Exploring Matrix Effects on Binding Properties and Characterization of Cotinine Molecularly Imprinted Polymer on Paper-Based Scaffold
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Cotinine-Imprinted Polymers
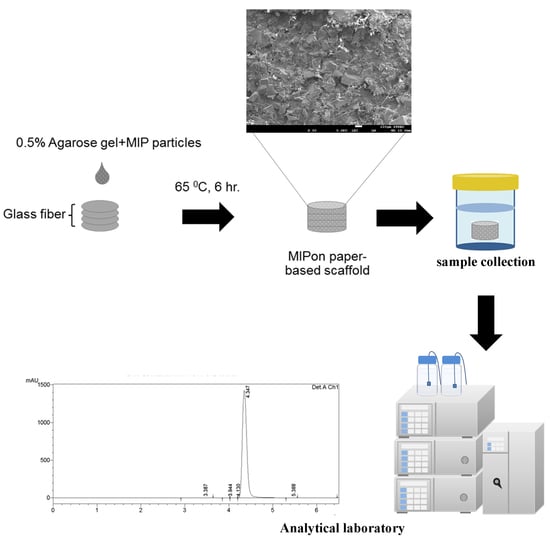
2.3. Preparation of Molecularly Imprinted Polymer (MIP) Paper-Based Scaffolds
2.4. Polymer Characterization
2.4.1. Study of Size and Shape of Synthesized Polymer
2.4.2. Optimization of the Cotinine Binding Experiment
2.4.3. Effect of pH and Salt Ions
2.4.4. Effect of Solvent on Binding
2.4.5. Kinetic and Adsorption Isotherm Experiment
2.4.6. Selectivity of the Synthesized Polymer
2.4.7. Interference Study on Adsorption Capacity of MIP and Recovery Study on Extraction Process
2.4.8. Application and Method Comparison of MIP Paper-Based Scaffold
3. Results
3.1. Morphological Characteristics
3.2. Attenuated Total Reflection Fourier Transform–Infrared (ATR-FT–IR) Analysis
3.3. Adsorption Isotherm and Adsorption Kinetics of Synthesized Polymers
3.4. Scatchard Analysis
3.5. Effect of NaCl and pH on Cotinine Adsorption and Extraction
3.6. Effect of Sample Matrix and Solvent on Cotinine Adsorption on MIP Paper-Based Scaffolds
3.7. Interference Study and Selectivity of Synthesized Polymers
3.8. Application and Comparison of MIP Paper-Based Scaffold
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gilliland, F.; Li, Y.-F.; Peters, J. Effects of Maternal Smoking during Pregnancy and Environmental Tobacco Smoke on Asthma and Wheezing in Children. Am. J. Respir. Crit. Care Med. 2001, 163, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Zevin, S.; Benowitz, N.L. Drug interactions with tobacco smoking. Clin. Pharmacokinet. 1999, 36, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef] [PubMed]
- Howanitz, J.H.; Howanitz, P.J.; Skrodzki, C.A.; Iwanski, J.A. Influences of specimen processing and storage conditions on results for plasma ammonia. Clin. Chem. 1984, 30, 906. [Google Scholar]
- Lee, A.; Gin, T.; Chui, P.T.; Tan, P.E.; Chiu, C.H.; Tam, T.P.; Samy, W. The Accuracy of Urinary Cotinine Immunoassay Test Strip as an Add-on Test to Self-Reported Smoking Before Major Elective Surgery. Nicotine Tob. Res. 2013, 15, 1690–1695. [Google Scholar] [CrossRef]
- Gronkjaer, M.; Eliasen, M.; Skov-Ettrup, L.S.; Tolstrup, J.S.; Christiansen, A.H.; Mikkelsen, S.S.; Becker, U.; Flensborg-Madsen, T. Preoperative smoking status and postoperative complications: a systematic review and meta-analysis. Ann. Surg. 2014, 259, 52–71. [Google Scholar] [CrossRef] [PubMed]
- SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob. Res. 2002, 4, 149–159. [Google Scholar] [CrossRef]
- Asha, V.; Dhanya, M. Immunochromatographic assessment of salivary cotinine and its correlation with nicotine dependence in tobacco chewers. J. Cancer Prev. 2015, 20, 159. [Google Scholar]
- Gonzalez, J.M.; Foley, M.W.; Bieber, N.M.; Bourdelle, P.A.; Niedbala, R.S. Development of an ultrasensitive immunochromatography test to detect nicotine metabolites in oral fluids. Anal. Bioanal. Chem. 2011, 400, 3655–3664. [Google Scholar] [CrossRef]
- Bernert, J.T.; Harmon, T.L.; Sosnoff, C.S.; McGuffey, J.E. Use of cotinine immunoassay test strips for preclassifying urine samples from smokers and nonsmokers prior to analysis by LC-MS-MS. J. Anal. Toxicol. 2005, 29, 814–818. [Google Scholar] [CrossRef]
- Langone, J.J.; Gjika, H.B.; Van Vunakis, H. Nicotine and its metabolites. Radioimmunoassays for nicotine and cotinine. Biochemistry 1973, 12, 5025–5030. [Google Scholar] [CrossRef] [PubMed]
- Knight, G.; Wylie, P.; Holman, M.; Haddow, J. Improved 125I radioimmunoassay for cotinine by selective removal of bridge antibodies. Clin. Chem. 1985, 31, 118–121. [Google Scholar] [PubMed]
- Bjercke, R.J.; Cook, G.; Rychlik, N.; Gjika, H.B.; Van Vunakis, H.; Langone, J.J. Stereospecific monoclonal antibodies to nicotine and cotinine and their use in enzyme-linked immunosorbent assays. J. Immunol. Methods 1986, 90, 203–213. [Google Scholar] [CrossRef]
- Ziegler, U.E.; Kauczok, J.; Dietz, U.A.; Reith, H.B.; Schmidt, K. Clinical correlation between the consumption of nicotine and cotinine concentrations in urine and serum by competitive enzyme-linked immunosorbent assay. Pharmacology 2004, 72, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Wielkoszyński, T.; Tyrpień, K.; Szumska, M. The enzyme-linked immunosorbent assay (ELISA) method for nicotine metabolites determination in biological fluids. J. Pharm. Biomed. Anal. 2009, 49, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Cope, G.; Nayyar, P.; Wilkinson, L.; Holder, R. Simple colorimetric test to quantify exposure to environmental tobacco smoke: occupational health study. Ann. Clin. Biochem. 2000, 37, 795–796. [Google Scholar] [CrossRef]
- Smith, R.F.; Mather, H.M.; Ellard, G.A. Assessment of simple colorimetric procedures to determine smoking status of diabetic subjects. Clin. Chem. 1998, 44, 275. [Google Scholar]
- Barlow, R.D.; Stone, R.B.; Wald, N.J.; Puhakainen, E.V. The direct barbituric acid assay for nicotine metabolites in urine: a simple colorimetric test for the routine assessment of smoking status and cigarette smoke intake. Clin. Chim. Acta 1987, 165, 45–52. [Google Scholar] [CrossRef]
- Russell, S.M.; Doménech-Sánchez, A.; de la Rica, R. Augmented Reality for Real-Time Detection and Interpretation of Colorimetric Signals Generated by Paper-Based Biosensors. ACS Sens. 2017, 2, 848–853. [Google Scholar] [CrossRef]
- Jacob, P., III; Wilson, M.; Benowitz, N.L. Improved gas chromatographic method for the determination of nicotine and cotinine in biologic fluids. J. Chromatogr. B Biomed. Sci. Appl. 1981, 222, 61–70. [Google Scholar] [CrossRef]
- Curvall, M.; Kazemi-Vala, E.; Enzell, C.R. Simultaneous determination of nicotine and cotinine in plasma using capillary column gas chromatography with nitrogen-sensitive detection. J. Chromatogr. B Biomed. Sci. Appl. 1982, 232, 283–293. [Google Scholar] [CrossRef]
- Greaves, R.; Trotter, L.; Brennecke, S.; Janus, E. A simple high-pressure liquid chromatography cotinine assay: validation of smoking status in pregnant women. Ann. Clin. Biochem. 2001, 38, 333–338. [Google Scholar] [CrossRef]
- Hariharan, M.; VanNoord, T.; Greden, J.F. A high-performance liquid-chromatographic method for routine simultaneous determination of nicotine and cotinine in plasma. Clin. Chem. 1988, 34, 724–729. [Google Scholar] [PubMed]
- Bernert, J.T.; Turner, W.E.; Pirkle, J.L.; Sosnoff, C.S.; Akins, J.R.; Waldrep, M.K.; Ann, Q.; Covey, T.R.; Whitfield, W.E.; Gunter, E.W. Development and validation of sensitive method for determination of serum cotinine in smokers and nonsmokers by liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Clin. Chem. 1997, 43, 2281–2291. [Google Scholar] [PubMed]
- Kataoka, H.; Inoue, R.; Yagi, K.; Saito, K. Determination of nicotine, cotinine, and related alkaloids in human urine and saliva by automated in-tube solid-phase microextraction coupled with liquid chromatography–mass spectrometry. J. Pharm. Biomed. Anal. 2009, 49, 108–114. [Google Scholar] [CrossRef]
- Mitra, S.; Brukh, R. Sample Preparation: An Analytical Perspective. In Sample Preparation Techniques in Analytical Chemistry; Mitra, S., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2003. [Google Scholar] [CrossRef]
- Pietsch, J.; Günther, J.; Henle, T.; Dreßler, J. Simultaneous determination of thirteen plant alkaloids in a human specimen by SPE and HPLC. J. Sep. Sci. 2008, 31, 2410–2416. [Google Scholar] [CrossRef]
- Mohamed, R.; Richoz-Payot, J.; Gremaud, E.; Mottier, P.; Yilmaz, E.; Tabet, J.-C.; Guy, P.A. Advantages of Molecularly Imprinted Polymers LC-ESI-MS/MS for the Selective Extraction and Quantification of Chloramphenicol in Milk-Based Matrixes. Comparison with a Classical Sample Preparation. Anal. Chem. 2007, 79, 9557–9565. [Google Scholar] [CrossRef] [PubMed]
- Ostovan, A.; Ghaedi, M.; Arabi, M.; Yang, Q.; Li, J.; Chen, L. Hydrophilic Multitemplate Molecularly Imprinted Biopolymers Based on a Green Synthesis Strategy for Determination of B-Family Vitamins. ACS Appl. Mater. Interfaces 2018, 10, 4140–4150. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef]
- Haupt, K.; Mosbach, K. Molecularly imprinted polymers and their use in biomimetic sensors. Chem. Rev. 2000, 100, 2495–2504. [Google Scholar] [CrossRef]
- Suwanwong, Y.; Kulkeratiyut, S.; Prachayasittikul, V.; Boonpangrak, S. Effects of Polymerization Methods and Functional Monomers on Curcumin Imprinted Polymer Properties. Sep. Sci. Technol. 2014, 49, 1086–1095. [Google Scholar] [CrossRef]
- Zander, Å.; Findlay, P.; Renner, T.; Sellergren, B.; Swietlow, A. Analysis of nicotine and its oxidation products in nicotine chewing gum by a molecularly imprinted solid-phase extraction. Anal. Chem. 1998, 70, 3304–3314. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Tian, X.-G.; Cai, L.-G.; Xu, Z.-L.; Lei, H.-T.; Wang, H.; Sun, Y.-M. Molecularly imprinted polymer based surface plasmon resonance sensors for detection of Sudan dyes. Anal. Methods 2014, 6, 3751–3757. [Google Scholar] [CrossRef]
- Hsu, H.-C.; Chen, L.-C.; Ho, K.-C. Colorimetric detection of morphine in a molecularly imprinted polymer using an aqueous mixture of Fe3+ and [Fe(CN)6]3−. Anal. Chim. Acta 2004, 504, 141–147. [Google Scholar] [CrossRef]
- Kostrewa, S.; Emgenbroich, M.; Klockow, D.; Wulff, G. Surface-Enhanced Raman Scattering on Molecularly Imprinted Polymers in Water. Macromol. Chem. Phys. 2003, 204, 481–487. [Google Scholar] [CrossRef]
- Kamra, T.; Zhou, T.; Montelius, L.; Schnadt, J.; Ye, L. Implementation of Molecularly Imprinted Polymer Beads for Surface Enhanced Raman Detection. Anal. Chem. 2015, 87, 5056–5061. [Google Scholar] [CrossRef]
- Yu, J.; Wan, F.; Zhang, C.; Yan, M.; Zhang, X.; Wang, S. Molecularly imprinted polymeric microspheres for determination of bovine serum albumin based on flow injection chemiluminescence sensor. Biosens. Bioelectron. 2010, 26, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Wang, S.; Yu, J.; Li, N.; Ge, S.; Yan, M. Molecularly Imprinted Polymer Grafted Porous Au-Paper Electrode for an Microfluidic Electro-Analytical Origami Device. Adv. Funct. Mater. 2013, 23, 3115–3123. [Google Scholar] [CrossRef]
- Mendes, T.P.P.; Pereira, I.; Ferreira, M.R.; Chaves, A.R.; Vaz, B.G. Molecularly imprinted polymer-coated paper as a substrate for highly sensitive analysis using paper spray mass spectrometry: quantification of metabolites in urine. Anal. Methods 2017, 9, 6117–6123. [Google Scholar] [CrossRef]
- Yang, Q.; Li, J.; Wang, X.; Peng, H.; Xiong, H.; Chen, L. Strategies of molecular imprinting-based fluorescence sensors for chemical and biological analysis. Biosens. Bioelectron 2018, 112, 54–71. [Google Scholar] [CrossRef]
- Wang, P.; Sun, G.; Ge, L.; Ge, S.; Yu, J.; Yan, M. Photoelectrochemical lab-on-paper device based on molecularly imprinted polymer and porous Au-paper electrode. Analyst 2013, 138, 4802–4811. [Google Scholar] [CrossRef]
- Zarejousheghani, M.; Schrader, S.; Möder, M.; Mayer, T.; Borsdorf, H. Negative electrospray ionization ion mobility spectrometry combined with paper-based molecular imprinted polymer disks: A novel approach for rapid target screening of trace organic compounds in water samples. Talanta 2018, 190, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Zhou, L.; Zhang, H.; Liu, L.; Gong, Z.-Y. A molecularly imprinted polymers/carbon dots-grafted paper sensor for 3-monochloropropane-1,2-diol determination. Food Chem. 2019, 274, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, Z.; Qi, J.; Zhou, N.; Qin, S.; Choo, J.; Chen, L. Quantum Dot-Based Molecularly Imprinted Polymers on Three-Dimensional Origami Paper Microfluidic Chip for Fluorescence Detection of Phycocyanin. ACS Sens. 2017, 2, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ge, L.; Li, L.; Yan, M.; Ge, S.; Yu, J. Molecularly imprinted polymer grafted paper-based multi-disk micro-disk plate for chemiluminescence detection of pesticide. Biosens. Bioelectron. 2013, 50, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Guo, Y.; Luo, J.; Kou, J.; Zheng, H.; Li, B.; Zhang, Z. A molecularly imprinted polymer based a lab-on-paper chemiluminescence device for the detection of dichlorvos. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 141, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Zhang, Z.; Wu, C.; Han, L.; Zhang, H. Molecularly imprinted polymer grafted paper-based method for the detection of 17β-estradiol. Food Chem. 2017, 221, 82–86. [Google Scholar] [CrossRef]
- Ren, K.; Zhou, J.; Wu, H. Materials for microfluidic chip fabrication. Acc. Chem. Res. 2013, 46, 2396–2406. [Google Scholar] [CrossRef]
- Tang, R.H.; Yang, H.; Choi, J.R.; Gong, Y.; Feng, S.S.; Pingguan-Murphy, B.; Huang, Q.S.; Shi, J.L.; Mei, Q.B.; Xu, F. Advances in paper-based sample pretreatment for point-of-care testing. Crit. Rev. Biotechnol. 2017, 37, 411–428. [Google Scholar] [CrossRef]
- Chow, S.C.; McConkey, D.J.; Orrenius, S.; Jondal, M. Quantitation of DNA fragmentation using fiberglass filters. Anal. Biochem. 1989, 183, 42–45. [Google Scholar] [CrossRef]
- Faraji, M.; Pourpak, Z.; Naddafi, K.; Nodehi, R.N.; Nicknam, M.H.; Shamsipour, M.; Rezaei, S.; Ghozikali, M.G.; Ghanbarian, M.; Mesdaghinia, A. Effects of airborne particulate matter (PM10) from dust storm and thermal inversion on global DNA methylation in human peripheral blood mononuclear cells (PBMCs) in vitro. Atmos. Environ. 2018, 195, 170–178. [Google Scholar] [CrossRef]
- de Almeida, O.N.; Luzardo, F.H.M.; Amorim, F.A.C.; Velasco, F.G.; González, L.N. Use of fiberglass support in the application of dried-spot technique with dispersion liquid-liquid microextraction for the determination of Co, Cr, Cu, Ni and Pb by Energy Dispersive X-Ray Fluorescence Spectrometry. Spectrochim. Acta B 2018, 150, 92–98. [Google Scholar] [CrossRef]
- Qi, J.; Li, B.; Wang, X.; Fu, L.; Luo, L.; Chen, L. Rotational Paper-Based Microfluidic-Chip Device for Multiplexed and Simultaneous Fluorescence Detection of Phenolic Pollutants Based on a Molecular-Imprinting Technique. Anal. Chem. 2018, 90, 11827–11834. [Google Scholar] [CrossRef]
- Sajid, M.; Kawde, A.-N.; Daud, M. Designs, formats and applications of lateral flow assay: A literature review. J. Saudi Chem. Soc. 2015, 19, 689–705. [Google Scholar] [CrossRef]
- Yang, J.; Hu, Y.; Cai, J.-B.; Zhu, X.-L.; Su, Q.-D. A new molecularly imprinted polymer for selective extraction of cotinine from urine samples by solid-phase extraction. Anal. Bioanal. Chem. 2006, 384, 761–768. [Google Scholar] [CrossRef]
- Demirbas, E.; Dizge, N.; Sulak, M.T.; Kobya, M. Adsorption kinetics and equilibrium of copper from aqueous solutions using hazelnut shell activated carbon. Chem. Eng. J. 2009, 148, 480–487. [Google Scholar] [CrossRef]
- Song, X.; Li, J.; Wang, J.; Chen, L. Quercetin molecularly imprinted polymers: Preparation, recognition characteristics and properties as sorbent for solid-phase extraction. Talanta 2009, 80, 694–702. [Google Scholar] [CrossRef]
- Long, J.-P.; Chen, Z.-B.; Liu, X.-J.; Du, X.-Y. Preparation and adsorption property of solanesol molecular imprinted polymers. Des. Monomers Polym. 2015, 18, 641–649. [Google Scholar] [CrossRef]
- Huang, C.; Hu, B. Silica-coated magnetic nanoparticles modified with γ-mercaptopropyltrimethoxysilane for fast and selective solid phase extraction of trace amounts of Cd, Cu, Hg, and Pb in environmental and biological samples prior to their determination by inductively coupled plasma mass spectrometry. Spectrochim. Acta B 2008, 63, 437–444. [Google Scholar]
- Pluen, A.; Netti, P.A.; Jain, R.K.; Berk, D.A. Diffusion of Macromolecules in Agarose Gels: Comparison of Linear and Globular Configurations. Biophys. J. 1999, 77, 542–552. [Google Scholar] [CrossRef]
- Zucca, P.; Fernandez-Lafuente, R.; Sanjust, E. Agarose and Its Derivatives as Supports for Enzyme Immobilization. Molecules 2016, 21, 1577. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, R.; Ma, Y.; Zheng, L.; Peng, A.; Fu, H.; Yao, J. Transparent and flexible phosphomolybdate–agarose composite thin films with visible-light photochromism. J. Mater. Chem. 2010, 20, 1107–1111. [Google Scholar] [CrossRef]
- Richardson, J.J.; Liang, K.; Kempe, K.; Ejima, H.; Cui, J.; Caruso, F. Immersive Polymer Assembly on Immobilized Particles for Automated Capsule Preparation. Adv. Mater. 2013, 25, 6874–6878. [Google Scholar] [CrossRef]
- Hashemi, P.; Zarjani, R.A.; Abolghasemi, M.M.; Olin, Å. Agarose film coated glass slides for preparation of pH optical sensors. Sens. Actuators B Chem. 2007, 121, 396–400. [Google Scholar] [CrossRef]
- Choi, J.R.; Yong, K.W.; Tang, R.; Gong, Y.; Wen, T.; Yang, H.; Li, A.; Chia, Y.C.; Pingguan-Murphy, B.; Xu, F. Lateral Flow Assay Based on Paper-Hydrogel Hybrid Material for Sensitive Point-of-Care Detection of Dengue Virus. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, J.; Zhang, Y.; Zhou, X.; Liu, S. Application of Molecularly Imprinted Polymers to Selective Removal of Clofibric Acid from Water. PLoS ONE 2013, 8, e78167. [Google Scholar] [CrossRef]
- Yu, L.; Yun, Y.; Zhang, W.; Wang, L. Preparation, recognition characteristics and properties for quercetin molecularly imprinted polymers. Desalin. Water Treat. 2011, 34, 309–314. [Google Scholar] [CrossRef]
- Vasapollo, G.; Sole, R.D.; Mergola, L.; Lazzoi, M.R.; Scardino, A.; Scorrano, S.; Mele, G. Molecularly imprinted polymers: present and future prospective. Int. J. Mol. Sci. 2011, 12, 5908–5945. [Google Scholar] [CrossRef]
- Kempe, H.; Kempe, M. Influence of salt ions on binding to molecularly imprinted polymers. Anal. Bioanal. Chem. 2010, 396, 1599–1606. [Google Scholar] [CrossRef]
- Matsunaga, T.; Hishiya, T.; Takeuchi, T. Surface plasmon resonance sensor for lysozyme based on molecularly imprinted thin films. Anal. Chim. Acta 2007, 591, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Massadeh, A.M.; Gharaibeh, A.A.; Omari, K.W. A single-step extraction method for the determination of nicotine and cotinine in Jordanian smokers’ blood and urine samples by RP-HPLC and GC-MS. J. Chromatogr. Sci. 2009, 47, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Rabbaa-Khabbaz, L.; Abi Daoud, R.; Karam-Sarkis, D. A simple, sensitive, and rapid method for the determination of cotinine in urine by high-performance liquid chromatography with UV detection. J. Chromatogr. Sci. 2006, 44, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, O.; Xu, Y.; Goodacre, R. Simultaneous multiplexed quantification of nicotine and its metabolites using surface enhanced Raman scattering. Analyst 2014, 139, 4820–4827. [Google Scholar] [CrossRef] [PubMed]
- Caro, E.; Marcé, R.M.; Borrull, F.; Cormack, P.A.G.; Sherrington, D.C. Application of molecularly imprinted polymers to solid-phase extraction of compounds from environmental and biological samples. Trends Anal. Chem. 2006, 25, 143–154. [Google Scholar] [CrossRef]
- Horemans, F.; Weustenraed, A.; Spivak, D.; Cleij, T.J. Towards water compatible MIPs for sensing in aqueous media. J. Mol. Recognit. 2012, 25, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, C.; Bravo, C.; Rivas, B.; Moczko, E.; Sáez, P.; García, Y.; Pereira, E. Molecularly Imprinted Polymers for the Selective Extraction of Bisphenol A and Progesterone from Aqueous Media. Polymers 2018, 10, 679. [Google Scholar] [CrossRef]
- Hecht, S.S. Biochemistry, Biology, and Carcinogenicity of Tobacco-Specific N-Nitrosamines. Chem. Res. Toxicol. 1998, 11, 559–603. [Google Scholar] [CrossRef]
- Pagana, K.D.; Pagana, T.J. Mosby’s Manual of Diagnostic and Laboratory Tests-E-Book; Elsevier Health Sciences: St. Louis, MI, USA, 2013; p. 936. [Google Scholar]









| Urine Matrix | Water | |||
|---|---|---|---|---|
| Cotinine: nicotine (2:1) | Cotinine: nicotine (1:1) | Cotinine: nicotine (2:1) | Cotinine: nicotine (1:1) | |
| MIP | 88 ± 0.77% | 68 ± 0.85% | 71 ± 1.6% | 70 ± 1.0% |
| NIP | 72 ± 2.6% | 77 + 3.0% | 41 ± 4.0% | 50 ± 4.8% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larpant, N.; Suwanwong, Y.; Boonpangrak, S.; Laiwattanapaisal, W. Exploring Matrix Effects on Binding Properties and Characterization of Cotinine Molecularly Imprinted Polymer on Paper-Based Scaffold. Polymers 2019, 11, 570. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11030570
Larpant N, Suwanwong Y, Boonpangrak S, Laiwattanapaisal W. Exploring Matrix Effects on Binding Properties and Characterization of Cotinine Molecularly Imprinted Polymer on Paper-Based Scaffold. Polymers. 2019; 11(3):570. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11030570
Chicago/Turabian StyleLarpant, Nutcha, Yaneenart Suwanwong, Somchai Boonpangrak, and Wanida Laiwattanapaisal. 2019. "Exploring Matrix Effects on Binding Properties and Characterization of Cotinine Molecularly Imprinted Polymer on Paper-Based Scaffold" Polymers 11, no. 3: 570. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11030570