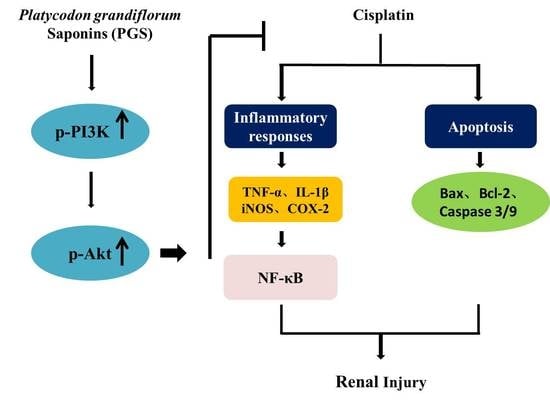
Platycodon grandiflorum Saponins Ameliorate Cisplatin-Induced Acute Nephrotoxicity through the NF-κB-Mediated Inflammation and PI3K/Akt/Apoptosis Signaling Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Compounds and Reagents
2.2. Experimental Design
- Control group: Control mice were orally administered physiological saline daily for 10 days, with no drug treatment.
- Cisplatin group: Mice were orally administered physiological saline daily for 10 days and received (25 mg/kg, i.p.) one hour after oral gavage on the seventh day.
- PGS (15 mg/kg) + cisplatin group: Mice were orally administered 15 mg/kg PGS, which was dissolved in physiological saline, daily for 10 days and they received cisplatin (25 mg/kg, i.p.) one hour after PGS administration on the seventh day.
- PGS (30 mg/kg) + cisplatin group: Mice were orally administered 30 mg/kg PGS, which was dissolved in physiological saline, daily for 10 days and they received cisplatin (25 mg/kg, i.p.) one hour after PGS administration on the seventh day.
2.3. Renal Function Tests
2.4. Histopathology Analysis
2.5. Immunohistochemical Staining
2.6. Immunofluorescence Staining
2.7. Western Blotting Analysis
2.8. TUNEL Staining Analysis
2.9. Statistical Analysis
3. Results
3.1. PGS-Attenuated Cisplatin-Induced Renal Dysfunction and Renal Histopathological Changes in Mice
3.2. PGS Alleviated Cisplatin-Induced Apoptosis
3.3. PGS Attenuated Cisplatin-Induced Renal Inflammation
3.4. PGS Regulated the NF-κB Signaling Pathway
3.5. PGS Regulated the PI3K/Akt Signaling Pathway
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, L.; Wei, X.H.; Pan, C.S.; Yan, L.; Gu, Y.Y.; Sun, K.; Liu, Y.Y.; Wang, C.S.; Fan, J.Y.; Han, J.Y. Qishenyiqi pills, a compound chinese medicine, prevented cisplatin induced acute kidney injury via regulating mitochondrial function. Front. Physiol. 2017, 8, 1090. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Lee, A.Y.; Kim, J.H.; Seong, S.H.; Jang, G.Y.; Cho, E.J.; Choi, J.S.; Kwon, J.; Kim, Y.O.; Lee, S.W.; et al. Protective effect of safflower seed on cisplatin-induced renal damage in mice via oxidative stress and apoptosis-mediated pathways. Am. J. Chin. Med. 2018, 46, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chirino, Y.I.; Pedraza-Chaverri, J. Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Exp. Toxicol. Pathol. 2009, 61, 223–242. [Google Scholar] [CrossRef] [PubMed]
- Malyszko, J.; Kozlowska, K.; Kozlowski, L. Nephrotoxicity of anticancer treatment. Nephrol. Dial. Transplant. 2017, 32, 924–936. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Panichpisal, K.; Kurtzman, N.; Nugent, K. Cisplatin nephrotoxicity: A review. Am. J. Med. Sci. 2007, 334, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Dang, C.; Kang, H.; Dai, Z.; Lin, S.; Guan, H.; Liu, X.; Wang, X.; Hui, W. Saikosaponin-d reduces cisplatin-induced nephrotoxicity by repressing ros-mediated activation of mapk and nf-kappab signalling pathways. Int. Immunopharmacol. 2015, 28, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Ajith, T.A.; Nivitha, V.; Usha, S. Zingiber officinale roscoe alone and in combination with alpha-tocopherol protect the kidney against cisplatin-induced acute renal failure. Food Chem. Toxicol. 2007, 45, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Ma, D.; Maze, M.; Franks, N.P. Effects of xenon on in vitro and in vivo models of neuronal injury. Anesthesiology 2002, 96, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Martelli, A.M.; Faenza, I.; Billi, A.M.; Manzoli, L.; Evangelisti, C.; Fala, F.; Cocco, L. Intranuclear 3’-phosphoinositide metabolism and akt signaling: New mechanisms for tumorigenesis and protection against apoptosis? Cell Signal 2006, 18, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Vallee, A.; Lecarpentier, Y.; Guillevin, R.; Vallee, J.N. Opposite interplay between the canonical wnt/beta-catenin pathway and ppar gamma: A potential therapeutic target in gliomas. Neurosci. Bull. 2018, 34, 573–588. [Google Scholar] [CrossRef] [PubMed]
- Kuwana, H.; Terada, Y.; Kobayashi, T.; Okado, T.; Penninger, J.M.; Irie-Sasaki, J.; Sasaki, T.; Sasaki, S. The phosphoinositide-3 kinase gamma-akt pathway mediates renal tubular injury in cisplatin nephrotoxicity. Kidney Int. 2008, 73, 430–445. [Google Scholar] [CrossRef] [PubMed]
- Cronin, J.G.; Turner, M.L.; Goetze, L.; Bryant, C.E.; Sheldon, I.M. Toll-like receptor 4 and myd88-dependent signaling mechanisms of the innate immune system are essential for the response to lipopolysaccharide by epithelial and stromal cells of the bovine endometrium. Biol. Reprod. 2012, 86, 51. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Yang, X.D.; Lamb, A.; Chen, L.F. Posttranslational modifications of nf-kappab: Another layer of regulation for nf-kappab signaling pathway. Cell Signal 2010, 22, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Sahin, K.; Tuzcu, M.; Gencoglu, H.; Dogukan, A.; Timurkan, M.; Sahin, N.; Aslan, A.; Kucuk, O. Epigallocatechin-3-gallate activates nrf2/ho-1 signaling pathway in cisplatin-induced nephrotoxicity in rats. Life Sci. 2010, 87, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.N.; Li, Y.Z.; Li, W.; Yan, X.T.; Yang, G.; Zhang, J.; Zhao, L.C.; Yang, L.M. Nephroprotective effects of saponins from leaves of panax quinquefolius against cisplatin-induced acute kidney injury. Int. J. Mol. Sci. 2017, 18, E1407. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, N.; Wang, Z.; Qi, Z.; Zheng, B.; Li, P.; Liu, J. Rapid characterization of chemical constituents of Platycodon grandiflorum and its adulterant adenophora stricta by uplc-qtof-ms/ms. J. Mass Spectrom. 2017, 52, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; You, H.J.; Park, S.J.; Kim, Y.S.; Chung, Y.C.; Jeong, T.C.; Jeong, H.G. Hepatoprotective effects of Platycodon grandiflorum on acetaminophen-induced liver damage in mice. Cancer Lett. 2001, 174, 73–81. [Google Scholar] [CrossRef]
- Ryu, C.S.; Kim, C.H.; Lee, S.Y.; Lee, K.S.; Choung, K.J.; Song, G.Y.; Kim, B.H.; Ryu, S.Y.; Lee, H.S.; Kim, S.K. Evaluation of the total oxidant scavenging capacity of saponins isolated from Platycodon grandiflorum. Food Chem. 2012, 132, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Wei, Z.; Wang, J.; Zhang, Y.; Shi, M.; Yang, Z.; Fu, Y. Platycodin d suppressed lps-induced inflammatory response by activating lxralpha in lps-stimulated primary bovine mammary epithelial cells. Eur. J. Pharmacol. 2017, 814, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Lin, C.H.; Yao, H.T.; Kuo, W.W.; Shen, C.Y.; Yeh, Y.L.; Ho, T.J.; Padma, V.V.; Huang, C.Y. Platycodon grandiflorum (pg) reverses angiotensin ii-induced apoptosis by repressing igf-iir expression. J. Ethnopharmacol. 2017, 205, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Y.; Wang, Z.; Han, Y.; Tian, Y.H.; Zhang, G.S.; Sun, Y.S.; Wang, Y.P. Platycodin d isolated from the aerial parts of Platycodon grandiflorum protects alcohol-induced liver injury in mice. Food Funct. 2015, 6, 1418–1427. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, Y.-S.; Wang, Z.; Zheng, Y.-N. Isolation and purification of saponins from Platycodon grandiflorum by semi-preparative high performance liquid chromatography and lc/esi-ms. J. Liquid Chromatogr. Relat. Technol. 2012, 35, 547–557. [Google Scholar] [CrossRef]
- Zheng, J.; He, J.; Ji, B.; Li, Y.; Zhang, X. Antihyperglycemic effects of Platycodon grandiflorum (jacq.) a. Dc. Extract on streptozotocin-induced diabetic mice. Plant Foods Hum. Nutr. 2007, 62, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.L.; Wang, Z.; Li, W.; Hou, J.G.; Liu, Y.; Li, X.D.; Li, H.P.; Wang, Y.P. Nephroprotective effects of anthocyanin from the fruits of panax ginseng (gfa) on cisplatin-induced acute kidney injury in mice. Phytother. Res. 2017, 31, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.N.; Liu, Z.; Wang, Z.; Ren, S.; Tang, S.; Wang, Y.P.; Xiao, S.Y.; Chen, C.; Li, W. Supplementation of american ginseng berry extract mitigated cisplatin-evoked nephrotoxicity by suppressing ros-mediated activation of mapk and nf-kappab signaling pathways. Food Chem. Toxicol. 2017, 110, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yan, M.H.; Liu, Y.; Liu, Z.; Wang, Z.; Chen, C.; Zhang, J.; Sun, Y.S. Ginsenoside rg5 ameliorates cisplatin-induced nephrotoxicity in mice through inhibition of inflammation, oxidative stress, and apoptosis. Nutrients 2016, 8, E566. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hu, S.; He, Y.; Zhang, J.; Zeng, X.; Gong, F.; Liang, L. The protective effects of zhen-wu-tang against cisplatin-induced acute kidney injury in rats. PLoS ONE 2017, 12, e0179137. [Google Scholar] [CrossRef] [PubMed]
- Potocnjak, I.; Broznic, D.; Kindl, M.; Kropek, M.; Vladimir-Knezevic, S.; Domitrovic, R. Stevia and stevioside protect against cisplatin nephrotoxicity through inhibition of erk1/2, stat3, and nf-kappab activation. Food Chem. Toxicol. 2017, 107, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Chen, Y.; Dubrulle, J.; Stossi, F.; Putluri, V.; Sreekumar, A.; Putluri, N.; Baluya, D.; Lai, S.Y.; Sandulache, V.C. Cisplatin generates oxidative stress which is accompanied by rapid shifts in central carbon metabolism. Sci. Rep. 2018, 8, 4306. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Z.; Jin, H.H.; Sun, H.X.; Zhang, Z.Z.; Zheng, J.X.; Li, S.H.; Han, S.H. Eriodictyol attenuates cisplatin-induced kidney injury by inhibiting oxidative stress and inflammation. Eur. J. Pharmacol. 2016, 772, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.M.; Sufka, K.J.; Gul, W.; ElSohly, M.A. Effects of delta-9-tetrahydrocannabinol and cannabidiol on cisplatin-induced neuropathy in mice. Planta Med. 2016, 82, 1169–1172. [Google Scholar] [CrossRef] [PubMed]
- Han, M.S.; Han, I.H.; Lee, D.; An, J.M.; Kim, S.N.; Shin, M.S.; Yamabe, N.; Hwang, G.S.; Yoo, H.H.; Choi, S.J.; et al. Beneficial effects of fermented black ginseng and its ginsenoside 20(s)-rg3 against cisplatin-induced nephrotoxicity in llc-pk1 cells. J. Ginseng Res. 2016, 40, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Nie, Y.; Hou, Y.; Ma, X.; Ding, G.; Gao, J.; Jiang, M.; Bai, G. Chemomics-integrated proteomics analysis of jie-geng-tang to ameliorate lipopolysaccharide-induced acute lung injury in mice. Evid. Based Complement. Alternat. Med. 2016, 2016, 7379146. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Jin, S.W.; Han, E.H.; Park, B.H.; Kim, H.G.; Khanal, T.; Hwang, Y.P.; Do, M.T.; Lee, H.S.; Chung, Y.C.; et al. Platycodon grandiflorum root-derived saponins attenuate atopic dermatitis-like skin lesions via suppression of nf-kappab and stat1 and activation of nrf2/are-mediated heme oxygenase-1. Phytomedicine 2014, 21, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Ha, I.J.; Ha, Y.W.; Kang, M.; Lee, J.; Park, D.; Kim, Y.S. Enzymatic transformation of platycosides and one-step separation of platycodin d by high-speed countercurrent chromatography. J. Sep. Sci. 2010, 33, 1916–1922. [Google Scholar] [CrossRef] [PubMed]
- Kaygusuzoglu, E.; Caglayan, C.; Kandemir, F.M.; Yildirim, S.; Kucukler, S.; Kilinc, M.A.; Saglam, Y.S. Zingerone ameliorates cisplatin-induced ovarian and uterine toxicity via suppression of sex hormone imbalances, oxidative stress, inflammation and apoptosis in female wistar rats. Biomed. Pharmacother. 2018, 102, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, G.; Fredriksson, L.; Herpers, B.; Meerman, J.; van de Water, B.; de Graauw, M. Tnf-alpha-mediated nf-kappab survival signaling impairment by cisplatin enhances jnk activation allowing synergistic apoptosis of renal proximal tubular cells. Biochem. Pharmacol. 2013, 85, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Jin, S.W.; Choi, C.Y.; Kim, H.G.; Kim, S.J.; Lee, H.S.; Chung, Y.C.; Kim, E.J.; Lee, Y.C.; Jeong, H.G. Saponins from the roots of Platycodon grandiflorum ameliorate high fat diet-induced non-alcoholic steatohepatitis. Biomed. Pharmacother. 2017, 86, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Shared principles in nf-kappab signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ghosh, S. Toll-like receptor-mediated nf-kappab activation: A phylogenetically conserved paradigm in innate immunity. J. Clin Investig. 2001, 107, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Shao, L.; Fu, Y.M.; Zou, Y. Effects of olmesartan on endothelial progenitor cell mobilization and function in carotid atherosclerosis. Med. Sci. Monit. 2015, 21, 1189–1193. [Google Scholar] [PubMed]
- Murthy, D.; Attri, K.S.; Singh, P.K. Phosphoinositide 3-kinase signaling pathway in pancreatic ductal adenocarcinoma progression, pathogenesis, and therapeutics. Front. Physiol. 2018, 9, 335. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.F.; Chen, X.F.; Zhang, J.; Di, D.L. Activity-screening-guided isolation and purification for vasodilative effects compounds from radix astragali by high-speed counter-current chromatography using gradient elution. Nat. Prod. Res. 2013, 27, 1020–1022. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Pan, X.; Fu, H.; Zheng, Y.; Dai, Y.; Yin, Y.; Chen, Q.; Hao, Q.; Bao, D.; Hou, D. Effect of curcumin on glycerol-induced acute kidney injury in rats. Sci. Rep. 2017, 7, 10114. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.J.; Cai, G.Y.; Ning, Y.C.; Liu, L.; Yang, J.R.; Dong, D.; Fu, B.; Lu, Y.; Cui, S.Y.; Chen, X.M. Dap5 ameliorates cisplatin-induced apoptosis of renal tubular cells. Am. J. Nephrol. 2012, 35, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Mukherjee, S.; Ray, D.; Raha, S. Involvement of the akt/pkb signaling pathway with disease processes. Mol. Cell Biochem. 2003, 253, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Gu, Q.; Xiang, L.; Dong, X.; Li, H.; Ni, J.; Wan, L.; Cai, G.; Chen, G. Curcumin inhibits apoptosis by modulating bax/bcl-2 expression and alleviates oxidative stress in testes of streptozotocin-induced diabetic rats. Ther. Clin. Risk Manag. 2017, 13, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Stepien, A.; Izdebska, M.; Grzanka, A. [the types of cell death]. Postepy Hig. Med. Dosw. 2007, 61, 420–428. [Google Scholar]
- Shang, Y.; Myers, M.; Brown, M. Formation of the androgen receptor transcription complex. Mol. Cell 2002, 9, 601–610. [Google Scholar] [CrossRef]
- Choudhary, G.S.; Al-Harbi, S.; Almasan, A. Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis. Methods Mol. Biol. 2015, 1219, 1–9. [Google Scholar] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Hou, J.; Yan, X.; Leng, J.; Li, R.; Zhang, J.; Xing, J.; Chen, C.; Wang, Z.; Li, W. Platycodon grandiflorum Saponins Ameliorate Cisplatin-Induced Acute Nephrotoxicity through the NF-κB-Mediated Inflammation and PI3K/Akt/Apoptosis Signaling Pathways. Nutrients 2018, 10, 1328. https://0-doi-org.brum.beds.ac.uk/10.3390/nu10091328
Zhang W, Hou J, Yan X, Leng J, Li R, Zhang J, Xing J, Chen C, Wang Z, Li W. Platycodon grandiflorum Saponins Ameliorate Cisplatin-Induced Acute Nephrotoxicity through the NF-κB-Mediated Inflammation and PI3K/Akt/Apoptosis Signaling Pathways. Nutrients. 2018; 10(9):1328. https://0-doi-org.brum.beds.ac.uk/10.3390/nu10091328
Chicago/Turabian StyleZhang, Weizhe, Jingang Hou, Xiaotong Yan, Jing Leng, Rongyan Li, Jing Zhang, Jingjing Xing, Chen Chen, Zi Wang, and Wei Li. 2018. "Platycodon grandiflorum Saponins Ameliorate Cisplatin-Induced Acute Nephrotoxicity through the NF-κB-Mediated Inflammation and PI3K/Akt/Apoptosis Signaling Pathways" Nutrients 10, no. 9: 1328. https://0-doi-org.brum.beds.ac.uk/10.3390/nu10091328