Identification and Characterization of Carboxylesterases from Brachypodium distachyon Deacetylating Trichothecene Mycotoxins
Abstract
:1. Introduction
2. Results and Discussion
2.1. Brachypodium distachyon Deacetylates 3-ADON and 15-ADON

2.2. The CXE Gene Family of B. Distachyon
| Gene Name | Gene Locus | * | Predicted cDNA Length (Bases) | Predicted Introns | Conserved Site Changed | Confidence Class | Results RT-Assay |
|---|---|---|---|---|---|---|---|
| BdCXE1 | (Bradi1g06240.1) | - | 1557 | 2 | - | 3 | constitutive |
| Bradi1g06241.1 ‡ | - | 1014 | 0 | - | - | ||
| BdCXE2 | Bradi1g17780.1 | - | 1038 | 0 | - | 5 | <LOD |
| BdCXE3 | (Bradi1g19715.1) | ↓ | 1935 | 3 | - | 3 | n.t. |
| BdCXE3a | Bradi1g19713.1 ‡ | ↓ | 1032 | 0 | - | - | n.t. |
| BdCXE3b | Bradi1g19716.1 ‡ | ↓ | 1068 | 0 | H316del | - | n.t. |
| BdCXE4 | Bradi1g19720.1 | ↓ | 1083 | 0 | - | 5 | n.t. |
| BdCXE5 | Bradi1g19730.1 | ↓ | 1047 | 0 | - | 4 | low constitutive expression |
| BdCXE6 | (Bradi1g19750.1) | ↓ | 1083 | 0 | - | 5 | constitutive |
| Bradi1g19750.2 ‡ | ↓ | 1233 | 1 | - | - | constitutive | |
| BdCXE7 | Bradi1g19760.1 | ↑ | 1011 | 0 | - | 4 | <LOD |
| BdCXE8 | Bradi1g21890.1 | - | 1038 | 0 | - | 5 | n.t. |
| BdCXE9 | Bradi1g38325.1 ‡ | - | 1053 | 0 | - | - | - |
| BdCXE10a | (Bradi1g45925.1) | ↓ | 942 | 0 | - | 5 | n.t. |
| BdCXE10b | Bradi1g45921.1 ‡ | ↓ | 2643 | 11 | - | - | - |
| BdCXE11 | Bradi1g45930.1 | ↑ | 987 | 0 | - | 5 | repressed |
| BdCXE12 | Bradi1g45945.1 | - | 924 | 0 | - | 4 | n.t. |
| BdCXE13 | Bradi1g45960.1 | - | 999 | 0 | - | 5 | induced |
| BdCXE14 | Bradi1g48173.1 ‡ | - | 1362 | 3 | G207S | - | - |
| BdCXE15 | Bradi1g48203.1 ‡ | - | 1107 | 1 | - | - | - |
| BdCXE16 | Bradi1g50705.1 ‡ | - | 1293 | 1 | - | - | - |
| BdCXE17 | Bradi1g56807.1 | ↑ | 1008 | 0 | - | 4 | n.t. |
| BdCXE18 | (Bradi1g56817.1) | ↑ | 984 | 0 | H296R | 3 | n.t. |
| Bradi1g56817.2 ‡ | ↑ | 990 | 0 | - | - | n.t. | |
| BdCXE19 | Bradi1g56830.1 | ↑ | 1086 | 0 | - | 4 | <LOD |
| BdCXE20 | Bradi1g56860.1 | ↓ | 1092 | 0 | - | 4 | repressed |
| BdCXE21 | Bradi1g56870.1 | ↑ | 1017 | 0 | - | 5 | low expression, repressed |
| BdCXE22 | Bradi1g56910.1 | - | 1038 | 0 | - | 4 | <LOD |
| BdCXE23 | Bradi1g67600.1 | - | 1116 | 0 | - | 5 | <LOD |
| BdCXE24 | Bradi1g67930.1 | - | 999 | 0 | S164H, H293Y | 5 | <LOD |
| BdCXE25 | Bradi1g74240.1 | - | 984 | 0 | D296C | 5 | n.t. |
| BdCXE26 | Bradi2g01817.1 | - | 987 | 0 | - | 4 | n.t. |
| BdCXE27 | Bradi2g25470.1 | - | 1062 | 0 | - | 5 | induced |
| BdCXE28 | Bradi2g25600.1 | - | 1068 | 1 | H328I | 5 | constitutive |
| BdCXE29 | Bradi2g27300.1 | - | 1026 | 0 | - | 5 | constitutive |
| BdCXE30 | Bradi2g57920.1 | - | 1209 | 1 | - | 5 | <LOD |
| BdCXE31 | Bradi3g21747.1 | - | 1053 | 0 | - | 4 | n.t. |
| BdCXE32 | Bradi3g38040.1 | ↑ | 984 | 0 | - | 5 | constitutive |
| BdCXE33 | Bradi3g38045.1 | ↑ | 990 | 0 | - | 5 | n.t. |
| BdCXE34 | Bradi3g38050.1 | ↑ | 951 | 0 | - | 5 | n.t. |
| BdCXE35 | Bradi3g38060.1 | ↓ | 1113 | 0 | - | 5 | n.t. |
| BdCXE36 | Bradi3g38070.1 | ↓ | 1002 | 0 | - | 5 | n.t. |
| BdCXE37 | Bradi3g38080.1 | ↑ | 1119 | 0 | - | 5 | <LOD |
| BdCXE38 | (Bradi3g38090.1) | ↓ | 1116 | 0 | - | 5 | <LOD |
| Bradi3g38090.2 ‡ | ↓ | 1257 | 0 | - | - | <LOD | |
| BdCXE39 | Bradi3g42207.1 | - | 1089 | 0 | H311F | 4 | n.t. |
| BdCXE40 | Bradi3g46450.1 | ↓ | 951 | 0 | - | 4 | constitutive |
| BdCXE41 | Bradi3g46460.1 | ↑ | 957 | 0 | - | 4 | <LOD |
| BdCXE42 | Bradi4g12370.1 | - | 1032 | 0 | - | 4 | n.t. |
| (Bradi4g12370.1) ‡ | - | 957 | 0 | D263del | - | n.t. | |
| BdCXE43 | Bradi4g21690.1 | - | 1065 | 0 | - | 5 | <LOD |
| BdCXE44 | Bradi4g21700.1 | - | 1047 | 0 | - | 5 | <LOD |
| BdCXE45 | Bradi4g24773.1 ‡ | - | 1380 | 0 | - | - | n.t. |
| BdCXE46 | Bradi4g32080.1 | - | 966 | 0 | - | 5 | repressed |
| Bradi4g32080.2 ‡ | - | 1113 | 0 | - | - | repressed | |
| BdCXE47 | Bradi4g32300.1 | ↓ | 1221 | 0 | - | 5 | repressed |
| BdCXE48 | Bradi4g32310.1 | ↑ | 1071 | 0 | - | 5 | n.t. |
| BdCXE49 | Bradi4g32320.1 | ↓ | 954 | 0 | - | 5 | low constitutive expression |
| BdCXE50 | (Bradi4g32330.1) | ↓ | 969 | 0 | D264C | 5 | repressed |
| Bradi4g32330.2 ‡ | ↓ | 1110 | 0 | D311C | repressed | ||
| BdCXE51 | Bradi4g32340.1 | ↓ | 1149 | 0 | - | 5 | constitutive |
| BdCXE52 | Bradi4g32350.1 | ↑ | 1002 | 0 | - | 5 | low constitutive expression |
| BdCXE53 | Bradi4g32360.1 | ↓ | 972 | 0 | - | 5 | constitutive |
| BdCXE54 | Bradi4g39410.1 | - | 1062 | 0 | HGGdel | 3 | <LOD |
| Bradi4g39410.2 ‡ | - | 1311 | 1 | - | - | <LOD | |
| BdCXE55 | Bradi5g08900.1 | - | 1041 | 0 | - | 4 | n.t. |
| BdCXE56 | Bradi5g11800.1 | - | 942 | 0 | - | 5 | n.t. |
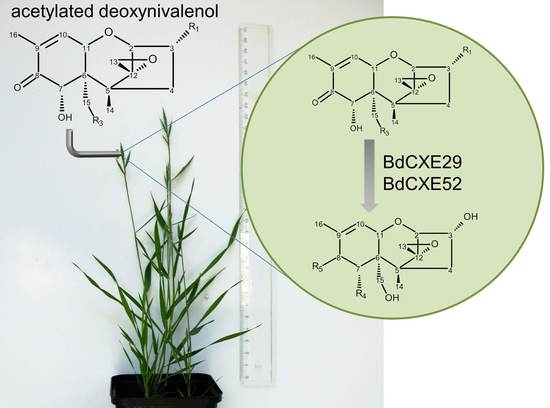
2.3. Screening for BdCXEs Acting on Trichothecenes

| Substrate | Product | BdCXE29 | BdCXE52 | ||
|---|---|---|---|---|---|
| 2 h | 24 h | 2 h | 24 h | ||
| 3-acetyldeoxynivalenol | deoxynivalenol | 77% | 100% | 5% | 34% |
| 15-acetyldeoxynivalenol | deoxynivalenol | 70% | 100% | 0% | 4% |
| T-2 toxin | HT-2 toxin | 100% | 100% | 0% | 0% |
| trichothecin | trichothecolone | 0% | 0% | 0% | 0% |
| HT-2 toxin | T-2 triol | 0% | 0% | 0% | 0% |
| fusarenon X | nivalenol | <1% | 4% | 0% | <1% |
| NX-2 | NX-3 | 91% | 100% | 3% | 17% |
 | R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|---|
| 3-acetyldeoxynivalenol | –OAc | –H | –OH | –OH | =O |
| 15-acetyldeoxynivalenol | –OH | –H | –OAc | –OH | =O |
| deoxynivalenol | –OH | –H | –OH | –OH | =O |
| trichothecin | –H | –OIsocrot | –CH3 | –H | =O |
| trichothecolone | –H | –OH | –CH3 | –H | =O |
| T-2 toxin | –OH | –OAc | –OAc | –H | –OIsoval |
| HT-2 toxin | –OH | –OH | –OAc | –H | –OIsoval |
| T-2 triol | –OH | –OH | –OH | –H | –OIsoval |
| fusarenon X | –OH | –OAc | –OH | –OH | =O |
| nivalenol | –OH | –OH | –OH | –OH | =O |
| NX-2 | –OAc | –H | –OH | –OH | –H |
| NX-3 | –OAc | –H | –OH | –OH | –H |
2.4. Characterization of Purified BdCXE29
| Substrate | Kinetic Constant | |||
|---|---|---|---|---|
| Km (µM) | Vmax (µmol/min/mg) | R2 | Ki (µM) | |
| 4-nitrophenylacetate | 1860 ± 288 | 48 ± 4 | 0.988 | - |
| 4-nitrophenylbutyrate | 5530 ± 757 | 1.7 ± 0.2 | 0.996 | - |
| 15-acetyl-deoxynivalenol | 421 ± 70 | 3.4 ± 0.2 | 0.973 | - |
| 3-acetyl-deoxynivalenol | 89 ± 26 | 1.6 ± 0.2 | 0.939 | 1800 ± 490 |

2.5. CXE Family of Crop Species and A. thaliana
3. Experimental Section
3.1. Chemicals and Reagents
3.2. B. distachyon Suspension Culture Assay
3.3. RNA Extraction, cDNA Synthesis and RT-PCR
3.4. Cloning, Heterologous Expression in Yeast: Phenotypic Testing and Protein Extract Assays
3.5. Cloning, Heterologous Expression and Purification from E. coli
3.6. Enzyme Assays
3.7. Liquid Chromatographic-Tandem Mass Spectrometric Conditions
| Analyte | Precursor Ion (DP) | Quantifier (CE; CXP) | Qualifier (CE; CXP) |
|---|---|---|---|
| deoxynivalenol | 355.1 (−40) | 59.2 (−40; −8) | 265.2 (−22; −13) |
| 3-acetyl-deoxynivalenol | 397.3 (−40) | 59.2 (−38; −8) | 307.1 (−20; −7) |
| 15-acetyl-deoxynivalenol | 397.3 (−40) | 59.2 (−38; −8) | 337.1 (−10; −7) |
| nivalenol | 371.1 (−45) | 59.1 (−42; −7) | 281.1 (−22; −15) |
| fusarenon X | 413.3.(−40) | 59.1 (−40; −9) | 262.9 (−22; −16) |
| HT-2 toxin | 442.2 (46) | 263.1 (21; 19) | - |
| 447.4 (101) | - | 345.1 (27; 20) | |
| T-2 toxin | 484.3 (56) | 215.2 (29; 18) | 185.1 (31; 11) |
| T-2 tetraol | 316.2 (31) | 215.3 (13; 16) | 281.4 (13; 8) |
| T-2 triol | 400.2 (41) | 215.2 (17; 12) | 281.3 (13; 16) |
| NX-2 | 383.1 (−45) | 59.0 (−36; −7) | 323 (−14; −7) |
| NX-3 | 341.1 (−50) | 59.0 (−36; −7) | 281 (−14; −7) |
3.8. In Vitro Translation Assays
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Brkljacic, J.; Grotewold, E.; Scholl, R.; Mockler, T.; Garvin, D.F.; Vain, P.; Brutnell, T.; Sibout, R.; Bevan, M.; Budak, H.; et al. Brachypodium as a Model for the Grasses: Today and the Future. Plant Physiol. 2011, 157, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Pasquet, J.-C.; Chaouch, S.; Macadré, C.; Balzergue, S.; Huguet, S.; Martin-Magniette, M.-L.; Bellvert, F.; Deguercy, X.; Thareau, V.; Heintz, D.; et al. Differential gene expression and metabolomic analyses of Brachypodium distachyon infected by deoxynivalenol producing and non-producing strains of Fusarium graminearum. BMC Genom. 2014, 15, 629. [Google Scholar] [CrossRef] [PubMed]
- Peraldi, A.; Beccari, G.; Steed, A.; Nicholson, P. Brachypodium distachyon: A new pathosystem to study Fusarium head blight and other Fusarium diseases of wheat. BMC Plant Biol. 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, W.; Pasquet, J.-C.; Nussbaumer, T.; Paris, M.P.K.; Wiesenberger, G.; Macadré, C.; Ametz, C.; Berthiller, F.; Lemmens, M.; Saindrenan, P.; et al. Functional Characterization of Two Clusters of Brachypodium distachyon UDP-Glycosyltransferases Encoding Putative Deoxynivalenol Detoxification Genes. Mol. Plant-Microbe Interact. 2013, 26, 781–792. [Google Scholar] [CrossRef] [PubMed]
- McMullen, M.; Jones, R.; Gallenberg, D. Scab of wheat and barley: A re-emerging disease of devastating impact. Plant Dis. 1997, 81, 1340–1348. [Google Scholar] [CrossRef]
- Van der Lee, T.; Zhang, H.; van Diepeningen, A.; Waalwijk, C. Biogeography of Fusarium graminearum species complex and chemotypes: A review. Food Addit. Contam. Part Chem. Anal. Control Expo. Risk Assess. 2015, 32, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Alexander, N.J.; McCormick, S.P.; Waalwijk, C.; van der Lee, T.; Proctor, R.H. The genetic basis for 3-ADON and 15-ADON trichothecene chemotypes in Fusarium. Fungal Genet. Biol. 2011, 48, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Han, Y.-K.; Kim, K.-H.; Yun, S.-H.; Lee, Y.-W. Tri13 and Tri7 Determine Deoxynivalenol- and Nivalenol-Producing Chemotypes of Gibberella zeae. Appl. Environ. Microbiol. 2002, 68, 2148–2154. [Google Scholar] [CrossRef] [PubMed]
- Varga, E.; Wiesenberger, G.; Hametner, C.; Ward, T.J.; Dong, Y.; Schöfbeck, D.; McCormick, S.; Broz, K.; Stückler, R.; Schuhmacher, R.; et al. New tricks of an old enemy: Isolates of Fusarium graminearum produce a type A trichothecene mycotoxin. Environ. Microbiol. 2015, 17, 2588–2600. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Kaneko, I.; Komiyama, M.; Takatsuki, A.; Koshino, H.; Yoneyama, K.; Yamaguchi, I. Trichothecene 3-O-Acetyltransferase Protects Both the Producing Organism and Transformed Yeast from Related Mycotoxins. J. Biol. Chem. 1998, 273, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- Ward, T.J.; Clear, R.M.; Rooney, A.P.; O’Donnell, K.; Gaba, D.; Patrick, S.; Starkey, D.E.; Gilbert, J.; Geiser, D.M.; Nowicki, T.W. An adaptive evolutionary shift in Fusarium head blight pathogen populations is driving the rapid spread of more toxigenic Fusarium graminearum in North America. Fungal Genet. Biol. 2008, 45, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Z.; van der Lee, T.; Chen, W.Q.; Xu, J.; Xu, J.S.; Yang, L.; Yu, D.; Waalwijk, C.; Feng, J. Population genetic analyses of Fusarium asiaticum populations from barley suggest a recent shift favoring 3ADON producers in southern China. Phytopathology 2010, 100, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; van der Lee, T.; Waalwijk, C.; Chen, W.; Xu, J.; Xu, J.; Zhang, Y.; Feng, J. Population analysis of the Fusarium graminearum species complex from wheat in China show a shift to more aggressive isolates. PLoS ONE 2012, 7, e31722. [Google Scholar] [CrossRef] [PubMed]
- Schmeitzl, C.; Warth, B.; Fruhmann, P.; Michlmayr, H.; Malachová, A.; Berthiller, F.; Schuhmacher, R.; Krska, R.; Adam, G. The Metabolic Fate of Deoxynivalenol and Its Acetylated Derivatives in a Wheat Suspension Culture: Identification and Detection of DON-15-O-Glucoside, 15-Acetyl-DON-3-O-Glucoside and 15-Acetyl-DON-3-Sulfate. Toxins 2015, 7, 3112–3126. [Google Scholar] [CrossRef] [PubMed]
- Ollis, D.L.; Cheah, E.; Cygler, M.; Dijkstra, B.; Frolow, F.; Franken, S.M.; Harel, M.; Remington, S.J.; Silman, I.; Schrag, J. The α/β hydrolase fold. Protein Eng. 1992, 5, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Holmes, R.S.; Wright, M.W.; Laulederkind, S.J.F.; Cox, L.A.; Hosokawa, M.; Imai, T.; Ishibashi, S.; Lehner, R.; Miyazaki, M.; Perkins, E.J.; et al. Recommended nomenclature for five mammalian carboxylesterase gene families: Human, mouse, and rat genes and proteins. Mamm. Genome Off. J. Int. Mamm. Genome Soc. 2010, 21, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.D.G.; Putterill, J.J.; Plummer, K.M.; Newcomb, R.D. The carboxylesterase gene family from Arabidopsis thaliana. J. Mol. Evol. 2003, 57, 487–500. [Google Scholar] [PubMed]
- Lee, S.; Hwang, S.; Seo, Y.W.; Jeon, W.B.; Oh, B.-J. Molecular characterization of the AtCXE8 gene, which promotes resistance to Botrytis cinerea infection. Plant Biotechnol. Rep. 2013, 7, 109–119. [Google Scholar] [CrossRef]
- Gershater, M.C.; Cummins, I.; Edwards, R. Role of a carboxylesterase in herbicide bioactivation in Arabidopsis thaliana. J. Biol. Chem. 2007, 282, 21460–21466. [Google Scholar] [CrossRef] [PubMed]
- Cummins, I.; Landrum, M.; Steel, P.G.; Edwards, R. Structure activity studies with xenobiotic substrates using carboxylesterases isolated from Arabidopsis thaliana. Phytochemistry 2007, 68, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Shimada, A.; Takashi, Y.; Kim, Y.-C.; Park, S.-H.; Ueguchi-Tanaka, M.; Suzuki, H.; Katoh, E.; Iuchi, S.; Kobayashi, M.; et al. Identification and characterization of Arabidopsis gibberellin receptors. Plant J. 2006, 46, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Gershater, M.C.; Edwards, R. Regulating biological activity in plants with carboxylesterases. Plant Sci. 2007, 173, 579–588. [Google Scholar] [CrossRef]
- Poppenberger, B.; Berthiller, F.; Lucyshyn, D.; Sieberer, T.; Schuhmacher, R.; Krska, R.; Kuchler, K.; Glössl, J.; Luschnig, C.; Adam, G. Detoxification of the Fusarium mycotoxin deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana. J. Biol. Chem. 2003, 278, 47905–47914. [Google Scholar] [CrossRef] [PubMed]
- Kluger, B.; Bueschl, C.; Lemmens, M.; Michlmayr, H.; Malachova, A.; Koutnik, A.; Maloku, I.; Berthiller, F.; Adam, G.; Krska, R.; et al. Biotransformation of the mycotoxin deoxynivalenol in fusarium resistant and susceptible near isogenic wheat lines. PLoS ONE 2015, 10, e0119656. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shin, S.; Heinen, S.; Dill-Macky, R.; Berthiller, F.; Nersesian, N.; Clemente, T.; McCormick, S.; Muehlbauer, G. Transgenic wheat expressing a barley UDP-glucosyltransferase detoxifies deoxynivalenol and provides high levels of resistance to Fusarium graminearum. Mol. Plant-Microbe Interact. 2015, 28, 1237–1246. [Google Scholar] [PubMed]
- Nussbaumer, T.; Martis, M.M.; Roessner, S.K.; Pfeifer, M.; Bader, K.C.; Sharma, S.; Gundlach, H.; Spannagl, M. MIPS PlantsDB: A database framework for comparative plant genome research. Nucleic Acids Res. 2013, 41, D1144–D1151. [Google Scholar] [CrossRef] [PubMed]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef] [PubMed]
- Marchler-Bauer, A.; Zheng, C.; Chitsaz, F.; Derbyshire, M.K.; Geer, L.Y.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Lanczycki, C.J.; et al. CDD: Conserved domains and protein three-dimensional structure. Nucleic Acids Res. 2013, 41, D348–D352. [Google Scholar] [CrossRef] [PubMed]
- Tükenmez, H.; Xu, H.; Esberg, A.; Byström, A.S. The role of wobble uridine modifications in +1 translational frameshifting in eukaryotes. Nucleic Acids Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- The International Brachypodium Initiative (IBI). Genome sequencing and analysis of the model grass Brachypodium distachyon. Nature 2010, 463, 763–768. [Google Scholar]
- Mitterbauer, R.; Adam, G.; YZGA2274. University of Natural Resources and Life Sciences Vienna, Austria. Personal communication, 2009.
- Clément, Y.; Fustier, M.-A.; Nabholz, B.; Glémin, S. The Bimodal Distribution of Genic GC Content Is Ancestral to Monocot Species. Genome Biol. Evol. 2014, 7, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Batard, Y.; Hehn, A.; Nedelkina, S.; Schalk, M.; Pallett, K.; Schaller, H.; Werck-Reichhart, D. Increasing expression of P450 and P450-reductase proteins from monocots in heterologous systems. Arch. Biochem. Biophys. 2000, 379, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Taylor, P.; Bosron, W.F.; Sanghani, S.P.; Hosokawa, M.; La Du, B.N. Current progress on esterases: From molecular structure to function. Drug Metab. Dispos. Biol. Fate Chem. 2002, 30, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Williams, E.T.; Bourgea, J.; Wong, Y.N.; Patten, C.J. Characterization of recombinant human carboxylesterases: Fluorescein diacetate as a probe substrate for human carboxylesterase 2. Drug Metab. Dispos. Biol. Fate Chem. 2011, 39, 1329–1333. [Google Scholar] [CrossRef] [PubMed]
- Michlmayr, H.; Malachová, A.; Varga, E.; Kleinová, J.; Lemmens, M.; Newmister, S.; Rayment, I.; Berthiller, F.; Adam, G. Biochemical Characterization of a Recombinant UDP-glucosyltransferase from Rice and Enzymatic Production of Deoxynivalenol-3-O-β-d-glucoside. Toxins 2015, 7, 2685–2700. [Google Scholar] [CrossRef] [PubMed]
- International Barley Genome Sequencing Consortium; Mayer, K.F.; Waugh, R.; Brown, J.W.; Schulman, A.; Langridge, P.; Platzer, M.; Fincher, G.B.; Muehlbauer, G.J.; Sato, K.; et al. A physical, genetic and functional sequence assembly of the barley genome. Nature 2012, 491, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Chandler, P.M.; Harding, C.A.; Ashton, A.R.; Mulcair, M.D.; Dixon, N.E.; Mander, L.N. Characterization of Gibberellin Receptor Mutants of Barley (Hordeum vulgare L.). Mol. Plant 2008, 1, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Nihashi, Y.; Lim, C.-H.; Tanaka, C.; Miyagawa, H.; Ueno, T. Phytotoxic sesterterpene, 11-epiterpestacin, from Bipolaris sorokiniana NSDR-011. Biosci. Biotechnol. Biochem. 2002, 66, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Moretti, A.; Mulè, G.; Ritieni, A.; Logrieco, A. Further data on the production of beauvericin, enniatins and fusaproliferin and toxicity to Artemia salina by Fusarium species of Gibberella fujikuroi species complex. Int. J. Food Microbiol. 2007, 118, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, A.E.; McCormick, S.P.; Appell, M. Structure-activity relationships of trichothecene toxins in an Arabidopsis thaliana leaf assay. J. Agric. Food Chem. 2007, 55, 6487–6492. [Google Scholar] [CrossRef] [PubMed]
- Fruhmann, P.; Skrinjar, P.; Weber, J.; Mikula, H.; Warth, B.; Sulyok, M.; Krska, R.; Adam, G.; Rosenberg, E.; Hametner, C.; et al. Sulfation of deoxynivalenol, its acetylated derivatives, and T2-toxin. Tetrahedron 2014, 70, 5260–5266. [Google Scholar] [CrossRef] [PubMed]
- Shams, M. Isolation of Trichothecene Mycotoxins and Radicicol-Metabolites and Their Characterization by LC-MS; Boku: Wien, Austria, 2012. [Google Scholar]
- Islam, M.N.; Chambers, J.P.; Ng, C.K.-Y. Lipid profiling of the model temperate grass, Brachypodium distachyon. Metabolomics 2011, 8, 598–613. [Google Scholar] [CrossRef]
- Vernet, T.; Dignard, D.; Thomas, D.Y. A family of yeast expression vectors containing the phage f1 intergenic region. Gene 1987, 52, 225–233. [Google Scholar] [CrossRef]
- McCormick, S.; Alexander, N.J. Fusarium Tri8 encodes a trichothecene C-3 esterase. Appl. Environ. Microbiol. 2002, 68, 2959–2964. [Google Scholar] [CrossRef] [PubMed]
- Warth, B.; Fruhmann, P.; Wiesenberger, G.; Kluger, B.; Sarkanj, B.; Lemmens, M.; Hametner, C.; Fröhlich, J.; Adam, G.; Krska, R.; et al. Deoxynivalenol-sulfates: Identification and quantification of novel conjugated (masked) mycotoxins in wheat. Anal. Bioanal. Chem. 2015, 407, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmeitzl, C.; Varga, E.; Warth, B.; Kugler, K.G.; Malachová, A.; Michlmayr, H.; Wiesenberger, G.; Mayer, K.F.X.; Mewes, H.-W.; Krska, R.; et al. Identification and Characterization of Carboxylesterases from Brachypodium distachyon Deacetylating Trichothecene Mycotoxins. Toxins 2016, 8, 6. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins8010006
Schmeitzl C, Varga E, Warth B, Kugler KG, Malachová A, Michlmayr H, Wiesenberger G, Mayer KFX, Mewes H-W, Krska R, et al. Identification and Characterization of Carboxylesterases from Brachypodium distachyon Deacetylating Trichothecene Mycotoxins. Toxins. 2016; 8(1):6. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins8010006
Chicago/Turabian StyleSchmeitzl, Clemens, Elisabeth Varga, Benedikt Warth, Karl G. Kugler, Alexandra Malachová, Herbert Michlmayr, Gerlinde Wiesenberger, Klaus F. X. Mayer, Hans-Werner Mewes, Rudolf Krska, and et al. 2016. "Identification and Characterization of Carboxylesterases from Brachypodium distachyon Deacetylating Trichothecene Mycotoxins" Toxins 8, no. 1: 6. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins8010006