Chitosan-Based Carbon Quantum Dots for Biomedical Applications: Synthesis and Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
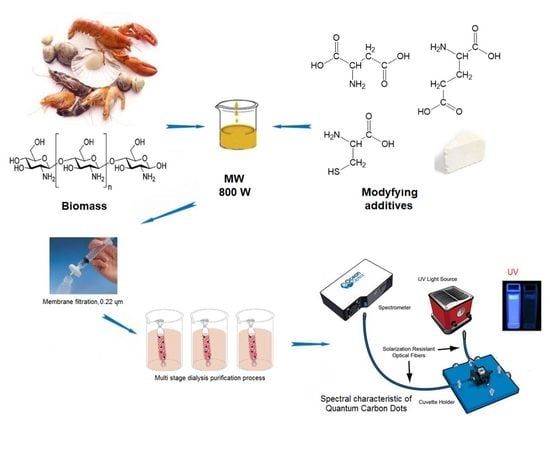
2.2.1. Chitosan-Based Carbon Quantum Dots Synthesis and Functionalization
2.2.2. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
2.2.3. Dynamic Light Scattering (DLS) Analysis
2.2.4. Scanning Electron Microscopy (SEM) Analysis
2.2.5. Chitosan-Based Carbon Quantum Dot Spectroscopic Characterization
2.2.6. Chitosan-Based Caron Quantum Dot Spectrofluorimetric Characterization
2.2.7. Quantum Yield Determination of Chitosan-Based Carbon Quantum Dots
2.2.8. Cytotoxicity of the Prepared Chitosan-Based CQDs
3. Results and Discussion
3.1. FT-IR Analysis of Chitosan-Based CQDs
3.2. Spectroscopic Studies on Chitosan-Based CQDs
3.3. Fluorescence Studies on Chitosan-Based CQDs
3.4. Quantum Yield, Size and Morphology of Chitosan-Based CQDs
3.5. Cytotoxicity Study
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liang, Z.; Kang, M.; Payne, G.F.; Wang, X.; Sun, R. Probing Energy and Electron Transfer Mechanisms in Fluorescence Quenching of Biomass Carbon Quantum Dots. ACS Appl. Mater. Interfaces 2016, 8, 17478–17488. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Chen, P.-C.; Periasamy, A.P.; Chen, Y.-N.; Chang, H.-T. Photoluminescent carbon nanodots: Synthesis, physicochemical properties and analytical applications. Elsevier 2015, 18, 447–458. [Google Scholar] [CrossRef]
- Panda, S.; Jadav, A.; Panda, N.; Mohapatra, S. A novel carbon quantum dot-based fluorescent nanosensor for selective detection of flumioxazin in real samples. N. J. Chem. 2018, 42, 2074–2080. [Google Scholar] [CrossRef]
- Gao, X.; Du, C.; Zhuang, Z.; Chen, W. Carbon quantum dot-based nanoprobes for metal ion detection. J. Mater. Chem. C 2016, 4, 6927–6945. [Google Scholar] [CrossRef]
- Cayuela, A.; Soriano, M.L.; Carrillo-Carrion, C.; Valcarcel, M. Semiconductor and carbon-based fluorescent nanodots: The need for consistency. Chem. Commun. 2015, 52, 1311–1326. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lu, K.-Q.; Tang, Z.-R.; Xu, Y.-J. Recent progress in carbon quantum dots: Synthesis, properties and applications in photocatalysis. J. Mater. Chem. A 2017, 5, 3717–3734. [Google Scholar] [CrossRef]
- Yu, T.; Wang, H.; Guo, C.; Zhai, Y.; Yang, J.; Yuan, J. A rapid microwave synthesis of green-emissive carbon dots with solid-state fluorescence and pH-sensitive properties. R. Soc. Open Sci. 2018, 5, 180245. [Google Scholar] [CrossRef]
- Prasannan, A.; Imae, T. One-Pot Synthesis of Fluorescent Carbon Dots from Orange Waste Peels. Ind. Eng. Chem. Res. 2013, 52, 15673–15678. [Google Scholar] [CrossRef]
- Zhang, M.; Fang, Z.; Zhao, X.; Niu, Y.; Lou, J.; Zhao, L.; Wu, Y.; Zou, S.; Du, F.; Shao, Q. Hyaluronic acid functionalized Nitrogen-doped carbon quantum dots for targeted specific bioimaging. RSC Adv. 2016, 6, 104979–104984. [Google Scholar] [CrossRef]
- Luo, P.G.; Yang, F.; Yang, S.T.; Sonkar, S.K.; Yang, L.; Broglie, J.J.; Liu, Y.; Sun, Y.P. Carbon-based quantum dots for fluorescence imaging of cells and tissues. RSC Adv. 2014, 4, 10791–10807. [Google Scholar] [CrossRef]
- Tang, C.; Zhou, J.; Qian, Z.; Ma, Y.; Huang, Y.; Feng, H. A universal fluorometric assay strategy for glycosidases based on functional carbon quantum dots: β-galactosidase activity detection in vitro and in living cells. J. Mater. Chem. B 2017, 5, 1971–1979. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef]
- Choi, Y.; Thongsai, N.; Chae, A.; Job, S.; Kang, E.B.; Paoprasert, P.; Park, S.Y.; In, I. Microwave-assisted synthesis of luminescent and biocompatible lysine-based carbon quantum dots. J. Ind. Eng. Chem. 2017, 47, 329–335. [Google Scholar] [CrossRef]
- Das, R.K.; Mohapatra, S. Highly luminescent, heteroatom-doped carbon quantum dots for ultrasensitive sensing of glucosamine and targeted imaging of liver cancer cells. J. Mater. Chem. B 2017, 5, 2190–2197. [Google Scholar] [CrossRef]
- Adams, L.A.; Fagbenro-Owoseni, K.A. Tunable carbon quantum dots from starch via microwave assisted Carbonization. Int. J. Nanoelectronics Mater. 2017, 10, 11–20. [Google Scholar]
- Rodríguez-Padrón, D.; Algarra, M.; Tarelho, L.A.C.; Frade, J.; Franco, A.; de Miguel, G.; Jiménez, J.; Rodríguez-Castellón, E.; Luque, R. Catalyzed Microwave-Assisted Preparation of Carbon Quantum Dots from Lignocellulosic Residues. ACS Sustain. Chem. Eng. 2018, 6, 7200–7205. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef]
- Chae, A.; Choi, Y.; Jo, S.; Paoprasert, P.; Park, S.Y.; In, I. Microwave-assisted synthesis of fluorescent carbon quantum dots from an A2/B3 monomer set. RSC Adv. 2017, 7, 12663–12669. [Google Scholar] [CrossRef]
- Pires, N.R.; Santos, C.M.W.; Sousa, R.R.; de Paula, R.C.M.; Cunha, P.L.R.; Feitosa, J.P.A. Novel and Fast Microwave-Assisted Synthesis of Carbon Quantum Dots from Raw Cashew Gum. J. Braz. Chem. Soc. 2015, 26, 1274–1282. [Google Scholar] [CrossRef]
- Zuo, J.; Jiang, T.; Zhao, X.; Xiong, X.; Xiao, S.; Zhu, Z. Preparation and Application of Fluorescent Carbon Dots. J. Nanomaterials 2015, 2015, 1–13. [Google Scholar] [CrossRef]
- Chandra, S.; Pathan, S.H.; Mitra, S.; Modha, B.H.; Goswami, A.; Pramanik, P. Tuning of photoluminescence on different surface functionalized carbon quantum dots. RSC Adv. 2012, 2, 3602–3606. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, M.; Huang, H.; Liu, Y.; Kang, Z. Advances, challenges and promises of carbon dots. Inorg. Chem. Front. 2017, 4, 1963–1986. [Google Scholar] [CrossRef]
- Liu, X.; Pang, J.; Xu, F.; Zhan, X. Simple Approach to Synthesize Amino-Functionalized Carbon Dots by Carbonization of Chitosan. Sci. Rep. 2016, 6, 31100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Zhao, L.; Wang, Y.; Xue, Y.; Deng, Y.; Zhao, X.; Li, Q. Carbon Quantum Dots Prepared with Chitosan for Synthesis of CQDs/AuNPs for Iodine Ions Detection. Nanomaterials 2018, 8, 1043. [Google Scholar] [CrossRef]
- Chowdhury, D.; Gogoi, N.; Majumdar, G. Fluorescent carbon dots obtained from chitosan gel. RSC Adv. 2012, 2, 12156–12159. [Google Scholar] [CrossRef]
- Iannazzo, D.; Ziccarelli, I.; Pistone, A. Graphene quantum dots: Multifunctional nanoplatforms for anticancer therapy. J. Mater. Chem. B 2017, 5, 6471–6489. [Google Scholar] [CrossRef]
- Namdari, P.; Negahdari, B.; Eatemadi, A. Synthesis, properties and biomedical applications of carbon-based quantum dots: An updated review. Biomed. Pharmacother. 2017, 87, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Atabaev, T. Doped carbon dots for sensing and bioimaging applications: A minireview. Nanomaterials 2018, 8, 342. [Google Scholar] [CrossRef]








| Sample | Time, min | Functionalizer, g |
|---|---|---|
| CQDs-1 | 5 | Lysine, 0.05 |
| CQDs-2 | 4 | Lysine, 0.05 |
| CQDs-3 | 3 | Lysine, 0.05 |
| CQDs-4 | 5 | Glutamic acid, 0.05 |
| CQDs-5 | 4 | Glutamic acid, 0.05 |
| CQDs-6 | 3 | Glutamic acid, 0.05 |
| CQDs-7 | 5 | Cysteine, 0.05 |
| CQDs-8 | 4 | Cysteine, 0.05 |
| CQDs-9 | 3 | Cysteine, 0.05 |
| CQDs-10 | 5 | Casein hydrolysate, 0.05 |
| CQDs-11 | 4 | Casein hydrolysate, 0.05 |
| CQDs-12 | 3 | Casein hydrolysate, 0.05 |
| Sample | Fluorescence Quantum Yield, % | Particle Diameter, nm |
|---|---|---|
| CQDs-1 | 3.6 | 5.0 |
| CQDs-2 | 3.8 | 5.5 |
| CQDs-3 | 11.5 | 5.5 |
| CQDs-4 | 7.4 | 5.5 |
| CQDs-5 | 6.5 | 6.5 |
| CQDs-6 | 4.4 | 8.0 |
| CQDs-7 | 4.3 | 8.5 |
| CQDs-8 | 4.7 | 11.5 |
| CQDs-9 | 2.5 | 12.0 |
| CQDs-10 | 6.6 | 11.0 |
| CQDs-11 | 2.3 | 12.5 |
| CQDs-12 | 4.6 | 14.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janus, Ł.; Piątkowski, M.; Radwan-Pragłowska, J.; Bogdał, D.; Matysek, D. Chitosan-Based Carbon Quantum Dots for Biomedical Applications: Synthesis and Characterization. Nanomaterials 2019, 9, 274. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9020274
Janus Ł, Piątkowski M, Radwan-Pragłowska J, Bogdał D, Matysek D. Chitosan-Based Carbon Quantum Dots for Biomedical Applications: Synthesis and Characterization. Nanomaterials. 2019; 9(2):274. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9020274
Chicago/Turabian StyleJanus, Łukasz, Marek Piątkowski, Julia Radwan-Pragłowska, Dariusz Bogdał, and Dalibor Matysek. 2019. "Chitosan-Based Carbon Quantum Dots for Biomedical Applications: Synthesis and Characterization" Nanomaterials 9, no. 2: 274. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9020274