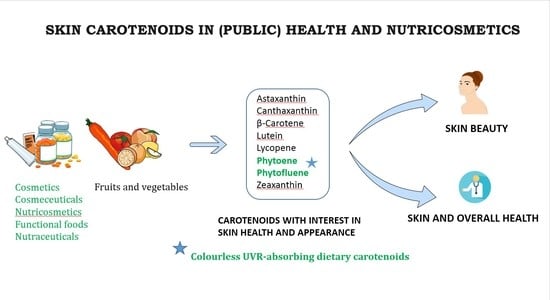
Skin Carotenoids in Public Health and Nutricosmetics: The Emerging Roles and Applications of the UV Radiation-Absorbing Colourless Carotenoids Phytoene and Phytofluene
Abstract
:1. Introduction
2. Structure and Functions of the Skin
2.1. Skin Structure
2.2. Skin Functions
3. Skin Disorders
3.1. UV Radiation Types and Consequences of Exposure
3.2. Skin Disorders Associated to UVR
3.2.1. Photosensitivity Disorders
3.2.2. Sunburn
3.2.3. Photoaging
3.2.4. Photoimmune Modulation
3.2.5. Photocarcinogenesis
4. Skin Beauty
4.1. Colour and Other Parameters Associated to Skin Beauty
4.1.1. Colour
4.1.2. Other Skin Aesthetic Parameters
4.2. Geographic and Ethnic Differences
5. Functional Foods, Nutraceuticals, Cosmetics, Cosmeceuticals, and Nutricosmetics: Definitions and Concepts
6. Dietary Carotenoids
6.1. Basics on Dietary Carotenoids
6.1.1. Sources and Intakes
6.1.2. Presence in Plasma, Other Biological Fluids and Tissues
6.1.3. Health-Promoting Biological Actions
6.2. The “Undercover” Colourless Carotenoids Phytoene and Phytofluene
- -
- They are present in widely consumed foods;
- -
- Their intakes and levels in plasma and tissues are comparable (or even superior in some cases) to those of other major dietary carotenoids; and
- -
- They are involved in biological actions that result in health and cosmetic benefits.
6.2.1. Distinctive Chemical Features among Carotenoids
UV Light Absorption
Susceptibility to Oxidation
Rigidity and Tendency to Aggregation
6.2.2. Sources and Intakes
6.2.3. Presence in Plasma, Other Biological Fluids, and Tissues
6.2.4. Health-Promoting Biological Actions
6.2.5. Safety of Phytoene and Phytofluene
7. Carotenoids in the Skin
7.1. Deposition Mechanism
7.2. Assessment of Skin Carotenoid Levels
7.2.1. HPLC Analysis
7.2.2. Non-Invasive Spectroscopic Methods
Light Reflection Spectroscopy (LRS)
Resonance Raman Spectroscopy (RRS)
7.2.3. Major Skin Carotenoids
7.2.4. Factors Affecting Skin Carotenoid Levels
Diet
Skin Location
Individual Characteristics
Stress Factors
7.2.5. Kinetic Aspects
8. Carotenoids and Photoprotection
8.1. Carotenoids and Protection Against Light in Diverse Organisms and Locations
8.2. Sunscreens vs. Dietary Approaches
8.3. Mechanisms of Skin Protection by Carotenoids
8.3.1. Inhibition of Lipid Peroxidation
8.3.2. Inhibition of UVA-Induced Expression of Heme Oxygenase 1
8.3.3. Prevention of Mitochondrial DNA Mutations
8.3.4. Metalloprotease Inhibition
8.4. Visible Light-Absorbing Coloured Carotenoids and Photoprotection in Humans
8.4.1. Astaxanthin
8.4.2. Canthaxanthin
8.4.3. Beta-Carotene
8.4.4. Lutein
8.4.5. Lycopene
8.4.6. Other Carotenoid-Containing Products
8.5. UV Light-Absorbing Colourless-Carotenoids (Phytoene and Phytofluene) and Photoprotection in Humans
Possible Mechanisms
9. Carotenoids and Cosmetic Benefits
9.1. Carotenoids and Colour Signalling in Animals
9.2. Carotenoids as Cosmetics
9.2.1. From Cleopatra to Tanning Pills
9.2.2. New Trends
9.3. The Colourless UV-Absorbing Carotenoids Phytoene and Phytofluene in Cosmetics
9.3.1. Skin Whitening by Phytoene and Phytofluene-Rich Products
9.3.2. Effect on Other Skin Aesthetical Parameters
9.4. Carotenoids and Aesthetic Benefits: Public Health Implications
10. Conclusions
Acknowledgments
Conflicts of Interest
References
- Lai-Cheong, J.E.; McGrath, J.A. Structure and function of skin, hair and nails. Medicine (Baltimore). 2017, 45, 347–351. [Google Scholar] [CrossRef]
- Rittie, L.; Fisher, G.J.; Rittié, L.; Fisher, G.J. Natural and sun-induced aging of human skin. Cold Spring Harb. Perspect. Med. 2015, 5, a015370. [Google Scholar] [CrossRef] [PubMed]
- Presland, R.B.; Dale, B.A. Epithelial Structural Proteins of the Skin and Oral Cavity: Function in Health and Disease. Crit. Rev. Oral Biol. Med. 2000, 11, 383–408. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, A.K.; Spano, F.; Derler, S.; Adlhart, C.; Spencer, N.D.; Rossi, R.M. The relationship between skin function, barrier properties, and body-dependent factors. Ski. Res. Technol. 2018, 24, 165–174. [Google Scholar] [CrossRef]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Nutraceuticals for skin care: A comprehensive review of human clinical studies. Nutrients 2018, 10, 403. [Google Scholar] [CrossRef]
- Fink, B.; Grammer, K.; Matts, P.J. Visible skin color distribution plays a role in the perception of age, attractiveness, and health in female faces. Evol. Hum. Behav. 2006, 27, 433–442. [Google Scholar] [CrossRef]
- Matts, P.J.; Fink, B.; Grammer, K.; Burquest, M. Color homogeneity and visual perception of age, health, and attractiveness of female facial skin. J. Am. Acad. Dermatol. 2007, 57, 977–984. [Google Scholar] [CrossRef]
- Little, A.C.; Jones, B.C.; Debruine, L.M. Facial attractiveness: Evolutionary based research. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 1638–1659. [Google Scholar] [CrossRef] [PubMed]
- Samson, N.; Fink, B.; Matts, P.J. Visible skin condition and perception of human facial appearance. Int. J. Cosmet. Sci. 2010, 32, 167–184. [Google Scholar] [CrossRef]
- Lee, C.-M. Fifty years of research and development of cosmeceuticals: A contemporary review. J. Cosmet. Dermatol. 2016, 15, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Goralczyk, R.; Wertz, K. Skin Photoprotection by Carotenoids. In Carotenoids. Volume 5. Nutrition and Health; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser: Basel, Switzerland, 2009; pp. 335–362. ISBN 978-3-7643-7501-0. [Google Scholar]
- Draelos, Z.D. Nutrition and enhancing youthful-appearing skin. Clin. Dermatol. 2010, 28, 400–408. [Google Scholar] [CrossRef]
- Anunciato, T.P.; da Rocha Filho, P.A. Carotenoids and polyphenols in nutricosmetics, nutraceuticals, and cosmeceuticals. J. Cosmet. Dermatol. 2012, 11, 51–54. [Google Scholar] [CrossRef]
- Madhere, S.; Simpson, P. A market overview of nutricosmetics. Cosmet. Dermatology 2010, 23, 268–274. [Google Scholar]
- Britton, G.; Khachik, F. Carotenoids in Food. In Carotenoids. Volume 5: Nutrition and Health; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser: Basel, Switzerland, 2009; pp. 45–66. ISBN 978-3-7643-7501-0. [Google Scholar]
- Mortensen, A. Supplements. In Carotenoids: Volume 5: Nutrition and Health; Britton, G., Pfander, H., Liaaen-Jensen, S., Eds.; Birkhäuser: Basel, Switzerland, 2009; pp. 67–82. ISBN 978-3-7643-7501-0. [Google Scholar]
- Meléndez-Martínez, A.J.; Pérez-Gálvez, A.; Roca, M.; Estévez Santiago, R.; Olmedilla Alonso, B.; Mercadante, A.Z.; Jesús Ornelas-Paz, J.D. Biodisponibilidad de carotenoides, factores que la determinan y métodos de estimación. In Carotenoides en Agroalimentación y Salud; Meléndez-Martínez, A.J., Ed.; Editorial Terracota: Ciudad de México, México, 2017; pp. 574–608. ISBN 978-84-15413-35-6. [Google Scholar]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids. Volume 5: Nutrition and Health; Birkhäuser: Basel, Switzerland, 2009; ISBN 978-3-7643-7501-0. [Google Scholar]
- Krinsky, N.I.; Johnson, E.J. Carotenoid actions and their relation to health and disease. Mol. Aspects Med. 2005, 26, 459–516. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, N.; Mayne, S.T.; Sies, H. Carotenoids in Health and Disease; Marcel Dekker: New York, NY, USA, 2004. [Google Scholar]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef]
- Engelmann, N.J.; Clinton, S.K.; Erdman, J.W. Nutritional aspects of phytoene and phytofluene, carotenoid precursors to lycopene. Adv. Nutr. 2011, 2, 51–61. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Mapelli-Brahm, P.; Benítez-González, A.; Stinco, C.M. A comprehensive review on the colorless carotenoids phytoene and phytofluene. Arch. Biochem. Biophys. 2015, 572, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J.; Mapelli-Brahm, P.; Stinco, C.M. The colourless carotenoids phytoene and phytofluene: From dietary sources to their usefulness for the functional foods and nutricosmetics industries. J. Food Compos. Anal. 2018, 67, 91–103. [Google Scholar] [CrossRef]
- De Spirt, S.; Lutter, K.; Stahl, W. Carotenoids in Photooxidative Stress. Curr. Nutr. Food Sci. 2010, 6, 36–43. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V.J.V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef]
- Darvin, M.E.; Fluhr, J.W.; Schanzer, S.; Richter, H.; Patzelt, A.; Meinke, M.C.; Zastrow, L.; Golz, K.; Doucet, O. Dermal carotenoid level and kinetics after topical and systemic administration of antioxidants: Enrichment strategies in a controlled in vivo study. J. Dermatol. Sci. 2011, 64, 53–58. [Google Scholar] [CrossRef]
- Beckenbach, L.; Baron, J.M.; Merk, H.F.; Amann, P.M. Retinoid treatment of skin diseases. Eur J Dermatol 2015, 25, 384–391. [Google Scholar] [CrossRef]
- Havas, F.; Krispin, S.; Meléndez-Martínez, A.J.; von Oppen-Bezalel, L. Preliminary data on the safety of phytoene and phytofluene-rich products for human use including topical application. J. Toxicol. 2018, 2018, 5475784. [Google Scholar] [CrossRef]
- Sorg, O.; Antille, C.; Kaya, G.; Saurat, J.-H. Retinoids in cosmeceuticals. Dermatol. Ther. 2006, 19, 289–296. [Google Scholar] [CrossRef]
- Bear, M.; Connors, B.W.; Paradiso, M.A. The somatic sensory system. In Neuroscience. Exploring the Brain; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; pp. 387–423. [Google Scholar]
- Chu, D.H.; Haake, A.R.; Holbrook, K.; Loomis, C.A. Nerves and receptors of the skin in Chapter 6: The structure and development of skin. In Fitzpatrick’s Dermatology General Medicine; Freedberg, I.M., Eisen, A.Z., Wolff, K., Austen, K.F., Goldsmith, L.A., Katz, S.I., Eds.; McGraw-Hill: New York, NY, USA, 2003; pp. 89–115. [Google Scholar]
- Kandel, E.R.; Schwartz, J.H.; Jessell, T.M.; Siegelbaum, S.A.; Hudspeth, A.J. Touch. In Principles of Neural Science; McGraw-Hill: New York, NY, USA, 2013. [Google Scholar]
- Bikle, D.D. Vitamin D and the skin: Physiology and pathophysiology. Rev. Endocr. Metab. Disord. 2012, 13, 3–19. [Google Scholar] [CrossRef]
- Fitzpatrick, T.B. The validity and practicality of sun-reactive skin types I through VI. Arch. Dermatol. 1988, 124, 869. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef]
- Sies, H.; Stahl, W. Nutritional protection against skin damage from sunlight. Annu. Rev. Nutr. 2004, 24, 173–200. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Photoprotection by dietary carotenoids: Concept, mechanisms, evidence and future development. Mol. Nutr. Food Res. 2012, 56, 287–295. [Google Scholar] [CrossRef]
- Mathews-Roth, M.M. Carotenoids in Erythropoietic Protoporphyria and Other Photosensitivity Diseases. Ann. N. Y. Acad. Sci. 1993, 691, 127–138. [Google Scholar] [CrossRef]
- Von Laar, J.; Stahl, W.; Bolsen, K.; Goerz, G.; Sies, H. β-Carotene serum levels in patients with erythropoietic protoporphyria on treatment with the synthetic all-trans isomer or a natural isomeric mixture of β-carotene. J. Photochem. Photobiol. B Biol. 1996, 33, 157–162. [Google Scholar] [CrossRef]
- Kou, J.; Dou, D.; Yang, L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, T.B. Soleil et peau. J Med Esthet. 1975, 2, 33–34. [Google Scholar]
- Fitzpatrick, T.B. Ultraviolet-induced pigmentary changes: Benefits and hazards. Curr. Probl. Dermatol. 1986, 15, 25–38. [Google Scholar]
- Pathak, M.A. Sunlight and melanin pigmentation. In Photochemical and Photobiological Reviews; Smith, K.C., Ed.; Plenum Press: New York, NY, USA, 1976; pp. 211–239. [Google Scholar]
- Schagen, S.K.; Zampeli, V.A.; Makrantonaki, E.; Zouboulis, C.C. Discovering the link between nutrition and skin aging. Dermatoendocrinol. 2012, 4, 298–307. [Google Scholar] [CrossRef]
- Terao, J.; Minami, Y.; Bando, N. Singlet molecular oxygen quenching activity of carotenoids: Relevance to protection of the skin from photoaging. J. Clin. Biochem. Nutr. 2011, 48, 57–62. [Google Scholar] [CrossRef]
- Kochevar, I.E.; Pathak, M.A.; Parrish, J.A. Photophysics, photochemistry, and phobiology. In Fitzpatrick’s Dermatology General Medicine; Freedberg, I., Eisen, A.Z., Wolff, K., Al, E., Eds.; McGraw-Hill: New York, NY, USA, 1999; pp. 220–229. [Google Scholar]
- Akhalaya, M.Y.; Maksimov, G.V.; Rubin, A.B.; Lademann, J.; Darvin, M.E. Molecular action mechanisms of solar infrared radiation and heat on human skin. Ageing Res. Rev. 2014, 16, 1–11. [Google Scholar] [CrossRef]
- Freitas, J.V.; Junqueira, H.C.; Martins, W.K.; Baptista, M.S.; Gaspar, L. Antioxidant role on the protection of melanocytes against visible light-induced photodamage. Free Radic. Biol. Med. 2019, 131, 399–407. [Google Scholar] [CrossRef]
- Ascenso, A.; Ribeiro, H.; Marques, H.C.; Oliveira, H.; Santos, C.; Simões, S. Chemoprevention of photocarcinogenesis by lycopene. Exp. Dermatol. 2014, 23, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Pullar, J.; Carr, A.; Vissers, M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef]
- Reich, P.; Shwachman, H.; Craig, J.M. Lycopenemia. N. Engl. J. Med. 1960, 262, 263–269. [Google Scholar] [CrossRef]
- Maharshak, N.; Shapiro, J.; Trau, H. Carotenoderma - a review of the current literature. Int. J. Dermatol. 2003, 42, 178–181. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. β-Carotene and other carotenoids in protection from sunlight. Am. J. Clin. Nutr. 2012, 96, 1179–1184. [Google Scholar] [CrossRef]
- Pezdirc, K.; Hutchesson, M.J.; Williams, R.L.; Rollo, M.E.; Burrows, T.L.; Wood, L.G.; Oldmeadow, C.; Collins, C.E. Consuming High-Carotenoid Fruit and Vegetables Influences Skin Yellowness and Plasma Carotenoids in Young Women: A Single-Blind Randomized Crossover Trial. J. Acad. Nutr. Diet. 2016, 116, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; Sterry, W.; Lademann, J.; Vergou, T. The Role of Carotenoids in Human Skin. Molecules 2011, 16, 10491–10506. [Google Scholar] [CrossRef]
- Darvin, M.E.; Fluhr, J.W.; Caspers, P.; van der Pool, A.; Richter, H.; Patzelt, A.; Sterry, W.; Lademann, J. In vivo distribution of carotenoids in different anatomical locations of human skin: Comparative assessment with two different Raman spectroscopy methods. Exp. Dermatol. 2009, 18, 1060–1063. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Aisa, H.A. Upregulation of Melanogenesis and Tyrosinase Activity: Potential Agents for Vitiligo. Molecules 2017, 22, 1303. [Google Scholar] [CrossRef] [PubMed]
- Foo, Y.Z.; Simmons, L.W.; Rhodes, G. Predictors of facial attractiveness and health in humans. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Hunter, M.L. If You’re Light You’re Alright: Light Skin Color as Social Capital for Women of Color. Gend. Soc. 2002, 16, 175–193. [Google Scholar] [CrossRef]
- Hill, M.E. Skin color and the perception of attractiveness among African Americans: Does gender make a difference? Soc. Psychol. Q. 2002, 65, 77–91. [Google Scholar] [CrossRef]
- Awad, G.H.; Norwood, C.; Taylor, D.S.; Martinez, M.; McClain, S.; Jones, B.; Holman, A.; Chapman-Hilliard, C. Beauty and Body Image Concerns Among African American College Women. J Black Psychol. 2015 2015, 41, 1–20. [Google Scholar] [CrossRef]
- Coley, M.K.; Alexis, A.F. Cosmetic concerns in skin of color, part 1. Cosmet. Dermatology 2009, 22, 360–370. [Google Scholar]
- Tranggono, R.I. Adityarini Cosmeceuticals for Asians who are living in the tropics. J. Appl. Cosmetol. 2010, 28, 71–86. [Google Scholar]
- Howlett, J. Functional foods from science To health and claims; ILSI Press: Brussels, Belgium, 2008; ISBN 0849313724. [Google Scholar]
- DeFelice, S.L. The nutraceutical revolution: Its impact on food industry. Trends Food Sci. Technol. 1995, 6, 59–61. [Google Scholar] [CrossRef]
- Santini, A.; Cammarata, S.M.; Capone, G.; Ianaro, A.; Tenore, G.C.; Pani, L.; Novellino, E. Nutraceuticals: Opening the debate for a regulatory framework. Br. J. Clin. Pharmacol. 2018, 84, 659–672. [Google Scholar] [CrossRef]
- The European Parliament and the Council of the European Union (institution) Regulation (EC) no 1223/2009 of The European Parliament and of the Council of 30 November 2009 on cosmetic products. Off. J. Eur. Union 2009, 342, 59–209.
- FDA. Federal Food, Drug, and Cosmetic Act; 2019. Available online: https://www.fda.gov/cosmetics/guidanceregulation/lawsregulations/ucm074201.htm (accessed on 14 May 2019).
- Saint-Leger, D. ‘Cosmeceuticals’. Of men, science and laws…. Int. J. Cosmet. Sci. 2012, 34, 396–401. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J. Carotenoides: Estructura, propiedades y funciones. In Carotenoides en agroalimentación y salud; Meléndez-Martínez, A., Ed.; Editorial Terracota: Ciudad de México, México, 2017; ISBN 978-84-15413-35-6. [Google Scholar]
- Dias, M.G.; Olmedilla-Alonso, B.; Hornero-Méndez, D.; Mercadante, A.Z.; Osorio, C.; Vargas-Murga, L.; Meléndez-Martínez, A.J. Comprehensive Database of Carotenoid Contents in Ibero-American Foods. A Valuable Tool in the Context of Functional Foods and the Establishment of Recommended Intakes of Bioactives. J. Agric. Food Chem. 2018, 66, 5055–5107. [Google Scholar] [CrossRef]
- Singh, J.; Fan, D.; Banskota, A.H.; Stefanova, R.; Khan, W.; Hafting, J.; Craigie, J.; Critchley, A.T.; Prithiviraj, B. Bioactive components of the edible strain of red alga, Chondrus crispus, enhance oxidative stress tolerance in Caenorhabditis elegans. J. Funct. Foods 2013, 5, 1180–1190. [Google Scholar] [CrossRef]
- Zarekarizi, A.; Hoffmann, L.; Burritt, D. Approaches for the sustainable production of fucoxanthin, a xanthophyll with potential health benefits. J. Appl. Phycol. 2018, 1–19. [Google Scholar] [CrossRef]
- Rasmussen, H.M.; Muzhingi, T.; Eggert, E.M.R.; Johnson, E. Lutein, zeaxanthin, meso-zeaxanthin content in egg yolk and their absence in fish and seafood. J. Food Compos. Anal. 2012, 27, 139–144. [Google Scholar] [CrossRef]
- Schlatterer, J.; Breithaupt, D.E. Xanthophylls in commercial egg yolks: Quantification and identification by HPLC and LC-(APCI)MS using a C30 phase. J. Agric. Food Chem. 2006, 54, 2267–2273. [Google Scholar] [CrossRef]
- Álvarez, R.; Meléndez-Martínez, A.J.; Vicario, I.M.; Alcalde, M.J. Carotenoid and Vitamin A Contents in Biological Fluids and Tissues of Animals as an Effect of the Diet: A Review. Food Rev. Int. 2015, 31, 319–340. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids in Marine Animals. Mar. Drugs 2011, 9, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Metusalach; Brown, J.A.; Taylor, P. Carotenoid pigments in seafoods and aquaculture. Crit. Rev. Food Sci. Nutr. 1998, 38, 1–67. [Google Scholar] [CrossRef]
- Lehto, S.; Buchweitz, M.; Klimm, A.; Straßburger, R.; Bechtold, C.; Ulberth, F. Comparison of food colour regulations in the EU and the US: A review of current provisions. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2017, 34, 335–355. [Google Scholar] [CrossRef]
- Martins, N.; Roriz, C.L.; Morales, P.; Barros, L.; Ferreira, I.C.F.R. Food colorants: Challenges, opportunities and current desires of agro-industries to ensure consumer expectations and regulatory practices. Trends Food Sci. Technol. 2016, 52, 1–15. [Google Scholar] [CrossRef]
- Phelan, D.; Prado-Cabrero, A.; Nolan, J.M. Stability of Commercially Available Macular Carotenoid Supplements in Oil and Powder Formulations. Nutrients 2017, 9, 1. [Google Scholar] [CrossRef]
- Khachik, F. Analysis of carotenoids in nutritional studies. In Carotenoids. Volume 5: Nutrition and Health; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser: Basel, Switzerland, 2009; pp. 7–44. ISBN 978-3-7643-7501-0. [Google Scholar]
- Canene-Adams, K.; Erdman, J.W., Jr. Absorption, Transport, Distribution in Tissues and Bioavailability. In Carotenoids. Volume 5. Nutrition and Health; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser: Basel, Switzerland, 2009; pp. 115–148. ISBN 978-3-7643-7501-0. [Google Scholar]
- Meléndez-Martínez, A.; Stinco, C.M.; Liu, C.; Wang, X.-D. A simple HPLC method for the comprehensive analysis of cis/trans (Z/E) geometrical isomers of carotenoids for nutritional studies. Food Chem. 2013, 138, 1341–1350. [Google Scholar] [CrossRef]
- Burrows, T.; Rollo, M.; Williams, R.; Wood, L.; Garg, M.; Jensen, M.; Collins, C. A Systematic Review of Technology-Based Dietary Intake Assessment Validation Studies That Include Carotenoid Biomarkers. Nutrients 2017, 9, 140. [Google Scholar] [CrossRef]
- Britton, G. Vitamin A and Vitamin A Deficiency. In Carotenoids. Volume 5. Nutrition and Health; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser: Basel, Switzerland, 2009; pp. 173–190. ISBN 978-3-7643-7501-0. [Google Scholar]
- Giuliano, G. Provitamin A biofortification of crop plants: A gold rush with many miners. Curr. Opin. Biotechnol. 2017, 44, 169–180. [Google Scholar] [CrossRef]
- Van Hoang, D.; Pham, N.; Lee, A.; Tran, D.; Binns, C. Dietary Carotenoid Intakes and Prostate Cancer Risk: A Case-Control Study from Vietnam. Nutrients 2018, 10, 70. [Google Scholar] [CrossRef]
- Yamaguchi, M. β-Cryptoxanthin and bone metabolism: The preventive role in osteoporosis. J. Heal. Sci. 2008, 54, 356–369. [Google Scholar] [CrossRef]
- Böhm, V. Lycopene and heart health. Mol. Nutr. Food Res. 2012, 56, 296–303. [Google Scholar] [CrossRef]
- Nishino, H.; Murakoshi, M.; Tokuda, H.; Satomi, Y. Cancer prevention by carotenoids. Arch. Biochem. Biophys. 2009, 483, 165–168. [Google Scholar] [CrossRef]
- Mares, J. Lutein and Zeaxanthin Isomers in Eye Health and Disease. Annu. Rev. Nutr. 2016, 36, 571–602. [Google Scholar] [CrossRef]
- Johnson, E.J. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr. Rev. 2014, 72, 605–612. [Google Scholar] [CrossRef]
- Bonet, M.; Canas, J.A.; Ribot, J.; Palou, A. Carotenoids and their conversion products in the control of adipocyte function, adiposity and obesity. Arch. Biochem. Biophys. 2015, 572, 112–125. [Google Scholar] [CrossRef]
- Coyne, T.; Ibiebele, T.I.; Baade, P.D.; McClintock, C.S.; Shaw, J.E. Metabolic syndrome and serum carotenoids: Findings of a cross-sectional study in Queensland, Australia. Br. J. Nutr. 2009, 102, 1668–1677. [Google Scholar] [CrossRef] [PubMed]
- Than, A.; Bramley, P.M.; Davies, B.H.; Rees, A.F. Stereochemistry of phytoene. Phytochemistry 1972, 11, 3187–3192. [Google Scholar] [CrossRef]
- Britton, G. Structure and properties of carotenoids in relation to function. FASEB J. 1995, 9, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J.; Britton, G.; Vicario, I.M.; Heredia, F.J. Relationship between the colour and the chemical structure of carotenoid pigments. Food Chem. 2007, 101, 1145–1150. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D. A Guide to Carotenoid Analysis in Foods; ILSI Press: Washington, DC, USA, 2001; ISBN 1578810728. [Google Scholar]
- Davies, B.H. Carotenoids. In Chemistry and Biochemistry of Plant Pigments; Goodwin, T.W., Ed.; Academic Press: London, UK, 1976; pp. 38–165. [Google Scholar]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids. Handbook; Birkhäuser: Basel, Switzerland, 2004. [Google Scholar]
- Martínez, A.; Stinco, C.M.; Meléndez-Martínez, A.J. Free radical scavenging properties of phytofluene and phytoene isomers as compared to lycopene: A combined experimental and theoretical study. J. Phys. Chem. B 2014, 118, 9819–9825. [Google Scholar] [CrossRef] [PubMed]
- Cooperstone, J.L.; Francis, D.M.; Schwartz, S.J. Thermal processing differentially affects lycopene and other carotenoids in cis-lycopene containing, tangerine tomatoes. Food Chem. 2016, 210, 466–472. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Paulino, M.; Stinco, C.M.; Mapelli-Brahm, P.; Wang, X.-D. Study of the Time-Course of cis/trans (Z/E) Isomerization of Lycopene, Phytoene, and Phytofluene from Tomato. J. Agric. Food Chem. 2014, 62, 12399–12406. [Google Scholar] [CrossRef] [PubMed]
- Desmarchelier, C.; Borel, P. Overview of carotenoid bioavailability determinants: From dietary factors to host genetic variations. Trends Food Sci. Technol. 2017, 69, 270–280. [Google Scholar] [CrossRef]
- Mapelli-Brahm, P.; Corte-Real, J.; Meléndez-Martínez, A.J.A.J.; Bohn, T. Bioaccessibility of phytoene and phytofluene is superior to other carotenoids from selected fruit and vegetable juices. Food Chem. 2017, 229, 304–311. [Google Scholar] [CrossRef]
- Mapelli-Brahm, P.; Stinco, C.M.; Rodrigo, M.J.; Zacarías, L.; Meléndez-Martínez, A.J. Impact of thermal treatments on the bioaccessibility of phytoene and phytofluene in relation to changes in the microstructure and size of orange juice particles. J. Funct. Foods 2018, 46, 38–47. [Google Scholar] [CrossRef]
- Mapelli-Brahm, P.; Desmarchelier, C.; Margier, M.; Reboul, E.; Meléndez Martínez, A.J.; Borel, P. Phytoene and Phytofluene Isolated from a Tomato Extract are Readily Incorporated in Mixed Micelles and Absorbed by Caco-2 Cells, as Compared to Lycopene, and SR-BI is Involved in their Cellular Uptake. Mol. Nutr. Food Res. 2018, 1800703. [Google Scholar] [CrossRef]
- Biehler, E.; Alkerwi, A.; Hoffmann, L.; Krause, E.; Guillaume, M.; Lair, M.L.; Bohn, T. Contribution of violaxanthin, neoxanthin, phytoene and phytofluene to total carotenoid intake: Assessment in Luxembourg. J. Food Compos. Anal. 2012, 25, 56–65. [Google Scholar] [CrossRef]
- Paetau, I.; Khachik, F.; Brown, E.D.; Beecher, G.R.; Kramer, T.R.; Chittams, J.; Clevidence, B.A. Chronic ingestion of lycopene-rich tomato juice or lycopene supplements significantly increases plasma concentrations of lycopene and related tomato carotenoids in humans. Int. J. Cancer 1998, 68, 1187–1195. [Google Scholar] [CrossRef]
- Müller, H.; Bub, A.; Watzl, B.; Rechkemmer, G.; Contribution, O. Plasma concentrations of carotenoids in healthy volunteers after intervention with carotenoid-rich foods. Eur. J. Nutr. 1999, 38, 35–44. [Google Scholar] [CrossRef]
- Aust, O.; Stahl, W.; Sies, H.; Tronnier, H.; Heinrich, U. Supplementation with tomato- based products increase lycopene, phytofluene, and phytoene levels in human serum and protects against UV-light-induced erythema. Int. J. Vitam. Nutr. Res. 2005, 75, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Khachik, F.; Spangler, C.J.; Smith, J.C., Jr.; Canfield, L.M.; Steck, A.; Pfander, H. Identification, quantification, and relative Concentrations of carotenoids and their metabolites in Human Milk and Serum. Anal. Chem. 1997, 69, 1873–1881. [Google Scholar] [CrossRef]
- Khachik, F.; Carvalho, L.; Bernstein, P.S.; Muir, G.J.; Zhao, D.-Y.; Katz, N.B. Chemistry, distribution, and metabolism of tomato carotenoids and their impact on human health. Exp. Biol. Med. (Maywood). 2002, 227, 845–851. [Google Scholar] [CrossRef]
- Basu, A.; Imrhan, V. Tomatoes versus lycopene in oxidative stress and carcinogenesis: Conclusions from clinical trials. Eur. J. Clin. Nutr. 2007, 61, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Jacques, P.F.; Lyass, A.; Massaro, J.M.; Vasan, R.S.; D’Agostino Sr, R.B. Relationship of lycopene intake and consumption of tomato products to incident CVD. Br. J. Nutr. 2013, 110, 545–551. [Google Scholar] [CrossRef]
- Ben-dor, A.; Steiner, M.; Gheber, L.; Danilenko, M.; Dubi, N.; Linnewiel, K.; Zick, A.; Sharoni, Y.; Levy, J. Carotenoids activate the antioxidant response element transcription system Carotenoids activate the antioxidant response element transcription system. Mol. Cancer Ther. 2005, 4, 177–186. [Google Scholar]
- Shaish, A.; Harari, A.; Kamari, Y.; Soudant, E.; Harats, D.; Ben-Amotz, A. A carotenoid algal preparation containing phytoene and phytofluene inhibited LDL oxidation in vitro. Plant Foods Hum. Nutr. 2008, 63, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Porrini, M.; Riso, P.; Brusamolino, A.; Berti, C.; Guarnieri, S.; Visioli, F. Daily intake of a formulated tomato drink affects carotenoid plasma and lymphocyte concentrations and improves cellular antioxidant protection. Br. J. Nutr. 2005, 93, 93. [Google Scholar] [CrossRef]
- Simmons, D.L.; Botting, R.M.; Hla, T. Cyclooxygenase isozymes: The biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 2004, 56, 387–437. [Google Scholar] [CrossRef] [PubMed]
- Fuller, B.; Smith, D.; Howerton, A.; Kern, D. Anti-inflammatory effects of CoQ10 and colorless carotenoids. J. Cosmet. Dermatol. 2006, 5, 30–38. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Nascimento, A.F.; Wang, Y.; Liu, C.; Mao, Y.; Wang, X.-D. Effect of tomato extract supplementation against high-fat diet-induced hepatic lesions. Hepatobiliary Surg. Nutr. 2013, 2, 198–208. [Google Scholar] [CrossRef]
- Kotake-Nara, E.; Kushiro, M.; Zhang, H.; Sugawara, T.; Miyashita, K.; Nagao, A. Carotenoids affect proliferation of human prostate cancer cells. J. Nutr. 2001, 131, 3303–3306. [Google Scholar] [CrossRef]
- Nara, E.; Hayashi, H.; Kotake, M.; Miyashita, K.; Nagao, A. Acyclic Carotenoids and Their Oxidation Mixtures Inhibit the Growth of HL-60 Human Promyelocytic Leukemia Cells Acyclic Carotenoids and Their Oxidation Mixtures Inhibit the Growth of HL-60 Human Promyelocytic Leukemia Cells. Nutr. Cancer 2009, 39, 37–41. [Google Scholar] [CrossRef]
- Hirsch, K.; Atzmon, A.; Danilenko, M.; Levy, J.; Sharoni, Y. Lycopene and other carotenoids inhibit estrogenic activity of 17-β-estradiol and genistein in cancer cells. Breast Cancer Res. Treat. 2007, 104, 221–230. [Google Scholar] [CrossRef]
- Boileau, T.W.-M.; Liao, Z.; Kim, S.; Lemeshow, S.; Erdman, J.; Clinton, S.K. Prostate Carcinogenesis in N-methyl-N-nitrosourea (NMU)-Testosterone-Treated Rats Fed Tomato Powder, Lycopene, or Energy-Restricted Diets. J. Natl. Cancer Inst. 2003, 95, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.K.; Stroud, C.K.; Nakamura, M.T.; Lila, M.A.; Erdman, J.W. Serum testosterone is reduced following short-term phytofluene, lycopene, or tomato powder consumption in F344 rats. J. Nutr. 2006, 136, 2813–2819. [Google Scholar] [CrossRef] [PubMed]
- FDA. GRAS Notice 163; 2005. Available online: https://www.accessdata.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=163 (accessed on 12 August 2018).
- FDA. GRAS Notice 185; 2006. Available online: https://www.accessdata.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=185 (accessed on 12 August 2018).
- EFSA Safety of Lycopene oleoresin from tomatoes. EFSA J. 2008, 675, 1–22. [CrossRef]
- Darvin, M.E.; Patzelt, A.; Knorr, F.; Blume-Peytavi, U.; Sterry, W.; Lademann, J. One-year study on the variation of carotenoid antioxidant substances in living human skin: Influence of dietary supplementation and stress factors. J. Biomed. Opt. 2008, 13, 044028-1–044028-9. [Google Scholar] [CrossRef]
- Mayne, S.T.; Cartmel, B.; Scarmo, S.; Lin, H.; Leffell, D.J.; Welch, E.; Ermakov, I.; Bhosale, P.; Bernstein, P.S.; Gellermann, W. Noninvasive assessment of dermal carotenoids as a biomarker of fruit and vegetable intake. Am. J. Clin. Nutr. 2010, 92, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Ermakov, I.V.; Gellermann, W. Validation model for Raman based skin carotenoid detection. Arch. Biochem. Biophys. 2010, 504, 40–49. [Google Scholar] [CrossRef]
- Ermakov, I.V.; Ermakova, M.; Sharifzadeh, M.; Gorusupudi, A.; Farnsworth, K.; Bernstein, P.S.; Stookey, J.; Evans, J.; Arana, T.; Tao-Lew, L.; et al. Optical assessment of skin carotenoid status as a biomarker of vegetable and fruit intake. Arch. Biochem. Biophys. 2018, 646, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Heinrich, U.; Jungmann, H.; von Laar, J.; Schietzel, M.; Sies, H.; Tronnier, H. Increased Dermal Carotenoid Levels Assessed by Noninvasive Reflection Spectrophotometry Correlate with Serum Levels in Women Ingesting Betatene. J. Nutr. 1998, 128, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Heinrich, U.; Jungmann, H.; Tronnier, H.; Sies, H. Carotenoids in human skin: Noninvasive measurement and identification of dermal carotenoids and carotenol esters. Methods Enzymol. 2000, 319, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Meinke, M.C.; Sterry, W.; Darvin, M.E. Carotenoids in human skin. Exp. Dermatol. 2011, 20, 377–382. [Google Scholar] [CrossRef]
- Ermakov, I.V.; Gellermann, W. Optical detection methods for carotenoids in human skin. Arch. Biochem. Biophys. 2015, 572, 101–111. [Google Scholar] [CrossRef]
- Meinke, M.C.; Lohan, S.B.; Köcher, W.; Magnussen, B.; Darvin, M.E.; Lademann, J. Multiple spatially resolved reflection spectroscopy to monitor cutaneous carotenoids during supplementation of fruit and vegetable extracts in vivo. Ski. Res. Technol. 2017, 23, 459–462. [Google Scholar] [CrossRef]
- Jilcott Pitts, S.B.; Jahns, L.; Wu, Q.; Moran, N.E.; Bell, R.A.; Truesdale, K.P.; Laska, M.N. A non-invasive assessment of skin carotenoid status through reflection spectroscopy is a feasible, reliable and potentially valid measure of fruit and vegetable consumption in a diverse community sample. Public Health Nutr. 2018, 21, 1664–1670. [Google Scholar] [CrossRef]
- Ashton, L.M.; Pezdirc, K.B.; Hutchesson, M.J.; Rollo, M.E.; Collins, C.E. Is skin coloration measured by reflectance spectroscopy related to intake of nutrient-dense foods? A cross-sectional evaluation in Australian young adults. Nutrients 2018, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Coyle, D.H.; Pezdirc, K.; Hutchesson, M.J.; Collins, C.E. Intake of specific types of fruit and vegetables is associated with higher levels of skin yellowness in young women: A cross-sectional study. Nutr. Res. 2018, 56, 23–31. [Google Scholar] [CrossRef]
- Stinco, C.M.; Rodríguez-Pulido, F.J.; Escudero-Gilete, M.L.; Gordillo, B.; Vicario, I.M.; Meléndez-Martínez, A.J. Lycopene isomers in fresh and processed tomato products: Correlations with instrumental color measurements by digital image analysis and spectroradiometry. Food Res. Int. 2013, 50, 111–120. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Gómez-Robledo, L.; Melgosa, M.; Vicario, I.M.; Heredia, F.J. Color of orange juices in relation to their carotenoid contents as assessed from different spectroscopic data. J. Food Compos. Anal. 2011, 24, 837–844. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Vicario, I.M.; Heredia, F.J. Influence of white reference measurement and background on the colour specification of orange juices by means of diffuse reflectance spectrophotometry. J. AOAC Int. 2006, 89, 452–457. [Google Scholar]
- Meléndez-Martínez, A.J.; Vicario, I.M.; Heredia, F.J. Instrumental measurement of orange juice colour: A review. J. Sci. Food Agric. 2005, 85, 894–901. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Vicario, I.M.; Heredia, F.J. Rapid assessment of vitamin A activity through objective color measurements for the quality control of orange juices with diverse carotenoid profiles. J. Agric. Food Chem. 2007, 55, 2808–2815. [Google Scholar] [CrossRef] [PubMed]
- Scarmo, S.; Cartmel, B.; Lin, H.; Leffell, D.J.; Ermakov, I.V.; Gellermann, W.; Bernstein, P.S.; Mayne, S.T. Single v. multiple measures of skin carotenoids by resonance Raman spectroscopy as a biomarker of usual carotenoid status. Br. J. Nutr. 2013, 110, 911–917. [Google Scholar] [CrossRef]
- Aguilar, S.S.; Wengreen, H.J.; Lefevre, M.; Madden, G.J.; Gast, J. Skin Carotenoids: A Biomarker of Fruit and Vegetable Intake in Children. J. Acad. Nutr. Diet. 2014, 114, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.M.; Scherr, R.E.; Linnell, J.D.; Ermakov, I.V.; Gellermann, W.; Jahns, L.; Keen, C.L.; Miyamoto, S.; Steinberg, F.M.; Young, H.M.; et al. Evaluating the relationship between plasma and skin carotenoids and reported dietary intake in elementary school children to assess fruit and vegetable intake. Arch. Biochem. Biophys. 2015, 572, 73–80. [Google Scholar] [CrossRef]
- Holt, E.W.; Wei, E.K.; Bennett, N.; Zhang, L.M. Low skin carotenoid concentration measured by resonance Raman spectroscopy is associated with metabolic syndrome in adults. Nutr. Res. 2014, 34, 821–826. [Google Scholar] [CrossRef]
- Stahl, W.; Heinrich, U.; Wiseman, S.; Eichler, O.; Sies, H.; Tronnier, H. Dietary Tomato Paste Protects against Ultraviolet Light–Induced Erythema in Humans. J. Nutr. 2001, 131, 1449–1451. [Google Scholar] [CrossRef]
- Hata, T.R.; Scholz, T.A.; Ermakov, I.V.; McClane, R.W.; Khachik, F.; Gellermann, W.; Pershing, L.K. Non-invasive Raman spectroscopic detection of carotenoids in human skin. J. Invest. Dermatol. 2000, 115, 441–448. [Google Scholar] [CrossRef]
- Wingerath, T.; Sies, H.; Stahl, W. Xanthophyll Esters in Human Skin. Arch. Biochem. Biophys. 1998, 355, 271–274. [Google Scholar] [CrossRef]
- Wingerath, T.; Stahl, W.; Sies, H. β-Cryptoxanthin selectively increases in human chylomicrons upon ingestion of tangerine concentrate rich in beta-cryptoxanthin esters. Arch. Biochem. Biophys. 1995, 324, 385–390. [Google Scholar] [CrossRef]
- Mercadante, A.Z.; Rodrigues, D.B.; Petry, F.C.; Mariutti, L.R.B. Carotenoid esters in foods—A review and practical directions on analysis and occurrence. Food Res. Int. 2017, 99, 830–850. [Google Scholar] [CrossRef]
- Bohn, T.; McDougall, G.J.; Alegría, A.; Alminger, M.; Arrigoni, E.; Aura, A.-M.; Brito, C.; Cilla, A.; El, S.N.; Karakaya, S.; et al. Mind the gap-deficits in our knowledge of aspects impacting the bioavailability of phytochemicals and their metabolites-a position paper focusing on carotenoids and polyphenols. Mol. Nutr. Food Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T.; Desmarchelier, C.; Dragsted, L.O.; Nielsen, C.S.; Stahl, W.; Rühl, R.; Keijer, J.; Borel, P. Host-related factors explaining interindividual variability of carotenoid bioavailability and tissue concentrations in humans. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Massenti, R.; Perrone, A.; Livrea, M.A.; Lo Bianco, R. Regular consumption of fresh orange juice increases human skin carotenoid content. Int. J. Food Sci. Nutr. 2015, 66, 718–721. [Google Scholar] [CrossRef]
- Jahns, L.; Johnson, L.K.; Mayne, S.T.; Cartmel, B.; Sr, M.J.P.; Ermakov, I.V.; Gellermann, W.; Whigham, L.D.; Picklo, M.J.; Ermakov, I.V.; et al. Skin and plasma carotenoid response to a provided intervention diet high in vegetables and fruit: Uptake and depletion kinetics. Am. J. Clin. Nutr. 2014, 100, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, S.S.; Wengreen, H.J.; Dew, J. Skin Carotenoid Response to a High-Carotenoid Juice in Children: A Randomized Clinical Trial. J. Acad. Nutr. Diet. 2015, 115, 1771–1778. [Google Scholar] [CrossRef]
- Whitehead, R.D.; Re, D.; Xiao, D.; Ozakinci, G.; Perrett, D.I. You Are What You Eat: Within-Subject Increases in Fruit and Vegetable Consumption Confer Beneficial Skin-Color Changes. PLoS ONE 2012, 7, e32988. [Google Scholar] [CrossRef]
- Sansone, R.A.; Sansone, L.A. Carrot Man: A Case of Excessive Beta-Carotene Ingestion. Int. J. Eat. Disord. 2012, 45, 816–818. [Google Scholar] [CrossRef]
- Meinke, M.C.; Lauer, A.; Taskoparan, B.; Gersonde, I.; Lademann, J.; Darvin, M.E. Influence on the carotenoid levels of skin arising from age, gender, body mass index in smoking/non-smoking individuals. Free Radicals Antioxidants 2011, 1, 15–20. [Google Scholar] [CrossRef]
- Biesalski, H.K.; Hemmes, C.; Hopfenmuller, W.; Schmid, C.; Gollnick, H.P. Effects of controlled exposure of sunlight on plasma and skin levels of beta-carotene. Free Radic. Res. 1996, 24, 215–224. [Google Scholar] [CrossRef]
- Vandersee, S.; Beyer, M.; Lademann, J.; Darvin, M.E. Blue-violet light irradiation dose dependently decreases carotenoids in human skin, which indicates the generation of free radicals. Oxid. Med. Cell. Longev. 2015, 2015. [Google Scholar] [CrossRef]
- Darvin, M.E.; Patzelt, A.; Meinke, M.; Sterry, W.; Lademann, J. Influence of two different IR radiators on the antioxidative potential of the human skin. Laser Phys. Lett. 2009, 6, 229–234. [Google Scholar] [CrossRef]
- Darvin, M.E.; Fluhr, J.W.; Meinke, M.C.; Zastrow, L.; Sterry, W.; Lademann, J. Topical β-carotene protects against infra-red-light-induced free radicals. Exp. Dermatol. 2011, 20, 125–129. [Google Scholar] [CrossRef]
- Lima, X.T.T.; Kimball, A.B.B. Skin carotenoid levels in adult patients with psoriasis. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Li, D.G.; LeCompte, G.; Golod, L.; Cecchi, G.; Irwin, D.; Harken, A.; Matecki, A. Dermal carotenoid measurement is inversely related to anxiety in patients with breast cancer. J. Investig. Med. 2018, 66, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Meinke, M.C.; Darvin, M.E.; Vollert, H.; Lademann, J. Bioavailability of natural carotenoids in human skin compared to blood. Eur. J. Pharm. Biopharm. 2010, 76, 269–274. [Google Scholar] [CrossRef]
- Takaichi, S.; Mochimaru, M.; Sciences, M.L. Carotenoids and carotenogenesis in cyanobacteria: Unique ketocarotenoids and carotenoid glycosides. Cell. Mol. Life Sci. 2007, 64, 2607–2619. [Google Scholar] [CrossRef] [PubMed]
- Britton, G. Functions of intact carotenoids. In Carotenoids. Volume 4: Natural functions; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser: Basel, Switzerland, 2008; pp. 189–212. ISBN 978-3-7643-7499-0. [Google Scholar]
- Schalch, W.; Landrum, J.T.; Bone, R.A. The eye. In Carotenoids. Volume 5: Nutrition and Health; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser: Basel, Switzerland, 2009; pp. 301–334. ISBN 978-3-7643-7501-0. [Google Scholar]
- Johnson, E.J.; Neuringer, M.; Russell, R.M.; Schalch, W.; Snodderly, D.M. Nutritional manipulation of primate retinas, III: Effects of lutein or zeaxanthin supplementation on adipose tissue and retina of xanthophyll-free monkeys. Investig. Ophthalmol. Vis. Sci. 2005, 46. [Google Scholar] [CrossRef]
- Bhosale, P.; Larson, A.J.; Frederick, J.M.; Southwick, K.; Thulin, C.D.; Bernstein, P.S. Identification and characterization of a Pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthin-binding protein in the macula of the human eye. J. Biol. Chem. 2004, 279, 49447–49454. [Google Scholar] [CrossRef]
- Li, B.; Vachali, P.; Frederick, J.M.; Bernstein, P.S. Identification of StARD3 as a lutein-binding protein in the macula of the primate retina. Biochemistry 2011, 50, 2541–2549. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Landrum, J.T.; Bone, R. a Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu. Rev. Nutr. 2003, 23, 171–201. [Google Scholar] [CrossRef]
- Kopec, R.E.; Schick, J.; Tober, K.L.; Riedl, K.M.; Francis, D.M.; Young, G.S.; Schwartz, S.J.; Oberyszyn, T.M. Sex differences in skin carotenoid deposition and acute UVB-induced skin damage in SKH-1 hairless mice after consumption of tangerine tomatoes. Mol. Nutr. Food Res. 2015, 59, 2491–2501. [Google Scholar] [CrossRef]
- Álvarez, R.; Vaz, B.; Gronemeyer, H.; ALera, Á.R.; Rosana, A.; Gronemeyer, H.; de Lera, A.R. Functions, therapeutic applications, and synthesis of retinoids and carotenoids. Chem. Rev. 2014, 114, 1–125. [Google Scholar] [CrossRef]
- Niki, E. Lipid peroxidation: Physiological levels and dual biological effects. Free Radic. Biol. Med. 2009, 47, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Böhm, F.; Edge, R.; Truscott, G. Interactions of dietary carotenoids with activated (singlet) oxygen and free radicals: Potential effects for human health. Mol. Nutr. Food Res. 2012, 56, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Skibsted, L.H. Carotenoids in Antioxidant Networks. Colorants or Radical Scavengers. J. Agric. Food Chem. 2012, 60, 2409–2417. [Google Scholar] [CrossRef]
- Meinke, M.C.; Friedrich, A.; Tscherch, K.; Haag, S.F.; Darvin, M.E.; Vollert, H.; Groth, N.; Lademann, J.; Rohn, S. Influence of dietary carotenoids on radical scavenging capacity of the skin and skin lipids. Eur. J. Pharm. Biopharm. 2013, 84, 365–373. [Google Scholar] [CrossRef]
- Yeum, K.-J.; Aldini, G.; Russell, R.M.; Krinsky, N.I. Antioxidant/Pro-oxidant Actions of Carotenoids. In Carotenoids. Volume 5. Nutrition and Health2; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser: Basel, Switzerland, 2009; pp. 235–268. [Google Scholar]
- Palozza, P. Prooxidant Actions of Carotenoids in Biologic Systems. Nutr. Rev. 2009, 56, 257–265. [Google Scholar] [CrossRef]
- Ribeiro, D.; Freitas, M.; Silva, A.M.S.; Carvalho, F.; Fernandes, E. Antioxidant and pro-oxidant activities of carotenoids and their oxidation products. Food Chem. Toxicol. 2018, 120, 681–699. [Google Scholar] [CrossRef]
- Grochot-Przeczek, A.; Dulak, J.; Jozkowicz, A. Haem oxygenase-1: Non-canonical roles in physiology and pathology. Clin. Sci. (Lond). 2012, 122, 93–103. [Google Scholar] [CrossRef]
- Malemud, C. Matrix metalloproteinases (MMPs) in health and disease: An overview. Front. Biosci. 2006, 11, 1696–1701. [Google Scholar] [CrossRef] [PubMed]
- Wertz, K.; Hunziker, P.B.; Seifert, N.; Riss, G.; Neeb, M.; Steiner, G.; Hunziker, W.; Goralczyk, R. beta-Carotene interferes with ultraviolet light A-induced gene expression by multiple pathways. J. Invest. Dermatol. 2005, 124, 428–434. [Google Scholar] [CrossRef]
- Marini, A.; Jaenicke, T.; Stahl, W.; Krutmann, J.; Grether-Beck, S.; Marini, A.; Jaenicke, T.; Stahl, W.; Krutmann, J. Molecular evidence that oral supplementation with lycopene or lutein protects human skin against ultraviolet radiation: Results from a double-blinded, placebo-controlled, crossover study. Br. J. Dermatol. 2017, 176, 1231–1240. [Google Scholar] [CrossRef]
- Yuan, J.P.; Peng, J.; Yin, K.; Wang, J.H. Potential health-promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 2011, 55, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Nielsen, M.; Scapagnini, G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef]
- EFSA Scientific Opinion on the re-evaluation of canthaxanthin (E 161 g) as a food additive. EFSA J. 2010, 8, 1852–1893. [CrossRef]
- Heinrich, U.; Gärtner, C.; Wiebusch, M.; Eichler, O.; Sies, H.; Tronnier, H.; Stahl, W. Supplementation with beta-carotene or a similar amount of mixed carotenoids protects humans from UV-induced erythema. J. Nutr. 2003, 133, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Carotenoids and Protection against Solar UV Radiation. Skin Pharmacol. Physiol. 2002, 15, 291–296. [Google Scholar] [CrossRef]
- Köpcke, W.; Krutmann, J. Protection from Sunburn with β-Carotene—A Meta-analysis. Photochem. Photobiol. 2008, 84, 284–288. [Google Scholar] [CrossRef]
- Stahl, W.; Heinrich, U.; Jungmann, H.; Sies, H.; Tronnier, H. Carotenoids and carotenoids plus vitamin E protect against ultraviolet light-induced erythema in humans. Am. Soc. Clin. Nutr. 2000, 71, 795–798. [Google Scholar] [CrossRef]
- Greenberger, S.; Harats, D.; Salameh, F.; Lubish, T.; Harari, A.; Trau, H.; Shaish, A. 9-cis-rich β-carotene powder of the alga Dunaliella reduces the severity of chronic plaque psoriasis: A randomized, double-blind, placebo-controlled clinical trial. J. Am. Coll. Nutr. 2012, 31, 320–326. [Google Scholar] [CrossRef]
- Murillo, E.; Meléndez-Martínez, A.J.; Portugal, F. Screening of vegetables and fruits from Panama for rich sources of lutein and zeaxanthin. Food Chem. 2010, 122, 167–172. [Google Scholar] [CrossRef]
- Rojas-Garbanzo, C.; Gleichenhagen, M.; Heller, A.; Esquivel, P.; Schulze-Kaysers, N.; Schieber, A. Carotenoid Profile, Antioxidant Capacity, and Chromoplasts of Pink Guava (Psidium guajava L. Cv. ’Criolla’) during Fruit Ripening. J. Agric. Food Chem. 2017, 65. [Google Scholar] [CrossRef]
- Beutner, S.; Bloedorn, B.; Frixel, S.; Blanco, I.H.; Hoffmann, T.; Martin, H.D.; Mayer, B.; Noack, P.; Ruck, C.; Schmidt, M.; et al. Quantitative assessment of antioxidant properties of natural colorants and phytochemicals: Carotenoids, flavonoids, phenols and indigoids. The role of β-carotene in antioxidant functions. J. Sci. Food Agric. 2001, 81, 559–568. [Google Scholar] [CrossRef]
- Stahl, W.; Heinrich, U.; Aust, O.; Tronnier, H.; Sies, H. Lycopene-rich products and dietary photoprotection. Photochem. Photobiol. Sci. 2006, 5, 238–242. [Google Scholar] [CrossRef]
- Cooperstone, J.L.; Tober, K.L.; Riedl, K.M.; Teegarden, M.D.; Cichon, M.J.; Francis, D.M.; Schwartz, S.J.; Oberyszyn, T.M. Tomatoes protect against development of UV-induced keratinocyte carcinoma via metabolomic alterations. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Nishino, A.; Sugimoto, K.; Sambe, H.; Ichihara, T.; Takaha, T.; Kuriki, T. Effects of Dietary Paprika Xanthophylls on Ultraviolet Light-Induced Skin Damage: A Double-Blind Placebo-Controlled Study. J. Oleo Sci. 2018, 67, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Mathews Roth, M.M.; Pathak, M.A.; Mathews-Roth, M.M.; Pathak, M.A. Phytoene as a protective agent against sunburn (>280nm) radiation in guinea pigs. Photochem. Photobiol. 1975, 21, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Mathews-Roth, M.M. Antitumor activity of β-carotene, canthaxanthin and phytoene. Oncology 1982, 39, 33–37. [Google Scholar] [CrossRef]
- Von Oppen-Bezalel, L.; Fishbein, D.; Havas, F.; Ben-Chitrit, O.; Khaiat, A. The photoprotective effects of a food supplement tomato powder rich in phytoene and phytofluene, the colorless carotenoids, a preliminary study. Glob. Dermatol. 2015, 2, 178–182. [Google Scholar]
- Von Oppen-Bezalel, L.; Shaish, A. Application of the colorless carotenoids, phytoene, and phytofluene in cosmetics, wellness, nutrition, and therapeutics. In The alga Dunaliella: Biodiversity, Physiology, Genomics & Biotechnology; Ben-Amotz, A., Polle, J., Rao, S., Eds.; Science Publishers: Enfield, NH, USA, 2009; pp. 423–444. ISBN 978-1-5780-8545-3. [Google Scholar]
- Blount, J.D.; McGraw, K.J. Signal functions of carotenoid colouration. In Carotenoids. Volume 4: Natural functions; Gritton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser: Basel, Switzerland, 2008; pp. 213–236. ISBN 978-3-7643-7499-0. [Google Scholar]
- Blount, J.D. Carotenoids and life-history evolution in animals. Arch. Biochem. Biophys. 2004, 430, 10–15. [Google Scholar] [CrossRef]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids: A Colourful History; CaroteNature GmbH: Bern, Switzerland, 2017. [Google Scholar]
- Stephen, I.D.; Coetzee, V.; Perrett, D.I. Carotenoid and melanin pigment coloration affect perceived. Evol. Hum. Behav. 2011, 32, 216–227. [Google Scholar] [CrossRef]
- Lefevre, C.E.; Perrett, D.I.; Lefevre, C.E.; Perrett, D.I. Fruit over sunbed: Carotenoid skin colouration is found more attractive than melanin colouration. Q. J. Exp. Psychol. 2015, 0218, 1–10. [Google Scholar] [CrossRef]
- Pezdirc, K.; Rollo, M.E.M.E.; Whitehead, R.; Hutchesson, M.J.M.J.; Ozakinci, G.; Perrett, D.; Collins, C.E.C.E. Perceptions of carotenoid and melanin colouration in faces among young Australian adults. Aust. J. Psychol. 2017, 70, 85–90. [Google Scholar] [CrossRef]
- Desmedt, B.; Courselle, P.; De Beer, J.O.; Rogiers, V.; Grosber, M.; Deconinck, E.; De Paepe, K. Overview of skin whitening agents with an insight into the illegal cosmetic market in Europe. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 943–950. [Google Scholar] [CrossRef]
- Von Oppen-Bezalel, L. Colorless Carotenoids, phytoene and phytofluene for the skin: For prevention of aging/photo-aging from inside and out. SOFW J. 2007, 133, 1–3. [Google Scholar]
- Von Oppen-Bezalel, L.; Havas, F.; Ramot, O.; Kalo, E.; Fishbein, D.; Ben-Chitrit, O. Phytoene and Phytofluene for (Photo) Protection, Anti Aging, Lightening and Evening of Skin Tone. SOFW J. 2014, 140, 8–12. [Google Scholar]
- CIE Recommendations on Uniform Color Spaces, Color-Difference Equations, Psychometric Color Terms; CIE Publication No. 15 (E-1.3.1) 1971, Supplement 2; Bureau Central de la CIE: Vienna, Austria, 1978.
- Stephen, I.D.; Law Smith, M.J.; Stirrat, M.R.; Perrett, D.I. Facial skin coloration affects perceived health of human faces. Int. J. Primatol. 2009, 30, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.-J.; Hoerr, S.; Levine, R.; Coleman, G. Processes underlying young women’s decisions to eat fruits and vegetables. J. Hum. Nutr. Diet. 2006, 19, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, R.D.; Ozakinci, G.; Stephen, I.D.; Perrett, D.I. Appealing to Vanity: Could Potential Appearance Improvement Motivate Fruit and Vegetable Consumption? Am. J. Public Health 2012, 102, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, R.D.; Ozakinci, G.; Perrett, D.I. Attractive skin coloration: Harnessing sexual selection to improve diet and health. Evol. Psychol. 2012, 10, 842–854. [Google Scholar] [CrossRef] [PubMed]
- WHO. Diet, Nutrition, and the Prevention of Chronic Diseases; Report of the Joint WHO/FAO Expert Consultation WHO Technical Report Series, No. 916 (TRS 916); World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Mahler, H.I.M.; Kulik, J.A.; Gerrard, M.; Gibbons, F.X. Long-term effects of appearance-based interventions on sun protection behaviors. Heal. Psychol. 2007, 26, 350–360. [Google Scholar] [CrossRef]






| Characteristics a | |||
|---|---|---|---|
| Skin type a | Colour b | Sunburn | Tan |
| I | White | Yes | No |
| II | White | Yes | Minimal |
| III | White | Yes | Yes |
| IV | White | No | Yes |
| V | Brown | No | Yes |
| VI | Black | No | Yes |
| Carotenoid | Colour | c.d.b. (in rings) | Absorption maxima (nm) | ||
|---|---|---|---|---|---|
| Canthaxanthin | Red | 13 (4) | 472 | ||
| Astaxanthin * | Red | 13 (4) | 468 | ||
| Lycopene | Red | 11 (0) | 446 | 474 | 504 |
| β-Carotene | Orange | 11 (2) | 454 | 480 | |
| Zeaxanthin | Orange | 11 (2) | 454 | 480 | |
| Lutein | Yellow | 10 (1) | 424 | 448 | 476 |
| Phytofluene * | Colourless | 5 (0) | 331 | 348 | 367 |
| Phytoene * | Colourless | 3 (0) | 286 | ||
| Body Fluid/ Tissue | Phytoene | Phytofluene | Reference |
|---|---|---|---|
| Blood | 0.11 ± 0.01 | 0.30 ± 0.02 | [111] |
| Blood | 0.14 ± 0.08 | 0.14 ± 0.08 | [112] |
| Blood | 0.06 ± 0.04 | 0.33 ± 0.15 | [113] |
| Blood | 0.04 | 0.17 | [114] |
| Breast | 69 | 416 | [115] |
| Cervix | - | 106 | [115] |
| Colon | 70 | 116 | [115] |
| Liver | 168 | 261 | [115] |
| Lung | 1275 | 195 | [115] |
| Milk | 0.002 | 0.016 | [114] |
| Prostate | 45 | 201 | [115] |
| Carotenoid | Tissue | Concentration | Reference |
|---|---|---|---|
| α-Carotene | Abdominal skin | 0.01 | [154] |
| β-Carotene | Epidermis | 0.39 | [11] |
| β-Carotene | Dermis | 0.01 | [11] |
| β-Carotene | Epidermis | 4.1 | [11] |
| β-Carotene | Dermis | 1.3 | [11] |
| β-Carotene | Subcutis | 3.5 | [11] |
| β-Carotene | Surface lipid | 10.0 | [11] |
| β-Carotene | Comedones | 14.5 | [11] |
| β-Carotene | Whole skin | 0.09 | [11] |
| β-Carotene | Whole skin | 1.41 | [11] |
| β-Carotene | Punch biopsy | 8.3 | [11] |
| β-Carotene | Abdominal skin | 0.05 | [154] |
| γ-Carotene | Abdominal skin | 0.04 | [154] |
| ζ-Carotene | Abdominal skin | 0.02 | [154] |
| Lycopene | Abdominal skin | 0.13 | [154] |
| Phytoene | Abdominal skin | 0.12 | [154] |
| Phytoene | Whole skin | 0.12 | [115] |
| Phytofluene | Abdominal skin | 0.03 | [154] |
| Phytofluene | Whole skin | 0.03 | [115] |
| Total carotenoids | Whole skin | 0.17 | [113] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meléndez-Martínez, A.J.; Stinco, C.M.; Mapelli-Brahm, P. Skin Carotenoids in Public Health and Nutricosmetics: The Emerging Roles and Applications of the UV Radiation-Absorbing Colourless Carotenoids Phytoene and Phytofluene. Nutrients 2019, 11, 1093. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11051093
Meléndez-Martínez AJ, Stinco CM, Mapelli-Brahm P. Skin Carotenoids in Public Health and Nutricosmetics: The Emerging Roles and Applications of the UV Radiation-Absorbing Colourless Carotenoids Phytoene and Phytofluene. Nutrients. 2019; 11(5):1093. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11051093
Chicago/Turabian StyleMeléndez-Martínez, Antonio J., Carla M. Stinco, and Paula Mapelli-Brahm. 2019. "Skin Carotenoids in Public Health and Nutricosmetics: The Emerging Roles and Applications of the UV Radiation-Absorbing Colourless Carotenoids Phytoene and Phytofluene" Nutrients 11, no. 5: 1093. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11051093