.; Díaz, R.; Aguilar, F.; Peña, I.; Presa, J.L.; Yardley, V.; Martin, J.J.; Coteron, J.M.; Croft, S.L.; Cantizani, J. A Replicative In Vitro Assay for Drug Discovery against Leishmania donovani. Antimicrob. Agents Chemother. 2016, 60, 3524–3532. [Google Scholar] [CrossRef] [Green Version]
- Nühs, A.; De Rycker, M.; Manthri, S.; Comer, E.; Scherer, C.A.; Schreiber, S.L.; Ioset, J.-R.; Gray, D.W. Development and Validation of a Novel Leishmania donovani Screening Cascade for High-Throughput Screening Using a Novel Axenic Assay with High Predictivity of Leishmanicidal Intracellular Activity. PLoS Negl. Trop. Dis. 2015, 9, e0004094. [Google Scholar] [CrossRef] [Green Version]
- Siqueira-Neto, J.L.; Song, O.-R.; Oh, H.; Sohn, J.-H.; Yang, G.; Nam, J.; Jang, J.; Cechetto, J.; Lee, C.B.; Moon, S.; et al. Antileishmanial high-throughput drug screening reveals drug candidates with new scaffolds. PLoS Negl. Trop. Dis. 2010, 4, e675. [Google Scholar] [CrossRef] [Green Version]
- Aulner, N.; Danckaert, A.; Rouault-Hardoin, E.; Desrivot, J.; Helynck, O.; Commere, P.-H.; Lè Ne Munier-Lehmann, H.; Spä Th, G.F.; Shorte, S.L.; Ve Milon, G.; et al. High Content Analysis of Primary Macrophages Hosting Proliferating Leishmania Amastigotes: Application to Anti-leishmanial Drug Discovery. PLoS Negl. Trop. Dis. 2013, 7, e2154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khraiwesh, M.; Leed, S.; Roncal, N.; Johnson, J.; Sciotti, R.; Smith, P.; Read, L.; Paris, R.; Hudson, T.; Hickman, M.; et al. Antileishmanial Activity of Compounds Derived from the Medicines for Malaria Venture Open Access Box against Intracellular Leishmania major Amastigotes. Am. J. Trop. Med. Hyg 2016, 94, 340–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alcântara, L.M.; Ferreira, T.C.S.; Gadelha, F.R.; Miguel, D.C. Challenges in drug discovery targeting TriTryp diseases with an emphasis on leishmaniasis. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Lamotte, S.; Aulner, N.; Späth, G.F.; Prina, E. Discovery of novel hit compounds with broad activity against visceral and cutaneous Leishmania species by comparative phenotypic screening. Sci. Rep. 2019, 9, 438. [Google Scholar] [CrossRef]
- Hefnawy, A.; Cantizani, J.; Peña, I.; Manzano, P.; Rijal, S.; Dujardin, J.-C.; De Muylder, G.; Martin, J. Importance of secondary screening with clinical isolates for anti-leishmania drug discovery. Sci. Rep. 2018, 8, 11765. [Google Scholar] [CrossRef] [Green Version]
- Sacks, D.L.; Barral, A.; Neva, F.A. Thermosensitivity patterns of Old vs. New World cutaneous strains of Leishmania growing within mouse peritoneal macrophages in vitro. Am. J. Trop. Med. Hyg. 1983, 32, 300–304. [Google Scholar] [CrossRef]
- Escobar, P.; Matu, S.; Marques, C.; Croft, S.L. Sensitivities of Leishmania species to hexadecylphosphocholine (miltefosine), ET-18-OCH(3) (edelfosine) and amphotericin B. Acta Trop. 2002, 81, 151–157. [Google Scholar] [CrossRef]
- Morais-Teixeira, E.D.; Damasceno, Q.S.; Galuppo, M.K.; Romanha, A.J.; Rabello, A. The in vitro leishmanicidal activity of hexadecylphosphocholine (miltefosine) against four medically relevant Leishmania species of Brazil. Mem. Inst. Oswaldo Cruz 2011, 106, 475–478. [Google Scholar] [CrossRef] [Green Version]
- Fernández, O.L.; Diaz-Toro, Y.; Ovalle, C.; Valderrama, L.; Muvdi, S.; Rodríguez, I.; Gomez, M.A.; Saravia, N.G. Miltefosine and Antimonial Drug Susceptibility of Leishmania Viannia Species and Populations in Regions of High Transmission in Colombia. PLoS Negl. Trop. Dis. 2014, 8, e2871. [Google Scholar] [CrossRef] [Green Version]
- Voak, A.A.; Gobalakrishnapillai, V.; Seifert, K.; Balczo, E.; Hu, L.; Hall, B.S.; Wilkinson, S.R. An essential type I nitroreductase from Leishmania major can be used to activate leishmanicidal prodrugs. J. Biol. Chem. 2013, 288, 28466–28476. [Google Scholar] [CrossRef] [Green Version]
- Voak, A.A.; Seifert, K.; Helsby, N.A.; Wilkinson, S.R. Evaluating Aziridinyl Nitrobenzamide Compounds as Leishmanicidal Prodrugs. Antimicrob. Agents Chemother. 2014, 58, 370–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bot, C.; Hall, B.S.; Bashir, N.; Taylor, M.C.; Helsby, N.A.; Wilkinson, S.R. Trypanocidal Activity of Aziridinyl Nitrobenzamide Prodrugs. Antimicrob. Agents Chemother. 2010, 54, 4246–4252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drugs for Neglected Diseases Initiative. European Medicines Agency Recommends Fexinidazole, the First All-Oral Treatment for Sleeping Sickness; Drugs for Neglected Diseases Initiative: Geneva, Switzerland, 2018. [Google Scholar]
- Musa, A.M.; Khalil, E.A. Trial to Determine Efficacy of Fexinidazole in Visceral Leihmaniasis Patients in Sudan. Identification Number NCT01980199; 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT01980199 (accessed on 26 May 2020).
- Patterson, S.; Wyllie, S.; Norval, S.; Stojanovski, L.; Simeons, F.R.; Auer, J.L.; Osuna-Cabello, M.; Read, K.D.; Fairlamb, A.H. The anti-tubercular drug delamanid as a potential oral treatment for visceral leishmaniasis. Elife 2016, 5, e09744. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compound CB1954 are available from the authors. |





| Screening Parameters | L. amazonensis | L. braziliensis | L. donovani |
|---|---|---|---|
| Z’-factor | 0.65 ± 0.04 | 0.76 ± 0.07 | 0.77 ± 0.05 |
| Correlation index | 0.86 | 0.88 | 0.89 |
| CV of infected control (%) | 11.74 | 11.4 | 13.5 |
| EC50 Amphotericin B (µM) | 2.10 ± 0.16 | 0.93 ± 0.45 | 0.82 ± 0.07 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcântara, L.M.; Ferreira, T.C.S.; Fontana, V.; Chatelain, E.; Moraes, C.B.; Freitas-Junior, L.H. A Multi-Species Phenotypic Screening Assay for Leishmaniasis Drug Discovery Shows That Active Compounds Display a High Degree of Species-Specificity. Molecules 2020, 25, 2551. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25112551
Alcântara LM, Ferreira TCS, Fontana V, Chatelain E, Moraes CB, Freitas-Junior LH. A Multi-Species Phenotypic Screening Assay for Leishmaniasis Drug Discovery Shows That Active Compounds Display a High Degree of Species-Specificity. Molecules. 2020; 25(11):2551. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25112551
Chicago/Turabian StyleAlcântara, Laura M., Thalita C. S. Ferreira, Vanessa Fontana, Eric Chatelain, Carolina B. Moraes, and Lucio H. Freitas-Junior. 2020. "A Multi-Species Phenotypic Screening Assay for Leishmaniasis Drug Discovery Shows That Active Compounds Display a High Degree of Species-Specificity" Molecules 25, no. 11: 2551. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25112551