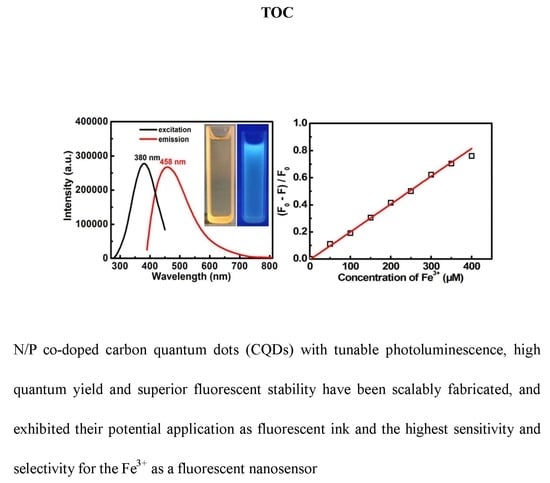
Green and Facile Synthesis of Nitrogen and Phosphorus Co-Doped Carbon Quantum Dots towards Fluorescent Ink and Sensing Applications
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals
2.2. Synthesis of N/P Co-Doped CQDs
2.3. QY Measurement
2.4. Fluorescence Ink Evaluation
2.5. Metal Ion Detection
2.6. Materials Characterizations
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Das, R.K.; Mohapatra, S. Highly luminescent, heteroatom-doped carbon quantum dots ultrasensitive sensing of glucosamine and targeted imaging of liver cancer cells. J. Mater. Chem. B 2017, 5, 2190–2197. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Edme, K.; Lian, S.C.; Weiss, E.A. Enhancing the rate of quantum-dot-photocatalyzed carbon-carbon coupling by tuning the composition of the dot’s ligand shell. J. Am. Chem. Soc. 2017, 139, 4246–4249. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.L.; Wang, Z.B.; Li, X.H.; Li, Y.C.; Tan, Z.A.; Fan, L.Z.; Yang, S.H. Bright multicolor bandgap fluorescent carbon quantum dots for electroluminescent light-emitting diodes. Adv. Mater. 2017, 29, 1604436. [Google Scholar] [CrossRef] [PubMed]
- Hess, S.C.; Permatasari, F.A.; Fukazawa, H.; Schneider, E.M.; Balgis, R.; Ogi, T.; Okuyama, K.; Stark, W.J. Direct synthesis of carbon quantum dots in aqueous polymer solution: One-pot reaction and preparation of transparent UV-blocking films. J. Mater. Chem. A 2017, 5, 5187–5194. [Google Scholar] [CrossRef]
- Yang, G.H.; Wan, X.J.; Liu, Y.J.; Li, R.; Su, Y.K.; Zeng, X.R.; Tang, J.N. Luminescent poly(vinyl alcohol)/carbon quantum dots composites with tunable water-induced shape memory behavior in different pH and temperature environments. ACS Appl. Mater. Interfaces 2016, 8, 34744–34754. [Google Scholar] [CrossRef] [PubMed]
- Gong, N.Q.; Wang, H.; Li, S.; Deng, Y.L.; Chen, X.A.; Ye, L.; Gu, W. Microwave-assisted polyol synthesis of gadolinium-doped green luminescent carbon dots as a bimodal nanoprobe. Langmuir 2014, 30, 10933–10939. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Hu, C.; Yu, C.; Wang, S.; Zhang, P.; Qiu, J.S. Organic amine-grafted carbon quantum dots with tailored surface and enhanced photoluminescence properties. Carbon 2015, 91, 291–297. [Google Scholar] [CrossRef]
- Sun, Y.P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.F.; et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Thongsai, N.; Chae, A.; Jo, S.; BiKang, E.; Paoprasert, P.; Park, S.Y.; In, I. Microwave-assisted synthesis of luminescent and biocompatible lysine-based carbon quantum dots. J. Ind. Eng. Chem. 2017, 47, 329–335. [Google Scholar] [CrossRef]
- Qu, S.N.; Wang, X.Y.; Lu, Q.P.; Liu, X.Y.; Wang, L.J. A biocompatible fluorescent ink based on water-soluble luminescent carbon nanodots. Angew. Chem. Int. Ed. 2012, 51, 12215–12218. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lu, K.Q.; Tang, Z.R.; Xu, Y.J. Recent progress in carbon quantum dots: Synthesis, properties and applications in photocatalysis. J. Mater. Chem. A 2017, 5, 3717–3734. [Google Scholar] [CrossRef]
- Pan, Y.; Yang, J.; Fang, Y.N.; Zheng, J.H.; Song, R.C.; Yi, Q. One-pot synthesis of gadolinium-doped carbon quantum dots for high-performance multimodal bioimaging. J. Mater. Chem. B 2017, 5, 92–101. [Google Scholar] [CrossRef]
- Han, Y.Z.; Huang, H.; Zhang, H.C.; Liu, Y.; Han, X.; Liu, R.H.; Li, H.T.; Kang, Z.H. Carbon quantum dots with photo enhanced hydrogen-bond catalytic activity in aldol condensations. ACS Catal. 2014, 4, 781–787. [Google Scholar] [CrossRef]
- Lu, Q.J.; Wu, C.Y.; Liu, D.; Wang, H.Y.; Su, W.; Li, H.T.; Zhang, Y.Y.; Yao, S.Z. A facile and simple method for synthesis of graphene oxide quantum dots from black carbon. Green Chem. 2017, 19, 900–904. [Google Scholar] [CrossRef]
- Guo, Y.M.; Zhang, L.F.; Cao, F.P.; Leng, Y.M. Thermal treatment of hair for the synthesis of sustainable carbon quantum dots and the applications for sensing Hg2+. Sci. Rep. 2016, 6, 35795. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.Q.; Lin, J.P.; Chen, Y.M.; Fu, F.F.; Chi, Y.W.; Chen, G.N. Graphene quantum dots, graphene oxide, carbon quantum dots and graphite nanocrystals in coals. Nanoscale 2014, 6, 7410–7415. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.L.; Gao, M.X.; Wang, T.T.; Wan, X.Y.; Zheng, L.L.; Huang, C.Z. A general quantitative pH sensor developed with dicyandiamide N-doped high quantum yield graphene quantum dots. Nanoscale 2014, 6, 3868–3874. [Google Scholar] [CrossRef] [PubMed]
- Algarra, M.; Pérez-Martín, M.; Cifuentes-Rueda, M.; Jiménez-Jiménez, J.; Esteves da Silva, J.C.; Bandosz, T.J.; Rodríguez-Castellón, E.; López Navarrete, J.T.; Casado, J. Carbon dots obtained using hydrothermal treatment of formaldehyde. Cell imaging in vitro. Nanoscale 2014, 6, 9071–9077. [Google Scholar] [CrossRef] [PubMed]
- Leturcq, R.; Stampfer, C.; Inderbitzin, K.; Durrer, L.; Hierold, C.; Mariani, E.; Schultz, M.G.; Oppen, F.V.; Ensslin, K. Franck-condon blockade in suspended carbon nanotube quantum dots. Nat. Phys. 2009, 5, 327–331. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, K.M.; Sonker, A.K.; Sonkar, S.K.; Sarkar, S. Pollutan soot of diesel engine exhaust transformed to carbon dots for multicoloured imaging of E-coli and sensing cholesterol. RSC Adv. 2014, 4, 30100–30107. [Google Scholar] [CrossRef]
- Mondal, T.K.; Gupta, A.; Shaw, B.K.; Mondal, S.; Ghorai, U.K.; Saha, S.K. Highly luminescent N-doped carbon quantum dots from lemon juice with porphyrin-like structures surrounded by graphitic network for sensing applications. RSC Adv. 2016, 6, 59927–59934. [Google Scholar] [CrossRef]
- Tyagi, A.; Tripathi, K.M.; Singh, N.; Choudhary, S.; Gupta, R.K. Green synthesis of carbon quantum dots from lemon peel waste: Applications in sensing and photocatalysis. RSC Adv. 2016, 6, 72423–72432. [Google Scholar] [CrossRef]
- Sachdev, A.; Gopinath, P. Green synthesis of multifunctional carbon dots from coriander leaves and their potential application as antioxidants, sensors and bioimaging agents. Analyst 2015, 14, 4260–4269. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.L.; Yin, Y.J.; Wang, C.F.; Chen, S. Plant leaf-derived fluorescent carbon dots for sensing, patterning and coding. J. Mater. Chem. C 2013, 1, 4925–4932. [Google Scholar] [CrossRef]
- Holá, K.; Sudolská, M.; Kalytchuk, S.; Nachtigallová, D.; Rogach, A.L.; Otyepka, M.; Zbořil, R. Graphitic nitrogen triggers red fluorescence in carbon dots. ACS Nano 2017, 11, 12402–12410. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Yu, S.B.; Wei, J.S.; Xiong, H.M. Full-color light-emitting carbon dots with a surface-state-controlled luminescence mechanism. ACS Nano 2016, 10, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.F.; Li, Z.H.; Sun, Y.Q.; Geng, X.; Hu, Y.L.; Meng, H.M.; Ge, J.; Qu, L.B. Synthesis of luminescent carbon dots with ultrahigh quantum yield and inherent folate receptor-positive cancer cell targetability. Sci. Rep. 2018, 8, 1086. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Cao, H.; Zhu, S.Q.; Hou, L.R.; Yuan, C.Z. Hierarchical micro-/mesoporous N- and O-enriched carbon derived from disposable cashmere: A competitive cost-effective material for high-performance electrochemical capacitors. Green Chem. 2015, 17, 2373–2382. [Google Scholar] [CrossRef]
- Wang, F.L.; Chen, P.; Feng, Y.P.; Xie, Z.J.; Liu, Y.; Su, Y.H.; Zhang, Q.X.; Wang, Y.F.; Yao, K.; Lv, W.Y. Facile synthesis of N-doped carbon dots/g-C3N4 photocatalyst with enhanced visible-light photocatalytic activity for the degradation of indomethacin. Appl. Catal. B 2017, 207, 103–113. [Google Scholar] [CrossRef]
- Hou, L.R.; Lian, L.; Li, D.K.; Pang, G.; Li, J.F.; Zhang, X.G.; Xiong, S.L.; Yuan, C.Z. Mesoporous N-containing carbon nanosheets towards high-performance electrochemical capacitors. Carbon 2013, 64, 141–149. [Google Scholar] [CrossRef]
- Cui, X.B.; Wang, Y.L.; Liu, J.; Yang, Q.Y.; Zhang, B.; Gao, Y.; Wang, Y.; Lu, G.Y. Dual functional N- and S-co-doped carbon dots as the sensor for temperature and Fe3+ ions. Sens. Actuators B 2017, 242, 1272–1280. [Google Scholar] [CrossRef]
- Pooja, D.; Anupma, T.; Shweta, C.; Navneet, K.; Praveen, K.; Narinder, S.; Mahesh, K.; Sonnada, M.S.; Nayak, M.K. Ultrasensitive and selective sensing of selenium using nitrogen-rich ligand interfaced carbon quantum dots. ACS Appl. Mater. Interfaces 2017, 9, 13448–13456. [Google Scholar]
- Liu, Y.H.; Duan, W.X.; Song, W.; Liu, J.J.; Ren, C.L.; Wu, J.; Liu, D.; Chen, H.L. Red emission B, N, S-co-doped carbon dots for colorimetric and fluorescent dual mode detection of Fe3+ ions in complex biological fluids and living cells. ACS Appl. Mater. Interfaces 2017, 9, 12663–12672. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.F.; Zhang, L.L.; Li, X.F.; Liu, R.J.; Lin, L.Y.; Zhao, S.L. Green preparationof S and N co-doped carbon dots from Water Chestnut and Onion as well as their use as an off–on fluorescent probe for the quantification and imaging of coenzyme A. ACS Sustain. Chem. Eng. 2017, 5, 4992–5000. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, C.Z.; Song, J.H.; Li, H.; Du, D.; Lin, Y.H. Drug-derived bright and color-tunable N-doped carbon dots for cell imaging and sensitive detection of Fe3+ in living cells. ACS Appl. Mater. Interfaces 2017, 9, 7399–7405. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.M.; Kong, W.Q.; Li, H.; Liu, J.; Yang, M.M.; Huang, H.; Liu, Y.; Wang, Z.Y.; Wang, Z.Q.; Sham, T.K.; et al. Fluorescent N-doped carbon dots as in vitro and in vivo nanothermometer. ACS Appl. Mater. Interfaces 2015, 7, 27324–27330. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Liu, Y.; Shu, Q.W.; Liang, J.M.; Zhang, F.; Chen, X.P.; Deng, X.Y.; Swihart, M.T.; Tan, K.J. One-pot hydrothermal synthesis of carbon dots with efficient up- and down-converted photoluminescence for the sensitive detection of morin in a dual-readout assay. Langmuir 2017, 33, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.B.; Zhou, L.; Li, Y.N.; Deng, R.P.; Zhang, H.J. Highly fluorescent nitrogen-doped carbon dots with excellent thermal and photo stability applied as invisible ink for loading important information and anti-counterfeiting. Nanoscale 2017, 9, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.X.; Ren, H.B.; Yan, X.P. Fluorescent metal-organic framework MIL-53(Al) for highly selective and sensitive detection of Fe3+ in aqueous solution. Anal. Chem. 2013, 85, 7441–7446. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.C.; Zhang, M.; Yang, F.Y.; Wang, F.; Wang, C.Y. Facile synthesis of water soluble fluorescent metal (Pt, Au, Ag and Cu) quantum clusters for the selective detection of Fe3+ ions as both fluorescent and colorimetric probes. J. Mater. Chem. C 2017, 5, 2466–2473. [Google Scholar] [CrossRef]
- Sui, B.L.; Tang, S.M.; Liu, T.H.; Kim, B.S.; Belfield, K.D. Novel BODIPY-based fluorescence turn-on sensor for Fe3+ and its bioimaging application in living cells. ACS Appl. Mater. Interfaces 2014, 6, 18408–18412. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.A.; Luo, F.X.; Khoshi, M.R.; Rabie, E.; Zhang, Q.; Flach, C.R.; Mendelsohn, R.; Garfunkel, E.; Szostak, M.; He, H.X. P-doped porous carbon as metal free catalysts for selective aerobic oxidation with an unexpected mechanism. ACS Nano 2016, 10, 2305–2315. [Google Scholar] [CrossRef] [PubMed]
- Barman, M.K.; Jana, B.; Bhattacharyya, S.; Patra, A. Photophysical properties of doped carbon dots (N, P, and B) and their influence on electron/hole transfer in carbon dots-nickel(II) phthalocyanine conjugates. J. Phys. Chem. C 2014, 118, 20034–20041. [Google Scholar] [CrossRef]
- Cao, X.C.; Wu, J.; Jin, C.; Tian, J.H.; Strasser, P.; Yang, R.Z. MnCo2O4 anchored on P-doped hierarchical porous carbon as an electrocatalyst for high-performance rechargeable Li-O2 batteries. ACS Catal. 2015, 5, 4890–4896. [Google Scholar] [CrossRef]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew. Chem. Int. Ed. 2013, 52, 3953–3957. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.N.; Jha, S.; Singhal, R.K.; Kailasa, S.K. Preparation of multicolor emitting carbon dots for HeLa cell imaging. New J. Chem. 2014, 38, 6152–6160. [Google Scholar] [CrossRef]
- Jaiswal, A.; Ghosh, S.S.; Chattopadhyay, A. One step synthesis of C-dots by microwave mediated caramelization of poly(ethylene glycol). Chem. Commun. 2012, 48, 407–409. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.Y.; Chen, Y.L.; Fan, H.; Wei, X.J.; Hu, C.G.; Wang, L.X.; Qu, L.T. A green one-arrow-two-hawks strategy for nitrogen-doped carbon dots as fluorescent ink and oxygen reduction electrocatalysts. J. Mater. Chem. A 2014, 2, 6320–6325. [Google Scholar] [CrossRef]
- Wang, C.F.; Sun, D.; Zhuo, K.L.; Zhang, H.C.; Wang, J.J. Simple and green synthesis of nitrogen-, sulfur-, and phosphorus-co-doped carbon dots with tunable luminescence properties and sensing application. RSC Adv. 2014, 4, 54060–54065. [Google Scholar] [CrossRef]
- Yang, S.W.; Sun, J.; Li, X.B.; Zhou, W.; Wang, Z.Y.; He, P.; Ding, G.Q.; Xie, X.M.; Kang, Z.H.; Jiang, M.H. Large-scale fabrication of heavy doped carbon quantum dots with tunable-photoluminescence and sensitive fluorescence detection. J. Mater. Chem. A 2014, 2, 8660–8667. [Google Scholar] [CrossRef]
- Zhang, Z.; Hao, J.H.; Zhang, J.; Zhang, B.L.; Tang, J.L. Protein as the source for synthesizing fluorescent carbon dots by a one-pot hydrothermal route. RSC Adv. 2012, 2, 8599–8601. [Google Scholar] [CrossRef]
- Wu, D.; Huang, X.M.; Deng, X.; Wang, K.; Liu, Q.Y. Preparation of photoluminescent carbon nanodots by traditional Chinese medicine and application as a probe for Hg2+. Anal. Methods 2013, 5, 3023–3027. [Google Scholar] [CrossRef]
- Hsu, P.-C.; Shih, Z.-Y.; Lee, C.-H.; Chang, H.-T. Synthesis and analytical applications of photoluminescent carbon nanodots. Green Chem. 2012, 14, 917–920. [Google Scholar] [CrossRef]
- Ray, S.C.; Saha, A.; Jana, N.R.; Sarkar, R. Fluorescent carbon nanoparticles: Synthesis, characterization, and bioimaging application. J. Phys. Chem. C 2009, 113, 18546–18551. [Google Scholar] [CrossRef]
- Liu, H.P.; Ye, T.; Mao, C.D. Fluorescent carbon nanoparticles derived from candle soot. Angew. Chem. Int. Ed. 2007, 46, 6473–6475. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.J.; Sun, Q.; Zhang, L.; Zhang, Y.; Lu, A.H. Photoluminescent carbon dots directly derived from polyethylene glycol and their application for cellular imaging. Carbon 2014, 71, 87–93. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, H.S. Green synthesis of luminescent nitrogen-doped carbon dots from milk and its imaging application. Anal. Chem. 2014, 86, 8902–8905. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xu, C.X.; Tian, Z.S.; Lin, Y.; Shi, Z.L. Facilely synthesized N-doped carbon quantum dots with high fluorescent yield for sensing Fe3+. New. J. Chem. 2016, 40, 2083–2088. [Google Scholar] [CrossRef]
- Sharma, A.; Gadly, T.; Gupta, A.; Ballal, A.; Ghosh, S.K.; Kumbhakar, M. Origin of excitation dependent fluorescence in carbon nanodots. J. Phys. Chem. Lett. 2016, 7, 3695–3702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Hu, Y.S.; Lin, J.; Fan, Y.; Li, Y.T.; Lv, Y.; Liu, X.Y. Excitation wavelength independence: Toward low-threshold amplified spontaneous emission from carbon nnodots. ACS Appl. Mater. Interfaces 2016, 8, 25454–25460. [Google Scholar] [CrossRef] [PubMed]
- Loukanov, A.; Sekiya, R.; Yoshikawa, M.; Kobayashi, N.; Moriyasu, Y.; Nakabayashi, S. Photosensitizer-conjugated ultra small carbon nanodots as multifunctional fluorescent probes for bioimaging. J. Phys. Chem. C 2016, 120, 15867–15874. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Sun, W.H.; Wu, P.Y. Highly photoluminescent carbon dots derived from egg white: Facile and green synthesis, photoluminescence properties, and multiple applications. ACS Sustain. Chem. Eng. 2015, 3, 1412–1418. [Google Scholar] [CrossRef]
- Liu, S.; Tian, J.Q.; Wang, L.; Luo, Y.L.; Zhai, J.F.; Sun, X.P. Preparation of photoluminescent carbon nitride dots from CCl4 and 1,2-ethylenediamine: A heat-treatment-based strategy. J. Mater. Chem. 2011, 21, 11726–11729. [Google Scholar] [CrossRef]
- Sahu, S.; Behera, B.; Maitib, T.K.; Mohapatra, S. Simple one-step synthesis of highly luminescent carbon dots from orange juice: Application as excellent bio-imaging agents. Chem. Commun. 2012, 48, 8835–8837. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, C.G.; Zhu, S.J.; Wang, H.L.; Chen, C.L.; Wang, Z.R.; Bai, T.Y.; Shi, Z.; Feng, S.H. Histidine-derived nontoxic nitrogen-doped carbon dots for sensing and bioimaging applications. Langmuir 2014, 30, 13542–13548. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, H.J.; Bian, T.; Zhao, Y.F.; Zhou, C.; Shang, L.; Liu, Y.H.; Wu, L.Z.; Tung, C.H.; Zhang, T.R. Highly luminescent nitrogen-doped carbon quantum dots as effective fluorescent probes for mercuric and iodide ions. J. Mater. Chem. C 2015, 3, 1922–1928. [Google Scholar] [CrossRef]
- Ding, H.; Wei, J.S.; Xiong, H.M. Nitrogen and sulfur co-doped carbon dots with strong blue luminescence. Nanoscale 2014, 6, 13817–13823. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, Z.; Kong, B.; Feng, S.; Wang, J.; Wang, L.; Yang, J.; Zhang, F.; Wu, P.; Zhao, D. Simple and green synthesis of nitrogen-doped photoluminescent carbonaceous nanospheres for bioimaging. Angew. Chem. Int. Ed. 2013, 52, 8151–8155. [Google Scholar] [CrossRef] [PubMed]
- Gedda, G.; Lee, C.Y.; Lin, Y.C.; Wu, H.F. Green synthesis of carbon dots from prawn shells for highly selective and sensitive detection of copper ions. Sens. Actuators B 2016, 224, 396–403. [Google Scholar] [CrossRef]
- Qu, K.; Wang, J.; Ren, J.; Qu, X. Carbon dots prepared by hydrothermal treatment of dopamine as an effective fluorescent sensing platform for the label-free detection of iron(III) ions and dopamine. Chem. Eur. J. 2013, 19, 7243–7249. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.; Zheng, E.; Chen, L.; Wang, X.; Kong, L.; You, C.; Ruan, Y.; Weng, X. Hybrid carbon source for producing nitrogen-doped polymernanodts: One-pot hydrothermal synthesis, fluorescence enhancement and highly selective detection of Fe(III). Nanoscale 2013, 5, 8015–8021. [Google Scholar] [CrossRef] [PubMed]
- Li, G.M.; Lv, N.; Bi, W.Z.; Zhang, J.L.; Ni, J.Z. Nitrogen-doped carbon dots as fluorescence probe suitable for sensing Fe3+ under acidic conditions. New J. Chem. 2016, 40, 10213–10218. [Google Scholar] [CrossRef]
- Li, S.H.; Li, Y.C.; Cao, J.; Zhu, J.; Fan, L.Z.; Li, X.H. Sulfur-doped grapheme quantum dots as novel fluorescent probe for highly selective and sensitive detection of Fe3+. Anal. Chem. 2014, 86, 10201–10207. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, J.F.; Huang, J.; He, D.G.; He, X.X.; Wang, K.M.; Ye, R.Z.; Yang, X.; Qing, T.P.; Tang, J.L. Highly Fe3+-selective fluorescent nanoprobe based on ultrabright N/P codoped carbon dots and its application in biological samples. Anal. Chem. 2017, 89, 7477–7484. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.Y.; Zhao, X.H.; Zhu, S.J.; Song, Y.B.; Yang, B. Novel cookie-with-chocolate carbon dotsdisplaying extremely acidophilic high luminescence. Nanoscale 2014, 6, 13939–13944. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.F.; Wang, L.P.; Xu, H.M.; Zhao, M.L. A fluorescein-based fluorogenic and chromogenic chemodosimeter for the sensitive detection of sulfide anion in aqueous solution. Anal. Chim. Acta 2009, 631, 91–95. [Google Scholar] [CrossRef] [PubMed]









© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, R.; Chen, Z.; Zhao, Z.; Sun, X.; Zhang, J.; Hou, L.; Yuan, C. Green and Facile Synthesis of Nitrogen and Phosphorus Co-Doped Carbon Quantum Dots towards Fluorescent Ink and Sensing Applications. Nanomaterials 2018, 8, 386. https://0-doi-org.brum.beds.ac.uk/10.3390/nano8060386
Bao R, Chen Z, Zhao Z, Sun X, Zhang J, Hou L, Yuan C. Green and Facile Synthesis of Nitrogen and Phosphorus Co-Doped Carbon Quantum Dots towards Fluorescent Ink and Sensing Applications. Nanomaterials. 2018; 8(6):386. https://0-doi-org.brum.beds.ac.uk/10.3390/nano8060386
Chicago/Turabian StyleBao, Ruiqi, Zhiyi Chen, Zhiwei Zhao, Xuan Sun, Jinyang Zhang, Linrui Hou, and Changzhou Yuan. 2018. "Green and Facile Synthesis of Nitrogen and Phosphorus Co-Doped Carbon Quantum Dots towards Fluorescent Ink and Sensing Applications" Nanomaterials 8, no. 6: 386. https://0-doi-org.brum.beds.ac.uk/10.3390/nano8060386