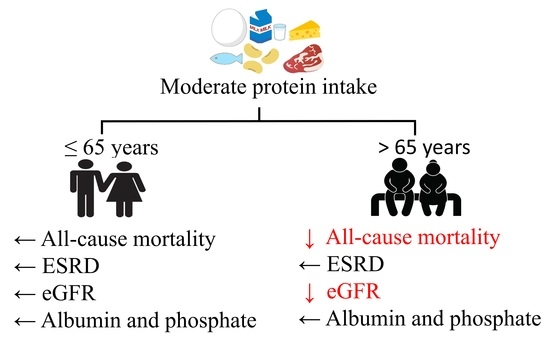
Age Modifies the Association of Dietary Protein Intake with All-Cause Mortality in Patients with Chronic Kidney Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Baseline Patient Characteristics and Comorbidities
2.3. Endpoints
2.4. Dietary Protein Intake
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Cohort
3.2. Protein Intake and Endpoints
3.3. Protein Intake and Secondary Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Hare, A.M.; Bertenthal, D.; Covinsky, K.E.; Landefeld, C.S.; Sen, S.; Mehta, K.; Steinman, M.A.; Borzecki, A.; Walter, L.C. Mortality risk stratification in chronic kidney disease: One size for all ages? J. Am. Soc. Nephrol. 2006, 17, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.Y.; Chiu, Y.W.; Lee, C.H.; Yu, H.Y.; Chen, H.C.; Wu, M.T.; Hwang, S.J. Factors associated with CKD in the elderly and nonelderly population. Clin. J. Am. Soc. Nephrol. 2013, 8, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.; Halter, J.B.; Hazzard, W.R.; Himmelfarb, J.; Horne, F.M.; Kaysen, G.A.; Kusek, J.W.; Nayfield, S.G.; Schmader, K.; Tian, Y.; et al. Prediction, progression, and outcomes of chronic kidney disease in older adults. J. Am. Soc. Nephrol. 2009, 20, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, A.M.; Choi, A.I.; Bertenthal, D.; Bacchetti, P.; Garg, A.X.; Kaufman, J.S.; Walter, L.C.; Mehta, K.M.; Steinman, M.A.; Allon, M.; et al. Age affects outcomes in chronic kidney disease. J. Am. Soc. Nephrol. 2007, 18, 2758–2765. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P.; Kopple, J.D.; Kalantar-Zadeh, K. Management of protein-energy wasting in non-dialysis-dependent chronic kidney disease: Reconciling low protein intake with nutritional therapy. Am. J. Clin. Nutr. 2013, 97, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Fouque, D.; Pelletier, S.; Mafra, D.; Chauveau, P. Nutrition and chronic kidney disease. Kidney Int. 2011, 80, 348–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikizler, T.A.; Cano, N.J.; Franch, H.; Fouque, D.; Himmelfarb, J.; Kalantar-Zadeh, K.; Kuhlmann, M.K.; Stenvinkel, P.; TerWee, P.; Teta, D.; et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: A consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013, 84, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Astor, B.C.; Fox, C.H.; Isakova, T.; Lash, J.P.; Peralta, C.A.; Kurella Tamura, M.; Feldman, H.I. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am. J. Kidney Dis. 2014, 63, 713–735. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Fouque, D. Nutritional management of chronic kidney disease. N. Engl. J. Med. 2017, 377, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- Iseki, K.; Yamagata, K. A practical approach of salt and protein restriction for CKD patients in Japan. BMC Nephrol. 2016, 17, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giovannetti, S.; Maggiore, Q. A low-nitrogen diet with proteins of high biological value for severe chronic uraemia. Lancet 1964, 1, 1000–1003. [Google Scholar] [CrossRef]
- Fouque, D.; Aparicio, M. Eleven reasons to control the protein intake of patients with chronic kidney disease. Nat. Clin. Pract. Nephrol. 2007, 3, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Fouque, D.; Laville, M. Low protein diets for chronic kidney disease in non diabetic adults. Cochrane Database Syst. Rev. 2009. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Greene, T.; Sarnak, M.J.; Wang, X.; Beck, G.J.; Kusek, J.W.; Collins, A.J.; Kopple, J.D. Effect of dietary protein restriction on the progression of kidney disease: Long-term follow-up of the Modification of Diet in Renal Disease (MDRD) Study. Am. J. Kidney Dis. 2006, 48, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Cianciaruso, B.; Pota, A.; Bellizzi, V.; Di Giuseppe, D.; Di Micco, L.; Minutolo, R.; Pisani, A.; Sabbatini, M.; Ravani, P. Effect of a low-versus moderate-protein diet on progression of CKD: Follow-up of a randomized controlled trial. Am. J. Kidney Dis. 2009, 54, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Halbesma, N.; Bakker, S.J.; Jansen, D.F.; Stolk, R.P.; De Zeeuw, D.; De Jong, P.E.; Gansevoort, R.T.; Group, P.S. High protein intake associates with cardiovascular events but not with loss of renal function. J. Am. Soc. Nephrol. 2009, 20, 1797–1804. [Google Scholar] [CrossRef] [PubMed]
- Rand, W.M.; Pellett, P.L.; Young, V.R. Meta-analysis of nitrogen balance studies for estimating protein requirements in healthy adults. Am. J. Clin. Nutr. 2003, 77, 109–127. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.W.; Crim, M.C.; Dallal, G.E.; Young, V.R.; Evans, W.J. Increased protein requirements in elderly people: New data and retrospective reassessments. Am. J. Clin. Nutr. 1994, 60, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med. 2007, 4, e296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.M.; Gallagher, D.; Nelson, M.E.; Matthews, D.E.; Heymsfield, S.B. Total-body skeletal muscle mass: Evaluation of 24-h urinary creatinine excretion by computerized axial tomography. Am. J. Clin. Nutr. 1996, 63, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maroni, B.J.; Steinman, T.I.; Mitch, W.E. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int. 1985, 27, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Masud, T.; Manatunga, A.; Cotsonis, G.; Mitch, W.E. The precision of estimating protein intake of patients with chronic renal failure. Kidney Int. 2002, 62, 1750–1756. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, A.; Asakura, K.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Todoriki, H.; Miura, A.; Fukui, M.; et al. Within- and between-individual variation in energy and nutrient intake in Japanese adults: Effect of age and sex differences on group size and number of records required for adequate dietary assessment. J. Epidemiol. 2013, 23, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.Y.; Roy, J.A.; Xie, D.; Yang, W.; Shou, H.; Anderson, A.H.; Landis, J.R.; Jepson, C.; Wolf, M.; Isakova, T.; et al. Statistical methods for cohort studies of CKD: Survival analysis in the setting of competing risks. Clin. J. Am. Soc. Nephrol. 2017, 12, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.E.; Suarez, J.A.; Brandhorst, S.; Balasubramanian, P.; Cheng, C.W.; Madia, F.; Fontana, L.; Mirisola, M.G.; Guevara-Aguirre, J.; Wan, J.; et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell. Metab. 2014, 19, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Buuren, S.V.; Groothuis-Oudshoorn, K. Mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45. [Google Scholar] [CrossRef]
- Rhee, C.M.; Ahmadi, S.F.; Kovesdy, C.P.; Kalantar-Zadeh, K. Low-protein diet for conservative management of chronic kidney disease: A systematic review and meta-analysis of controlled trials. J. Cachexia Sarcopenia Muscle 2018, 9, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Shinaberger, C.S.; Kilpatrick, R.D.; Regidor, D.L.; McAllister, C.J.; Greenland, S.; Kopple, J.D.; Kalantar-Zadeh, K. Longitudinal associations between dietary protein intake and survival in hemodialysis patients. Am. J. Kidney Dis. 2006, 48, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Giordano, M.; Ciarambino, T.; Castellino, P.; Paolisso, G. Light and shadows of dietary protein restriction in elderly with chronic kidney disease. Nutrition 2013, 29, 1090–1093. [Google Scholar] [CrossRef] [PubMed]
- Isoyama, N.; Qureshi, A.R.; Avesani, C.M.; Lindholm, B.; Barany, P.; Heimburger, O.; Cederholm, T.; Stenvinkel, P.; Carrero, J.J. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 1720–1728. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P.; Carrero, J.J.; Von Walden, F.; Ikizler, T.A.; Nader, G.A. Muscle wasting in end-stage renal disease promulgates premature death: Established, emerging and potential novel treatment strategies. Nephrol. Dial. Transplant. 2016, 31, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Jesudason, D.R.; Pedersen, E.; Clifton, P.M. Weight-loss diets in people with type 2 diabetes and renal disease: A randomized controlled trial of the effect of different dietary protein amounts. Am. J. Clin. Nutr. 2013, 98, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Knight, E.L.; Stampfer, M.J.; Hankinson, S.E.; Spiegelman, D.; Curhan, G.C. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann. Intern. Med. 2003, 138, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Coresh, J.; Turin, T.C.; Matsushita, K.; Sang, Y.; Ballew, S.H.; Appel, L.J.; Arima, H.; Chadban, S.J.; Cirillo, M.; Djurdjev, O.; et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 2014, 311, 2518–2531. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Chen, J.; Sang, Y.; Ballew, S.H.; Shimazaki, R.; Fukagawa, M.; Imai, E.; Coresh, J.; Hishida, A. Risk of end-stage renal disease in Japanese patients with chronic kidney disease increases proportionately to decline in estimated glomerular filtration rate. Kidney Int. 2016, 90, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Iff, S.; Wong, G.; Webster, A.C.; Flood, V.; Wang, J.J.; Mitchell, P.; Craig, J.C. Relative energy balance, CKD, and risk of cardiovascular and all-cause mortality. Am. J. Kidney Dis. 2014, 63, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.L.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition-A consensus report from the global clinical nutrition community. Clin. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Sacks, G.; Chandramohan, D.; Chow, C.C.; Wang, Y.C.; Gortmaker, S.L.; Swinburn, B.A. Quantification of the effect of energy imbalance on bodyweight. Lancet 2011, 378, 826–837. [Google Scholar] [CrossRef] [Green Version]
- Lew, Q.J.; Jafar, T.H.; Koh, H.W.; Jin, A.; Chow, K.Y.; Yuan, J.M.; Koh, W.P. Red Meat Intake and Risk of ESRD. J. Am. Soc. Nephrol. 2017, 28, 304–312. [Google Scholar] [CrossRef] [PubMed]



| All | Protein Intake | p Value 3 | |||
|---|---|---|---|---|---|
| Very Low | Low | Moderate | |||
| (n = 352) | (n = 75) | (n = 161) | (n = 116) | ||
| Age (years) | 70.2 ± 11.4 | 70.6 ± 11.9 | 71.2 ± 11.0 | 68.6 ± 11.5 | 0.173 |
| ≤65 (n (%)) | 93 (26.4) | 16 (21.3) | 39 (24.2) | 38 (32.8) | 0.150 |
| >65 (n (%)) | 259 (73.6) | 59 (78.7) | 122 (75.8) | 78 (67.2) | |
| Male (n (%)) | 102 (29.0) | 24 (32.0) | 49 (30.4) | 29 (25.0) | 0.499 |
| Smoking (n (%)) | 58 (16.5) | 11 (14.7) | 26 (16.2) | 21 (18.1) | 0.614 |
| Body weight (kg) | 64.2 ± 13.6 | 60.6 ± 13.2 | 62.0 ± 12.3 | 69.6 ± 15.4 | <0.001 |
| BMI (kg/m2) 4 | 24.7 ± 4.4 | 23.1 ± 4.1 | 24.0 ± 3.5 | 26.9 ± 4.9 | <0.001 |
| Skeletal muscle mass (kg) 5,6 | 19.3 ± 6.5 | 14.7 ± 5.8 | 18.6 ± 5.1 | 23.2 ± 6.4 | <0.001 |
| CKD stage (n (%)) | |||||
| 3a | 20 (5.7) | 2 (2.7) | 6 (3.7) | 12 (10.3) | <0.001 |
| 3b | 72 (20.5) | 13 (17.3) | 29 (18.0) | 30 (25.9) | |
| 4 | 149 (42.3) | 23 (30.7) | 76 (47.2) | 50 (43.1) | |
| 5 | 111 (31.5) | 37 (49.3) | 50 (31.1) | 24 (20.7) | |
| eGFR (mL/min/1.73 m2) 7 | 22.9 ± 11.3 | 19.5 ± 11.0 | 21.8 ± 10.2 | 26.7 ± 12.0 | <0.001 |
| UPE (g/24 h) 6 | 0.81 (0.18 to 2.00) | 1.2 (0.26 to 2.61) | 0.73 (0.19 to 1.77) | 0.72 (0.16 to 1.83) | 0.089 |
| Serum phosphate (mg/dL) | 3.7 ± 0.8 | 3.9 ± 1.0 | 3.7 ± 0.7 | 3.7 ± 0.7 | 0.030 |
| Hypertension history (n (%)) | 315 (89.5) | 67 (89.3) | 145 (90.1) | 103 (88.8) | 0.943 |
| CVD history (n (%)) | 151 (42.9) | 34 (45.3) | 75 (46.6) | 42 (36.2) | 0.204 |
| Systolic blood pressure (mmHg) | 135 ± 21 | 137 ± 21 | 132 ± 21 | 137 ± 19 | 0.161 |
| LVEF (%) | 64.0 ± 10.6 | 63.8 ± 11.2 | 62.9 ± 10.9 | 65.6 ± 9.4 | 0.119 |
| DM history (n (%)) | 157 (44.6) | 38 (50.7) | 69 (42.9) | 50 (43.1) | 0.492 |
| Anemia (n (%)) | 177 (50.3) | 45 (60.0) | 85 (52.8) | 47 (40.5) | 0.021 |
| Serum albumin (g/dL) | 3.9 ± 0.4 | 3.7 ± 0.4 | 3.9 ± 0.5 | 4.0 ± 0.4 | <0.001 |
| CRP (mg/dL) | 0.09 (0.04 to 0.22) | 0.10 (0.03 to 0.35) | 0.07 (0.04 to 0.18) | 0.10 (0.04 to 0.20) | 0.330 |
| BNP (pg/mL) | 90 (39 to 221) | 132 (66 to 238) | 89 (42 to 224) | 62 (29 to 182) | 0.002 |
| Protein intake (g/day) 6,8 | 42.0 ± 10.7 | 30.5 ± 5.6 | 39.7 ± 5.9 | 52.7 ± 8.3 | <0.001 |
| Protein intake (g/kg IBW/day) 6,8 | 0.74 ± 0.08 | 0.52 ± 0.07 | 0.70 ± 0.06 | 0.93 ± 0.10 | <0.001 |
| Sodium intake (mmol/day) 6 | 62.9 ± 21.4 | 57.4 ± 21.3 | 61.7 ± 19.6 | 68.0 ± 22.7 | 0.002 |
| VLPI | LPI | MPI | 0.1 g/kg IBW/day Increment | p Value 2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| 0.29 to 0.59 (g/kg IBW/day) | 0.60 to 0.80 (g/kg IBW/day) | 0.81 to 1.35 (g/kg IBW/day) | |||||||
| Mean (SD) protein intake (g/kg IBW/day) | 0.52 (0.07) | 0.70 (0.06) | 0.93 (0.10) | ― | ― | ||||
| All-cause mortality | |||||||||
| No of deaths/patient years | 10/284.9 | 22/613.3 | 4/426.6 | ― | ― | ||||
| Rate/1000 patient years | 35.1 | (23.7 to 46.5) | 35.9 | (24.4 to 47.4) | 9.4 | (3.4 to 15.4) | ― | ― | |
| All | |||||||||
| Model 1 3 | 0.89 | (0.39 to 1.88) | 1.00 (ref) | 0.29 | (0.08 to 0.77) | 0.89 | (0.80 to 0.99) | <0.001 | |
| Model 2 4 | 1.42 | (0.55 to 3.44) | 1.00 (ref) | 0.29 | (0.07 to 0.94) | 0.76 | (0.60 to 0.95) | <0.001 | |
| Model 3 5 | 1.45 | (0.57 to 3.48) | 1.00 (ref) | 0.27 | (0.07 to 0.81) | 0.83 | (0.70 to 0.98) | <0.001 | |
| ≤65 years 6 | |||||||||
| Model 1 3 | 2.54 | (0.09 to 70.13) | 1.00 (ref) | 3.73 | (0.31 to 94.60) | 1.00 | (0.01 to 82.07) | 0.879 | |
| Model 2 4 | ― | ― | ― | ― | ― | ||||
| Model 3 5 | ― | ― | ― | ― | ― | ||||
| >65 years 6 | |||||||||
| Model 1 3 | 0.84 | (0.35 to 1.85) | 1.00 (ref) | 0.16 | (0.03 to 0.55) | 0.57 | (0.49 to 0.67) | <0.001 | |
| Model 2 4 | 1.52 | (0.51 to 4.27) | 1.00 (ref) | 0.14 | (0.02 to 0.69) | 0.65 | (0.47 to 0.89) | <0.001 | |
| Model 3 5 | 1.64 | (0.58 to 4.45) | 1.00 (ref) | 0.14 | (0.02 to 0.59) | 0.69 | (0.51 to 0.93) | <0.003 | |
| ESRD7 | |||||||||
| No. of events/patient years | 22/171.6 | 48/436.0 | 27/340.8 | ― | ― | ||||
| Rate/1000 patient years | 128 | (107 to 149) | 110 | (90 to 129) | 79 | (63 to 96) | ― | ― | |
| All | |||||||||
| Model 1 3 | 0.97 | (0.58 to 1.60) | 1.00 (ref) | 0.70 | (0.43 to 1.16) | 1.00 | (0.21 to 4.85) | 0.689 | |
| Model 2 4 | 0.87 | (0.49 to 1.56) | 1.00 (ref) | 0.72 | (0.40 to 1.29) | 1.00 | (0.54 to 1.88) | 0.631 | |
| Model 3 5 | 0.83 | (0.47 to 1.47) | 1.00 (ref) | 0.62 | (0.35 to 1.09) | 1.00 | (0.62 to 1.64) | 0.722 | |
| ≤65 years 6 | |||||||||
| Model 1 3 | 1.20 | (0.43 to 2.93) | 1.00 (ref) | 0.71 | (0.28 to 1.69) | 1.00 | (0.01 to 81.05) | 0.508 | |
| Model 2 4 | 8.31 | (0.78 to 88.42) | 1.00 (ref) | 0.92 | (0.14 to 6.07) | 1.01 | (0.21 to 4.83) | 0.039 | |
| Model 3 5 | 4.12 | (0.47 to 36.26) | 1.00 (ref) | 0.78 | (0.14 to 4.22) | 1.01 | (0.24 to 4.31) | 0.054 | |
| >65 years 6 | |||||||||
| Model 1 3 | 0.80 | (0.42 to 1.47) | 1.00 (ref) | 0.85 | (0.45 to 1.57) | 1.00 | (0.17 to 5.87) | 0.719 | |
| Model 2 4 | 0.46 | (0.20 to 1.06) | 1.00 (ref) | 0.60 | (0.27 to 1.37) | 1.00 | (0.49 to 2.04) | 0.778 | |
| Model 3 5 | 0.47 | (0.21 to 1.03) | 1.00 (ref) | 0.70 | (0.33 to 1.48) | 1.00 | (0.49 to 2.06) | 0.757 | |
| VLPI | LPI | MPI | p Value 2 | ||||
|---|---|---|---|---|---|---|---|
| Change in eGFR (mL/min/1.73 m2/year) | |||||||
| All | 0.84 | (−3.69 to 5.37) | −0.23 | (−4.80 to 4.34) | −0.97 | (−5.63 to 3.70) | 0.032 |
| ≤65 years | −0.34 | (−8.37 to 7.69) | −1.34 | (−10.15 to 7.47) | −1.88 | (−10.85 to 7.09) | 0.812 |
| >65 years | 1.06 | (−4.24 to 6.36) | −0.69 | (−6.02 to 4.63) | −0.98 | (−6.46 to 4.49) | 0.028 |
| Change in spot urine protein (g/gCr/year) | |||||||
| All | −0.87 | (−2.36 to 0.62) | −0.36 | (−1.87 to 1.14) | −0.58 | (−2.12 to 0.95) | 0.787 |
| ≤65 years | −0.81 | (−3.38 to 1.76) | −0.15 | (−2.97 to 2.66) | −1.31 | (−4.18 to 1.56) | 0.338 |
| >65 years | −0.82 | (−2.61 to 0.97) | −0.37 | (−2.16 to 1.42) | −0.28 | (−2.13 to 1.56) | 0.375 |
| Change in body weight (%/year) | |||||||
| All | 1.70 | (−4.08 to 7.49) | 2.74 | (−3.14 to 8.61) | 1.54 | (−4.47 to 7.54) | 0.312 |
| ≤65 years | −7.79 | (−23.37 to 7.80) | −7.75 | (−24.00 to 8.50) | −7.44 | (−23.66 to 8.78) | 0.986 |
| >65 years | 2.54 | (−3.99 to 9.06) | 3.89 | (−2.71 to 10.48) | 2.66 | (−4.19 to 9.50) | 0.308 |
| Change in serum albumin (g/dL/year) | |||||||
| All | 0.08 | (-0.56 to 0.71) | 0.15 | (−0.50 to 0.79) | 0.25 | (−0.41 to 0.91) | 0.109 |
| ≤65 years | −1.00 | (−1.67 to −0.33) | −0.61 | (−1.34 to 0.13) | −0.56 | (−1.31 to 0.19) | 0.048 |
| >65 years | 0.64 | (−0.20 to 1.48) | 0.64 | (−0.21 to 1.49) | 0.73 | (−0.14 to 1.60) | 0.338 |
| Change in serum phosphate (mg/dL/year) | |||||||
| All | −0.08 | (−0.71 to 0.55) | 0.04 | (−0.59 to 0.68) | 0.01 | (−0.63 to 0.66) | 0.804 |
| ≤65 years | 0.34 | (−0.82 to 1.50) | 0.41 | (−0.86 to 1.68) | 0.20 | (−1.09 to 1.50) | 0.396 |
| >65 years | −0.13 | (−0.88 to 0.61) | 0.05 | (−0.70 to 0.80) | 0.08 | (−0.69 to 0.85) | 0.283 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, D.; Machida, S.; Matsumoto, N.; Shibagaki, Y.; Sakurada, T. Age Modifies the Association of Dietary Protein Intake with All-Cause Mortality in Patients with Chronic Kidney Disease. Nutrients 2018, 10, 1744. https://0-doi-org.brum.beds.ac.uk/10.3390/nu10111744
Watanabe D, Machida S, Matsumoto N, Shibagaki Y, Sakurada T. Age Modifies the Association of Dietary Protein Intake with All-Cause Mortality in Patients with Chronic Kidney Disease. Nutrients. 2018; 10(11):1744. https://0-doi-org.brum.beds.ac.uk/10.3390/nu10111744
Chicago/Turabian StyleWatanabe, Daiki, Shinji Machida, Naoki Matsumoto, Yugo Shibagaki, and Tsutomu Sakurada. 2018. "Age Modifies the Association of Dietary Protein Intake with All-Cause Mortality in Patients with Chronic Kidney Disease" Nutrients 10, no. 11: 1744. https://0-doi-org.brum.beds.ac.uk/10.3390/nu10111744