Published online Nov 7, 2008. doi: 10.3748/wjg.14.6360
Revised: October 27, 2008
Accepted: September 3, 2008
Published online: November 7, 2008
AIM: To investigate the therapeutic potential of an RNA ligand (aptamer) specific for the catalytic ricin A-chain (RTA), the protective effects of a 31-nucleotide RNA aptamer (31RA), which formed a high affinity complex with RTA, against ricin-induced toxicity in cell-based luciferase translation and cell cytotoxicity assays were evaluated.
METHODS: To test the therapeutic potential of anti-RTA aptamers in Chinese hamster ovary (CHO) AA8 cells stably transfected with a tetracycline regulatable promoter, ricin ribotoxicity was measured using luciferase and ricin-induced cytotoxicity was ascertained by MTS cell proliferation assay with tetrazolium compound [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium].
RESULTS: Inhibition of protein synthesis by ricin in CHO AA8 cells resulted in diminished luciferase activity and treatment with polyclonal antibody against deglycosylated RTA (dgA) neutralized the inhibitory effects of ricin on luciferase activity and protected against ricin-induced cytotoxicity as measured by MTS assay. The 31RA anti-RTA aptamer inhibited the translation of luciferase mRNA in cell-free reticulocyte translation assay. 31RA aptamer also partially neutralized the inhibitory effects of ricin on luciferase activity and partially protected against ricin-induced cytotoxicity in CHO AA8 cells.
CONCLUSION: We have shown that anti-RTA RNA aptamer can protect against ricin ribotoxicity in cell-based luciferase and cell cytotoxicity assays. Hence, RNA aptamer that inhibits RTA enzymatic activity represents a novel class of nucleic acid inhibitor that has the potential to be developed as a therapeutic agent for the treatment of ricin intoxication.
- Citation: Fan S, Wu F, Martiniuk F, Hale ML, Ellington AD, Tchou-Wong KM. Protective effects of anti-ricin A-chain RNA aptamer against ricin toxicity. World J Gastroenterol 2008; 14(41): 6360-6365
- URL: https://www.wjgnet.com/1007-9327/full/v14/i41/6360.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6360
Ricin, a lectin from the castor bean plant R. communis is considered one of the most potent plant toxins. Ricin poisoning can cause severe tissue damage and inflammation and can result in death. More than 750 cases of accidental or deliberate ricin poisoning have been described in humans. Most accidental exposures occur by ingestion of the seeds of castor beans whereby the toxin is released after the seed coat is damaged. The ingested toxin causes severe gastrointestinal damage with symptoms including nausea, vomiting, diarrhea, and abdominal pain and may progress to hypotension, liver failure, renal dysfunction, and death due to multiorgan failure or cardiovascular collapse[1]. Ricin administered intragastrically to mice induced villus atrophy and epithelial damage in the proximal small intestine[2]. In experimental exposed animals, intravenous injection of ricin to mice induced severe inflammatory responses and clinical symptoms resembling hemolytic uremic syndrome (HUS), including thrombotic microangiopathy, hemolytic anemia, thrombocytopenia, and acute renal failure[3].
Ricin belongs to a group of toxins referred to as ribosome-inactivating proteins (RIPs)[4,5] and is composed of two glycoproteins, the A-chain (RTA) and the B-chain (RTB), linked by a disulfide bond[6]. RTB binds to galactose residues on cell surface receptors and cell binding is followed by endocytic uptake. A proportion of the ricin moves from early endosomes to the trans-Golgi network and to the endoplasmic reticulum (ER) lumen. In the ER lumen, RTA and RTB dissociate and RTA is retrograde transported across the ER membrane into the cytosol[7]. RTA is a ribotoxin that possesses RNA N-glycosidase activity that disables protein translation by depurinating a single adenine in the 28 S eukaryotic ribosomal RNA[8] which prevents the binding of elongation factor 2, thereby terminating protein synthesis[9,10]. The irreversible poisoning of the ribosome and inhibition of protein synthesis may lead to eventual cell death.
Since currently there is no antidote or specific therapy available for ricin poisoning, the discovery of antitoxins is a high priority. Ricin ribotoxicity can be counteracted by several different types of antitoxins including neutralizing anti-ricin antibodies, small molecule RTA inhibitors, polynucleotide active site inhibitors and polynucleotide substrate analogues[11,12]. In vitro selection had been used to generate RNA ligands (aptamers) specific for the catalytic ricin A-chain[13]. An initial 80-nucleotide RNA ligand was minimized to a 31-nucleotide RNA aptamer (31RA) that contained all sequences and structures necessary for forming high affinity complexes with RTA and blocking enzymatic activity of RTA in vitro. A transient cell-based luciferase assay had been utilized for quantifying protein synthesis inhibition by bacterial toxins[14]. In this report, we utilized a stable cell-based luciferase assay and showed that 31RA aptamer also neutralized the inhibitory effects of ricin on translation inhibition in cell-free and cell-based luciferase assays and ricin-induced cytotoxicity assay. The use of a stably transfected cell-based luciferase assay will facilitate the development of high throughput screening for inhibitors of ricin as potential antidotes for the treatment of ricin intoxication.
Ricin (Ricinus communis agglutinin II) and ricin A-chain (RTA) were purchased from Vector Laboratories, Inc. (Burlington, CA). Anti-deglycosylated ricin A-chain (anti-dgA) antibody was IgG purified by protein-A sepharose from pooled polyclonal antisera obtained from mice hyperimmunized with dgA (USAMRIID, Fort Detrick, MD). The anti-RTA RNA aptamer (31RA) (GGCGAAUUCAGGGGACGUAGCAAUGACUGCC)[13] was synthesized by Sigma-Genosys. Rabbit reticulocyte lysate (nuclease treated), amino acid (complete) mixture, luciferase control RNA, RNasin inhibitor, nuclease-free water, and luciferin substrate (CFT luciferase reporter buffer) were purchased from Promega (Madison, WI). 31RA aptamer was diluted and heated for 3 min at 65°C, cooled to 25°C and incubated at 25°C for 10 min. After incubation, aptamer and toxin were mixed together and incubated at 25°C for an additional 10 min. As a standard control, ricin (1.6 to 200 ng/mL) and RTA (0.4 to 50 ng/mL) diluted in PBS buffer were added to a V-shaped 96-well microtiter plate. Rabbit reticulocyte lysate, RNAsin, amino acid complete mixture, nuclease-free deionized water, and luciferase mRNA were mixed together and kept on ice. Five μL of each standard and treatment group were added to microtiter plate, and then 25 μL of the lysate mixture was added to each well. The plate was wrapped in a damp paper towel, placed in a plastic bag and incubated for 90 min at 37°C. After incubation, 5 μL of reaction mixture was transferred to a black microtiter plate and 45 μL of the luciferin reaction buffer was added to each well. Luminescence was measured as counts per second (CPS) using a SpectraMax Luminometer (Molecular Devices). Data were presented as the % of control (PBS only or no treatment) [CPS experimental/CPS PBS control × 100] as previously described[15]. Statistical analyses were calculated using Microsoft Excel 7.0 and SigmaPlotTM V3.01. Three separate assays were performed for each experimental group.
Chinese Hamster Ovary AA8 (CHO AA8) cells offered a stably transfected luciferase reporter cell system, whereby expression of the luciferase gene was under the transcriptional control of a tetracycline-repressible promoter system (Tet-Off™ Expression System from Clontech/BD Biosciences). CHO AA8 cells were cultured in Dulbecco’s modified Eagle’s Medium (DMEM) supplemented with 10% FBS, penicillin and streptomycin and incubated at 37°C in a humidified 5% CO2 incubator. Isotype control mouse IgG, anisomycin and doxycycline were obtained from Sigma-Aldrich.
To suppress luciferase expression, CHO AA8 cells were cultured in DMEM containing 10% FBS and doxycycline (Dox) (1 μg/mL). For the kinetics of induction of luciferase activity, cells were trypsinized and seeded at 2 × 105 cells/well in 6-well plate without Dox and 3 h after cell plating, residual and cell-attached Dox was removed by several washes with PBS. Cells were subjected to the indicated treatment and incubated for different lengths of time before cell lysis. As control, cells were treated with anisomycin (10 μg/mL). Equal amounts of protein (20 μg) were assayed for luciferase activity using the Bright-Glo™ Luciferase Assay System (Promega) and luminescence was measured with EG&G Berthold microplate luminometer (MicroLumat Plus LB96V).
To evaluate the protective effects of anti-dgA Ab and 31RA aptamer, 5000 CHO AA8 cells were plated in 96-well plates in the absence of Dox overnight. Ricin was co-incubated with anti-dgA IgG or control IgG at a ratio of 1 μg ricin to 10 μg antibody for 30 min at 37°C in 5% CO2 as previously described[16]. The ricin-antibody mixture was added to CHO AA8 cells in triplicate wells. Prior to use, the 31RA aptamer was heated at 65°C for 3 min and cool at 25°C for 10 min. Various dilutions of the 31RA aptamer were added to ricin and incubated at 37°C for 30-40 min before incubation with cells. Cells were harvested 24 h after treatment for luciferase assay.
For ricin-induced cytotoxicity assay, CHO AA8 cells were seeded in 96-well flat-bottom plates at 5000 cells/well in 100 μL DMEM plus 10% FBS and incubated at 37°C overnight. Ricin was pre-incubated with anti-ricin Ab or aptamer as described above and cell viability was assayed at 48 h post-treatment. Cytotoxicity of CHO AA8 cells induced by ricin was quantitated in triplicate wells using the CellTiter 96® Aqueous Non-Radioactive Cell Proliferation (MTS) Assay (Promega) and the plate was read using a 492 nm absorbance filter in a Perkin Elmer HTS700 BioAssay plate reader.
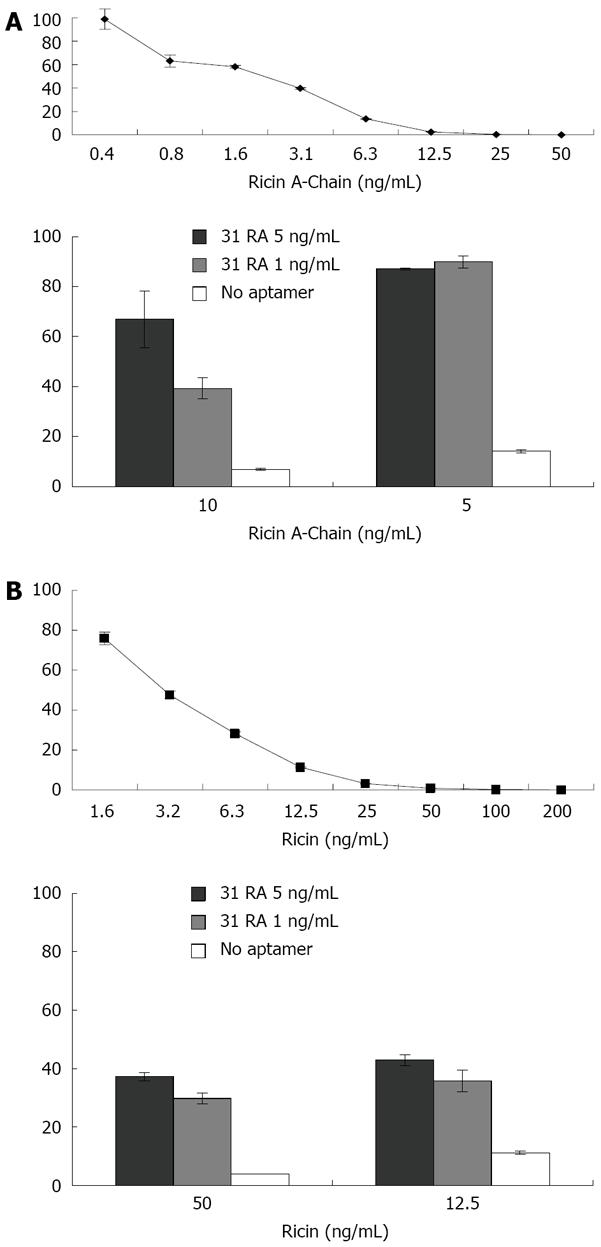
Hale ML[15] developed a cell-free in vitro translation assay for measuring the ribotoxicity of ribosome-inactivating toxins based on inhibition of protein translation of the luciferase mRNA in a rabbit reticulocyte assay. As shown in Figure 1 (upper panels), the amount of luciferase translated, as measured by luminescence, was inversely proportional to the concentration of RTA and ricin holoenzyme. The protective effects of 31RA aptamer were first evaluated using the cell-free translation assay. Preincubation of RTA (5 ng/mL) with 31RA aptamer at both 1 and 5 ng/mL protected equally against RTA-induced translation inhibition while dose-dependent protection was observed with a higher dose of RTA (10 ng/mL) (Figure 1A, lower panel). When preincu-bated with the ricin holoenzyme, 31RA aptamer partially neutralized protein synthesis inhibition by ricin (Figure 1B, lower panel).
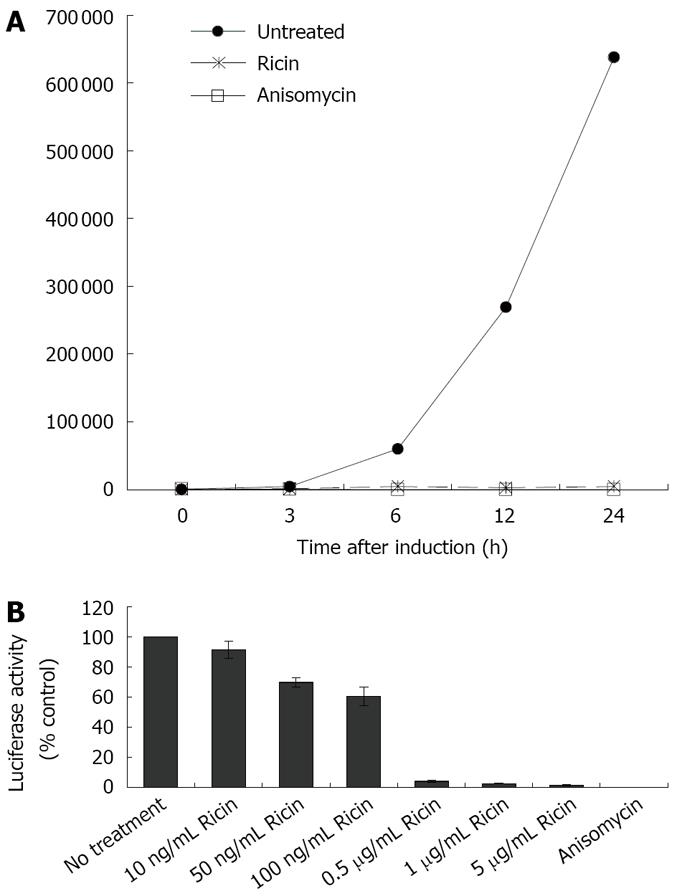
To evaluate the protective effects of 31RA aptamer against ricin ribotoxicity in cells, we utilized a cell-based luciferase assay to complement the cell-free luciferase translation assay. For the cell-based luciferase assay, CHO AA8 cells stably transfected with a luciferase reporter gene under a tetracycline-repressible promoter were used to measure the ribotoxicity of ricin. In the presence of ATP, the luciferase enzyme catalyzes the oxidation of D-luciferin to produce light and the light output corresponds to the concentration of the luciferase enzyme and activity. The light output (Relative light unit, RLU) was used to measure the dose-dependent inhibition of luciferase activity by ricin compared to anisomycin, a small molecule protein synthesis inhibitor known to target ribosomal RNA (rRNA) at an adjacent site distinct from that targeted by ricin[10]. First, the kinetics of induction of expression of the luciferase reporter gene after removal of doxycycline was determined by measuring luciferase activity over a 24-h period in the absence and presence of ricin or anisomycin (Figure 2A) The increase in luciferase activity was observed at 3 h after induction and ricin (1 μg/mL) and anisomycin (10 μg/mL) completely inhibited the increase in luciferase activity. Treatment of CHO AA8 cells with increasing concentrations of ricin resulted in increased inhibition of luciferase activity in a dose-dependent manner compared to untreated control and maximal inhibition was obtained with > 0.5 μg/mL ricin (Figure 2B).
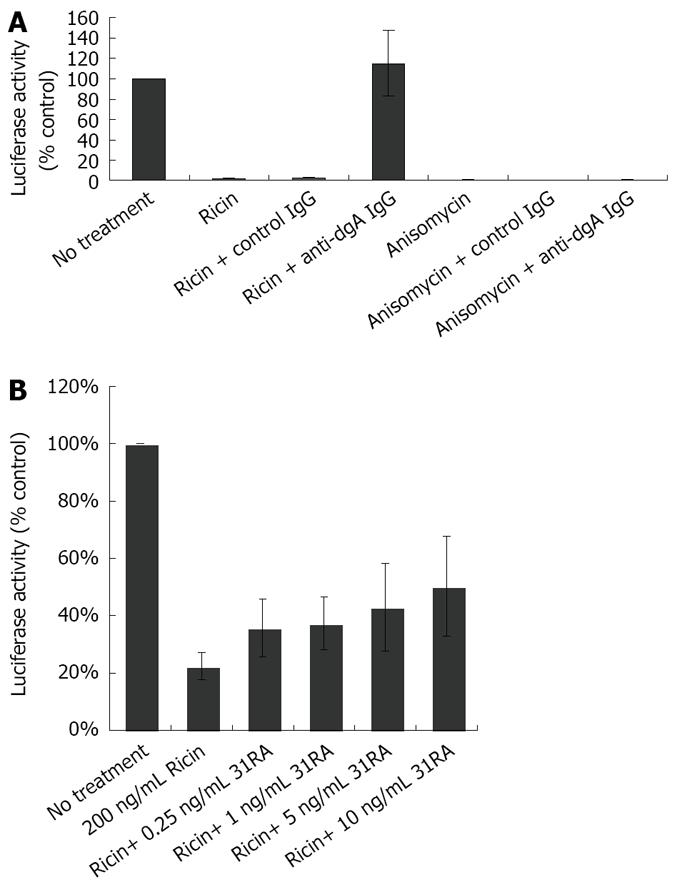
To determine the utility of this cell-based assay for testing specificity of antitoxins against ricin toxicity, the effects of polyclonal anti-deglycosylated ricin A chain (dgA) antibody in neutralizing the inhibitory effects of ricin on luciferase activity were determined. We have previously shown that anti-dgA Ab protected against ricin-induced cytotoxicity of RAW 264.7 mouse macrophage cells and ricin-induced lung injury and lethality[17]. CHO AA8 cells were treated with ricin or anisomycin in the presence of control IgG or IgG purified from the sera from dgA-immunized mice (anti-dgA IgG) and luciferase activity was measured 24 h later. As depicted in Figure 3A, anti-dgA IgG specifically neutralized the inhibitory effects of ricin on luciferase activity, but not that of anisomycin. Compared to anti-dgA IgG, 31RA aptamer partially protected against ricin-induced ribotoxicity as assessed by luciferase assay (Figure 3B).
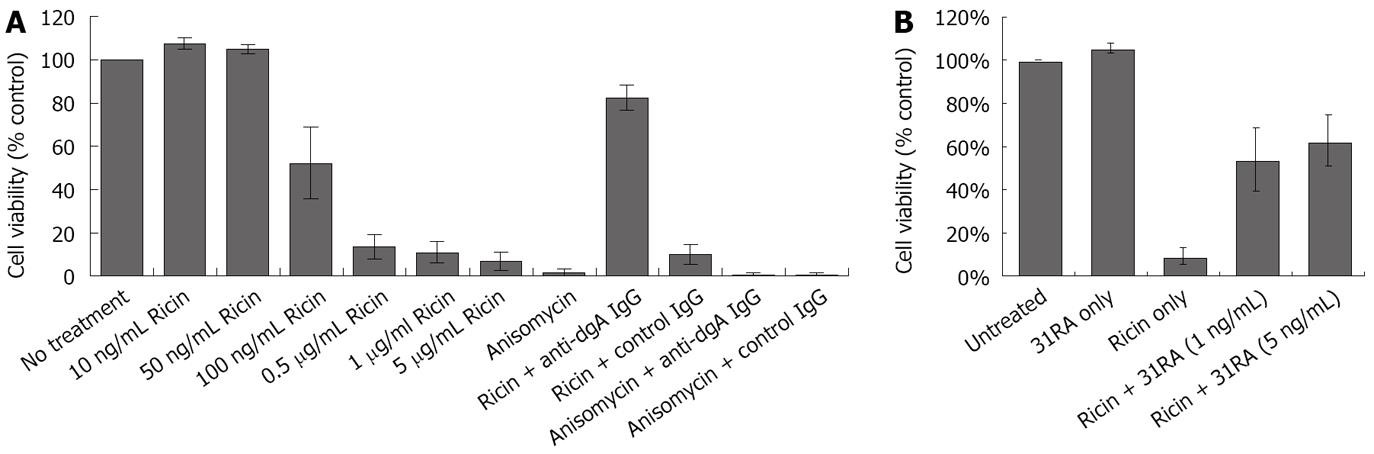
To examine the effects of increasing doses of ricin on viability of CHO AA8 cells using the MTS assay. As shown in Figure 4A, treatment of CHO AA8 cells with increasing concentrations of ricin resulted in decreased cell survival. Interestingly, similar to the dose-dependent inhibition of luciferase activity (Figure 2B), ricin (100 ng/mL) induced ~50% cell death while > 0.5 μg/mL induced > 80% cell death (Figure 4A). Treatment with anti-dgA IgG, but not control IgG protected against cytotoxicity induced by ricin, and not anisomycin in CHO AA8 cells (Figure 4A). Pretreatment of ricin with 31RA aptamer also neutralized ricin-induced cytotoxicity in CHO AA8 cells (Figure 4B) and RAW264.7 mouse macrophage cells (data not shown).
In vitro selection is a powerful molecular tool for the generation of ligands for a wide variety of targets for therapeutic purposes. RNA aptamers that bind to human immunodeficiency virus typeIRev also inhibit viral replication[18]. Therefore, aptamers that recognize and inhibit ricin might be useful therapeutic agents. Interestingly, although the 31RA aptamer specific for the catalytic RTA bore no resemblance to the normal RTA substrate, i.e. the sarcin-ricin loop (SRL) and was not depurinated by RTA[13], it contained all sequences and structures necessary for interacting with RTA. This minimal 31-nucleotide RNA formed high affinity complexes with RTA (Kd = 7.3 nmol/L) which could compete with SRL for binding to RTA and inhibited RTA depurination of the SRL and could partially protect protein translation from RTA inhibition in in vitro translation assay. The IC50 of the aptamer for RTA in the latter assay was 100 nmol/L, roughly 3 orders of magnitude lower than a small molecule inhibitor of ricin, pteroic acid[19]. Here we showed that 31RA aptamer can inhibit ricin ribotoxicity in cell-free and cell-based luciferase translation assays and cell cytotoxicity assay. It will also be interesting to determine if the 31RA aptamer will be effective against ricin after cell internalization which will be relevant for post-exposure treatment. We are currently testing various methods for intracellular delivery of the 31RA aptamer to determine if internalized aptamer can block toxicity of intracellular ricin A-chain. Hence, anti-RTA aptamers have the potential to be developed as ricin inhibitors and the therapeutic effects of aptamers in vivo in animal models of ricin intoxication remain to be determined.
Luciferase and luminescence assays, commonly used readouts for small molecule screens because of high sensitivity and linear signal response, can readily be adapted for high throughput screening of large libraries of compounds[20]. We have utilized a CHO Tet-Off luciferase system for measuring the biological activity of ricin based on protein synthesis inhibition and have shown that this assay can be used for measuring the protective effects of ricin inhibitors including anti-RTA neutralizing antibodies and an anti-RTA RNA aptamer. Compared to the transient luciferase-based assay described by Zhao et al[14] which utilized adenoviral transduction to deliver the luciferase gene, our cell-based assay is a stable cell system whereby the luciferase gene is stably integrated and its expression can be regulated by removal of tetracycline or doxycycline. Another advantage of using a stable cell system compared to adenoviral transduction is that the variability due to batch-to-batch variations of viral titers and infection efficiency are avoided. The conventional assays for protein synthesis utilize radioactive amino acids, but these assays suffer from significant sample-to-sample variability and insufficient sensitivity for high throughput assays. Hence, the adaptation of the stable luciferase-based assay in combination with cell cytotoxicity assay will be useful for high throughput screening of compounds for inhibitors of ricin and other related ribotoxins.
Ricin, a lectin from the castor bean plant Ricinus communis is considered one of the most potent plant toxins. Ricin poisoning can cause severe tissue damage and inflammation and can result in death. More than 750 cases of accidental or deliberate ricin poisoning have been described in humans. Most accidental exposures occur by ingestion of the seeds of castor beans whereby the toxin is released after the seed coat is damaged. The ingested toxin causes severe gastrointestinal damage with symptoms including nausea, vomiting, diarrhea, and abdominal pain and may progress to hypotension, liver failure, renal dysfunction, and death due to multiorgan failure or cardiovascular collapse.
Since currently there is no antidote or specific therapy available for ricin poisoning, the discovery of antitoxins is a high priority. Ricin ribotoxicity can be counteracted by several different types of antitoxins including neutralizing anti-ricin antibodies, small molecule RTA inhibitors, polynucleotide active site inhibitors and polynucleotide substrate analogues. The development of specific inhibitors of RTA will offer novel insights into the development of effective therapeutics against ricin poisoning.
In vitro selection had been used to generate RNA ligands (aptamers) specific for the catalytic ricin A-chain. An initial 80-nucleotide RNA ligand was minimized to a 31-nucleotide RNA aptamer (31RA) that contained all sequences and structures necessary for forming high affinity complexes with RTA and blocking enzymatic activity of RTA in vitro. In this report, authors utilized a stable cell-based luciferase assay and showed that 31RA aptamer also neutralized the inhibitory effects of ricin on translation inhibition in cell-free and cell-based luciferase assays and ricin-induced cytotoxicity assay.
The use of a stably transfected cell-based luciferase assay will facilitate the development of high throughput screening for inhibitors of ricin as potential antidotes for the treatment of ricin intoxication.
The manuscript deals with the inhibitory effects of a previously described aptamer against ricin toxicity. The authors employ an in vitro translation and a cell-based luciferase assay. This is an interesting paper.
Peer reviewer: Kostas Pantopoulos, Professor, Lady Davis Institute for Medical Research, McGill University, 3755 Cote-Ste-Catherine Road, Montreal H3T 1E2, Canada
S- Editor Li DL E- Editor Ma WH
| 1. | Audi J, Belson M, Patel M, Schier J, Osterloh J. Ricin poisoning: a comprehensive review. JAMA. 2005;294:2342-2351. [Cited in This Article: ] |
| 2. | Yoder JM, Aslam RU, Mantis NJ. Evidence for widespread epithelial damage and coincident production of monocyte chemotactic protein 1 in a murine model of intestinal ricin intoxication. Infect Immun. 2007;75:1745-1750. [Cited in This Article: ] |
| 3. | Korcheva V, Wong J, Corless C, Iordanov M, Magun B. Administration of ricin induces a severe inflammatory response via nonredundant stimulation of ERK, JNK, and P38 MAPK and provides a mouse model of hemolytic uremic syndrome. Am J Pathol. 2005;166:323-339. [Cited in This Article: ] |
| 4. | Olsnes S, Refsnes K, Pihl A. Mechanism of action of the toxic lectins abrin and ricin. Nature. 1974;249:627-631. [Cited in This Article: ] |
| 5. | Stirpe F, Barbieri L. Ribosome-inactivating proteins up to date. FEBS Lett. 1986;195:1-8. [Cited in This Article: ] |
| 6. | Lord JM, Roberts LM, Robertus JD. Ricin: structure, mode of action, and some current applications. FASEB J. 1994;8:201-208. [Cited in This Article: ] |
| 7. | Lord JM, Deeks E, Marsden CJ, Moore K, Pateman C, Smith DC, Spooner RA, Watson P, Roberts LM. Retrograde transport of toxins across the endoplasmic reticulum membrane. Biochem Soc Trans. 2003;31:1260-1262. [Cited in This Article: ] |
| 8. | Endo Y, Tsurugi K. The RNA N-glycosidase activity of ricin A-chain. The characteristics of the enzymatic activity of ricin A-chain with ribosomes and with rRNA. J Biol Chem. 1988;263:8735-8739. [Cited in This Article: ] |
| 9. | Olsnes S, Pihl A. Different biological properties of the two constituent peptide chains of ricin, a toxic protein inhibiting protein synthesis. Biochemistry. 1973;12:3121-3126. [Cited in This Article: ] |
| 10. | Wool IG, Gluck A, Endo Y. Ribotoxin recognition of ribosomal RNA and a proposal for the mechanism of translocation. Trends Biochem Sci. 1992;17:266-269. [Cited in This Article: ] |
| 11. | Rainey GJ, Young JA. Antitoxins: novel strategies to target agents of bioterrorism. Nat Rev Microbiol. 2004;2:721-726. [Cited in This Article: ] |
| 12. | Mantis NJ. Vaccines against the category B toxins: Staphylococcal enterotoxin B, epsilon toxin and ricin. Adv Drug Deliv Rev. 2005;57:1424-1439. [Cited in This Article: ] |
| 13. | Hesselberth JR, Miller D, Robertus J, Ellington AD. In vitro selection of RNA molecules that inhibit the activity of ricin A-chain. J Biol Chem. 2000;275:4937-4942. [Cited in This Article: ] |
| 14. | Zhao L, Haslam DB. A quantitative and highly sensitive luciferase-based assay for bacterial toxins that inhibit protein synthesis. J Med Microbiol. 2005;54:1023-1030. [Cited in This Article: ] |
| 15. | Hale ML. Microtiter-based assay for evaluating the biological activity of ribosome-inactivating proteins. Pharmacol Toxicol. 2001;88:255-260. [Cited in This Article: ] |
| 16. | Dertzbaugh MT, Rossi CA, Paddle BM, Hale M, Poretski M, Alderton MR. Monoclonal antibodies to ricin: in vitro inhibition of toxicity and utility as diagnostic reagents. Hybridoma (Larchmt). 2005;24:236-243. [Cited in This Article: ] |
| 17. | Pratt TS, Pincus SH, Hale ML, Moreira AL, Roy CJ, Tchou-Wong KM. Oropharyngeal aspiration of ricin as a lung challenge model for evaluation of the therapeutic index of antibodies against ricin A-chain for post-exposure treatment. Exp Lung Res. 2007;33:459-481. [Cited in This Article: ] |
| 18. | Symensma TL, Giver L, Zapp M, Takle GB, Ellington AD. RNA aptamers selected to bind human immunodeficiency virus type 1 Rev in vitro are Rev responsive in vivo. J Virol. 1996;70:179-187. [Cited in This Article: ] |
| 19. | Miller DJ, Ravikumar K, Shen H, Suh JK, Kerwin SM, Robertus JD. Structure-based design and characterization of novel platforms for ricin and shiga toxin inhibition. J Med Chem. 2002;45:90-98. [Cited in This Article: ] |
| 20. | de Wet JR, Wood KV, DeLuca M, Helinski DR, Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987;7:725-737. [Cited in This Article: ] |