Published online Feb 14, 2009. doi: 10.3748/wjg.15.697
Revised: December 11, 2008
Accepted: December 18, 2008
Published online: February 14, 2009
AIM: To examine the expressions of N-cadherin and E-cadherin in specimens of 62 normal esophageal epithela, 31 adjacent atypical hyperplastic epithelia and 62 esophageal squamous cell carcinomas (ESCCs), and to investigate the roles of N-cadherin in the invasiveness of ESCC cell line EC9706 transfected by N-cadherin shRNA.
METHODS: PV immunohistochemistry was used to detect the expression pattern of N-cadherin and E-cadherin in specimens of 62 normal esophageal epithelia, 31 adjacent atypical hyperplastic epithelia and 62 ESCCs. The invasiveness of ESCC line EC9706 was determined by transwell assay after EC9706 was transfected by N-cadherin shRNA.
RESULTS: The positive rates of N-cadherin decreased in the carcinoma, adjacent atypical hyperplastic and normal esophageal tissues (75.8%, 61.3% and 29.0%, P < 0.05), respectively, while those of E-cadherin increased (40.3%, 71.0% and 95.2%, P < 0.05). The increased expression of N-cadherin and decreased expression of E-cadherin were related to invasion, differentiation, and lymph node metastasis (P < 0.05). The expression level of N-cadherin decreased in the N-cadherin knocked down cells, and the invasiveness of those cells decreased significantly as well. The number of cells which crossed the basement membrane filter decreased from 123.40 ± 8.23 to 49.60 ± 6.80 (P < 0.05).
CONCLUSION: E-cadherin and N-cadherin expression is correlated with the invasion and aggravation of ESCC. The down-regulation of N-cadherin lowers the invasiveness of EC9706 cell line.
-
Citation: Li K, He W, Lin N, Wang X, Fan QX. N-cadherin knock-down decreases invasiveness of esophageal squamous cell carcinoma
in vitro . World J Gastroenterol 2009; 15(6): 697-704 - URL: https://www.wjgnet.com/1007-9327/full/v15/i6/697.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.697
The malignancy of cancer cells depends largely on their proliferative, invasive and metastatic activities, and invasive and metastatic activities are most closely associated with cell-to-cell and cell-to-extracellular matrix adhesion. As a class of transmembrane proteins, cadherins play an important role in cell adhesion. Among the members of cadherin family, E-cadherin and N-cadherin have been extensively studied about their biological activities and associations with cancer cell invasion. It was reported that the invasion and metastasis were present in the esophageal squamous cell carcinoma (ESCC) with a low level of E-cadherin[12]. Recent studies on prostate cancer and breast cancer proved that the up-regulated N-cadherin plays an important role in cell progression and metastasis[34]. In addition, N-cadherin is involved in angiogenesis and tumor growth regulation, and contributes to the invasive morphology in squamous tumor cells, and stimulates migration, invasion and metastasis[5]. However, the association of E-cadherin and N-cadherin expression with the malignancy of the ESCC is unknown.
In the current study, we analyzed the expression of E-cadherin and N-cadherin in the ESCC tissues, adjacent atypical hyperplastic epithelium and normal esophageal epithelium. The roles of N-cadherin in the invasiveness were investigated in ESCC cell line (EC9706) transfected by N-cadherin shRNA.
In this study, we randomly selected 62 esophageal cancer patients who underwent potentially curative surgery without preoperative chemotherapy or radiotherapy between February 26 and March 16, 2006 in Anyang Tumor Hospital, Henan, China. Among them, 36 were men and 26 were women, ranging from 38-75 years of age with a mean age of 52.6 years. Overall, 22 cases were poorly differentiated, 25 were moderately differentiated, and 15 were well differentiated. For the lymphatic node metastasis, 20 cases were positive and 42 cases were negative. Seven cases were classified as T1 and T2, and 55 as T3 and T4 in terms of T stages. Surgically removed specimens were routinely fixed in buffered formalin and embedded in paraffin blocks for clinical diagnosis and reclassification for this study. The normal esophageal squamous tissues were taken from mucosae 3 cm away from carcinomas, and the adjacent atypical hyperplastic epithelium 2 cm away from the carcinomas. The final pathological diagnosis was based on the result of histological examination.
Immunohistochemical analysis for E-cadherin and N-cadherin was performed on 5-&mgr;m sections made from tissue microarray blocks. The Envision Plus detection system (Dako, Carpinteria, CA, USA) was used for the immunostaining. The sections were deparaffinized in xylene and then were microwaved in 10 mmol/L citrate buffer (pH 6.0) to unmask the epitopes. Endogenous peroxidase activity was blocked by incubation with 0.03% hydrogen peroxide in methanol for 5 min. Slides were incubated with mouse anti-human E-cadherin antibody (microwaving retrieval in citrate buffer at 1:100 concentration, Abcam) and mouse anti-human N-cadherin antibody (microwaving retrieval in low pH buffer at 1:100 concentration; Abcam), respectively. Then, polyperoxidase anti-mouse IgG (Beijing Zhongshan Golden Bridge Biotechnology Co. Ltd., China) was added for incubation for another 30 min followed by gentle rinsing with washing buffer for three times. Thereafter, the sections were stained for 5 min with 3,3-diaminobenzidine (DAB) (Beijing Zhongshan Golden Bridge Biotechnology), counterstained by hematoxylin, dehydrated, and mounted in Diatex. Expression of E-cadherin and N-cadherin in breast cancer was used as a positive control, while the same concentration of PBS was applied as a negative control for E-cadherin and N-cadherin.
For both E-cadherin and N-cadherin, only membrane and cytoplasm staining was considered as positive. The intensity and percentage of immunostained carcinoma cells were all taken into consideration according to the previously published method with modification[6]. Briefly, the extent of positivity was scored as 0 when no positive cell was observed; 1 when the percentage of positive cells was < 30%; 2 when it was 30%-60%; and 3 when it was > 60%. The intensity was scored as 0 when no positive cells were identified; 1, weak; 2, moderate; and 3, strong staining. Multiplying the extent by intensity gave the following immunohistochemical staining grades as 0, 1, 2, 3, 4, 6 and 9. For statistical analyses, grades 0, 1 and 2 were considered as negatively stained, and grades > 2 were considered as positively stained.
PT67 packaging cells were seeded onto a six-well plate at 1 × 105 cells per well and incubated for 24-48 h. The cells were allowed to grow to 60%-70% confluence and then rinsed with fresh DMEM medium. The control vector pEGFP-MSCVneo and recombinant retroviral vector pMSCVneo/N-cadherin plasmids (kindly provided by Dr. Ma Jie, Chinese Academy of Medical Sciences, China) (The former contained the enhanced green fluorescent protein and neomycin resistance genes, the latter contained the N-cadherin shRNA, U6-promotor, enhanced green fluorescent protein and neomycin resistance genes) were transfected into the packaging cell line PT67 by lipofectamine 2000 (Invitrogen Corp, UK) and incubated for 6 h following the manufacturer’s instructions. The mixture was replaced with fresh medium to stop transfection and the transfected PT67 cells were further cultured with G418 (1000 mg/L) selecting medium for 2 wk. Viral supernatant of the drug-resistant clones was filtered through a 0.45-&mgr;m filter and condensed by ultracentrifugation at low temperature, then preserved at -80°C. The supernatant, from the fresh PT67 packaging cells containing recombinant retrovirus, was used to infect NIH3T3 cells to determine viral titer as previously described[7]. The highest titer clones were selected for further experiments.
EC9706 cells were plated in a six-well plate at 5 × 104 cells/mL and cultured with 5% CO2 at 37°C. Twenty-four hours later, the cells were exposed to 2 mL viral supernatant at a consecutive multiplicity of infection once for 12 h, in the presence of 8 &mgr;g/mL polybrene (Sigma). Subsequently, viral supernatant was replaced with fresh medium. After being incubated for another 24 h, the infected EC9706 cells were screened with G418 (600 mg/L) (Sigma) selecting medium for 2 wk. Drug-resistant clones were expanded with G418 (300 mg/L) (Sigma) selecting medium. Two days after culturing, the expression of EGFP in infected EC9706 cells was observed under fluorescence microscope.
Total RNA and protein isolation was performed using the Macherey-Nagel total RNA and protein isolation kit according to the user manual. About 5 × 106 EC9706 cells transfected with N-cadherin RNAi, control vector, or the untreated cells were collected and lysed. Through the NucleoSpin RNA/Protein column, RNA and DNA were bound to the column and protein was contained in the flow-through. After digestion of DNA, total RNA was isolated by washing the column. Protein was isolated from the flow-through and incubated for 3 min at 98°C for dissolving and denaturation, and stored at -20°C until used. All of the preparation and handling steps of RNA took place in a laminar flow hood under RNase-free conditions.
RNA quality and quantity were determined by absorbance readings at 260 and 280 nm with the Nano Drop (ND-1000) spectrophotometer. RNA integrity was tested by PCR amplification of the GAPDH gene. Reverse transcription of RNA was performed using Transcriptor First Strand cDNA Synthesis Kit (Roche). cDNA was synthesized from 5 &mgr;g total RNA isolated from EC9706 cells transfected with N-cadherin siRNA, control vector, or the untreated cells according to the manufacturer’s handbook.
The primer pairs and hydrolysis probes for N-cadherin, E-cadherin, matrix metalloproteinase-9 (MMP-9) and GAPDH were designed by Universal ProbeLibrary Assay Design Center (Roche). All primer sequences listed in Table 1 were synthesized by Shanghai Sangon (China). The hydrolysis probes were designed and synthesized by Roche Diagnostics.
| Target gene | Primers | Length of product (bp) |
| N-cadherin | Sense: 5’-GGTGGAGGAGAAGAAGACCAG-3’ | 72 |
| Antisense: 5’-GGCATCAGGCTCCACAGT-3’ | ||
| E-cadherin | Sense: 5’-CCCGGGACAACGTTTATTAC-3’ | 72 |
| Antisense: 5’-GCTGGCTCAAGTCAAAGTCC-3’ | ||
| MMP9 | Sense: 5’-GAACCAATCTCACCGACAGG-3’ | 67 |
| Antisense: 5’-GCCACCCGAGTGTAACCATA-3’ | ||
| GAPDH | Sense: 5’-AGCCACATCGCTCAGACA-3’ | 66 |
| Antisense: 5’-GCCCAATACGACCAAATCC-3’ |
Real-time PCR was performed with the ABI Prism 7500 Sequence Detection System (ABI) in a total volume of 20 &mgr;L in glasscapillaries containing 2 &mgr;L of cDNA, 0.5 &mgr;mol/L of each primer, 0.1 &mgr;mol/L of hydrolysis probe and 4 &mgr;L of LightCycler TaqMan Master Mix (Roche Diagnostics). PCR reaction was initiated with a 12-min denaturation at 95°C and terminated with a 30-s cooling step at 40°C. The cycling protocol consisted of denaturation at 95°C for 10 s, annealing at 54°C for 10 s, and extension at 72°C for 10 s, and was cycled 45 times. Fluorescence detection was performed at the end of each extension step. The housekeeping genes GAPDH and DEPC-H2O were set as internal control and negative control, respectively.
Twenty microliters of protein samples were separated on a 10% SDS-acrylamide gel (Bio-Rad) for 1 h at 150 V, and the proteins were transferred to nitrocellulose membrane (Whatman). After blocking in 5% fat-free milk, the membrane was treated with the dilution of the primary antibody overnight at 4°C and the dilution of the secondary IgG-horseradish peroxidase (HRP) conjugated antibody for 1 h at room temperature. All dilutions were in PBS containing 5% Blotto (Santa Cruz) and 0.1% Tween-20. The stained membranes were visualized by enhanced chemiluminescence reaction using the ECL Plus (GE Healthcare). Western blot experiments were repeated at least three times on every sample, with similar results.
Matrigel-coated filter inserts with 8-&mgr;m pores that fit into 24-well invasion chambers were obtained from Becton Dickinson. EC9706 cells transfected with N-cadherin RNAi, control vector, or the untreated cells were detached from the tissue culture plates, washed, resuspended in conditioned medium (106 cells/mL), and then added to the upper compartment of the invasion chamber with or without plasmin (1.8 mg). Conditioned medium (500 &mgr;L) was added to the lower compartment of the invasion chamber. The Matrigel invasion chambers were incubated at 37°C for 24 h in 5% CO2. After incubation, the filter inserts were removed from the wells, and the cells on the upper side of the filter were removed using cotton swabs. The filters were fixed, mounted, and stained according to the manufacturer’s instructions. The cells that invaded through the Matrigel were counted on the underside of the filter. Three to five invasion chambers were used for each experimental condition. The values obtained were calculated by averaging the total number of cells from three filters.
χ2 test and Spearman rank correlation coefficient analysis were used to assess the univariate association between the immunohistochemical status and the clinicopathological characteristics. Results were expressed as mean ± SD. Statistical analysis was made using one way ANOVA or paired-samples t test of SPSS 11.0. P < 0.05 was considered statistically significant.
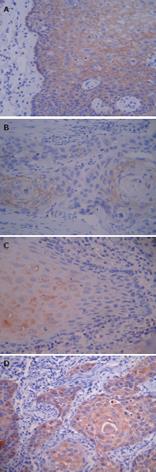
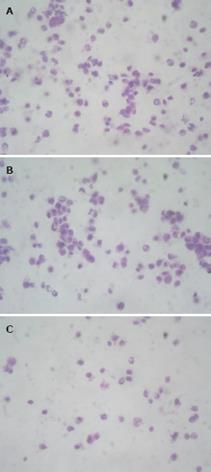
For E-cadherin, positive immunostaining was observed on the membrane of cancer cells and the intercellular junctions. It was strongly expressed in the normal esophageal squamous tissues (95.2%), moderately expressed in the adjacent atypical hyperplastic epithelium (71.0%), and weakly expressed in the ESCC tissues (40.3%) (Figure 1A, B and Table 2). Contrary to the E-cadherin, N-cadherin, which existed in the cytoplasm, was strongly expressed in the ESCC (75.8%) and moderately expressed in the adjacent atypical hyperplastic epithelium (61.3%), but weakly expressed in the normal esophageal squamous tissues (29.0%) (Figure 1C, D and Table 2). The correlations between the clinicopathological features and the expressions of E-cadherin and N-cadherin in the primary tumors are summarized in Table 2. Higher level of N-cadherin expression was significantly associated with higher histological grade, deeper invasion and more lymph node metastasis, while E-cadherin expression was associated with totally opposite sides.
In order to know whether the expression of E-cadherin and N-cadherin in these tumors was associated, a crosstable analysis was performed (Table 3), which showed that their expression was significantly negatively correlated.
| Items | n | E-cadherin | N-cadherin | ||||
| Cases (%) | χ2 | P | Cases (%) | χ2 | P | ||
| Histological classification | |||||||
| NEE | 62 | 59 (95.2) | 18 (29.0) | ||||
| AH | 31 | 22 (71.0) | 48.426 | 0.000 | 19 (61.3) | 29.091 | 0.000 |
| ESCC | 62 | 25 (40.3) | 47 (75.8) | ||||
| Histological grade | |||||||
| I | 15 | 11 (73.3) | 8 (53.3) | ||||
| II | 25 | 9 (36.0) | 9.962 | 0.007 | 19 (76.0) | 6.924 | 0.031 |
| III | 22 | 5 (22.7) | 20 (90.9) | ||||
| Depth of invasion | |||||||
| Not to serosa | 7 | 6 (85.7) | 4.797 | 0.029 | 2 (28.6) | 6.916 | 0.009 |
| To serosa | 55 | 19 (34.5) | 45 (81.8) | ||||
| Lymph node metastasis | |||||||
| Yes | 42 | 21 (50.0) | 3.897 | 0.048 | 28 (66.7) | 4.486 | 0.034 |
| No | 20 | 4 (20.0) | 19 (95.0) | ||||
| E-cadherin | N-cadherin (+) | N-cadherin (-) | γ | P | |
| + | 25 | 12 | 13 | -0.534 | 0.000 |
| - | 37 | 35 | 2 |
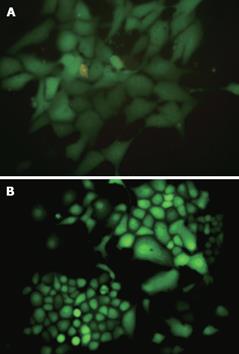
After G418 selection for 30 d, the stable colonies of pEGFP-MSCVneo plasmid (control vector) with viral titer 1 × 107 cfu/L and colonies of pMSCVneo/N-cadherin (RNAi vector) with viral titer 3 × 107 cfu/L were picked up to infect EC9706 cells (Figure 2A).
EC9706 cells were infected with the concentrated viral supernatant and selected with G418 as described above. After screening with G418 for 2 wk, the isolated G418-resistant clones (Figure 2B) were transferred into larger culture vessels to expand for further experiments.
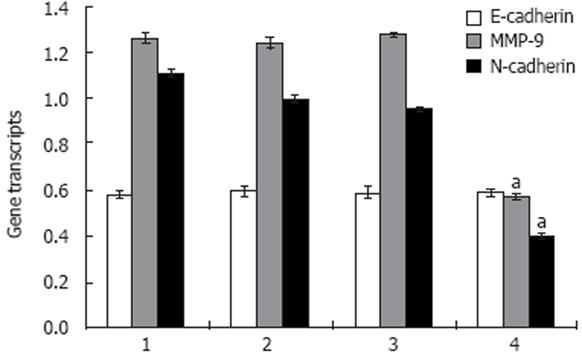
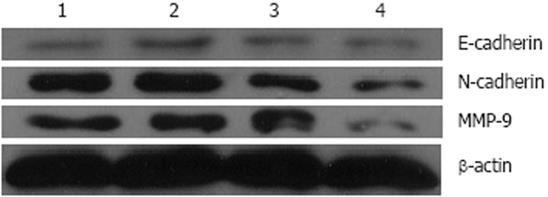
To investigate the expression phenotypes of E-cadherin and MMP-9 when N-cadherin was down-regulated, real-time PCR and Western blotting were employed to examine the expressions of the N-cadherin, E-cadherin and MMP-9 in ESCC cell line EC9706. Transfection of N-cadherin RNAi lowered N-cadherin mRNA level (0.397 ± 0.013) to less than 40%, compared with the untreated cells (1.000 ± 0.016) (Figure 3), and the N-cadherin protein (0.342 ± 0.006) to 50%, compared with the untreated cells (0.679 ± 0.004) (Figure 4).
The levels of MMP-9 mRNA and protein lowered by about 50% in the N-cadherin depleted cells compared with the untreated cells (Figures 3 and 4). The MMP-9 mRNA reduced from 1.241 ± 0.023 in the untreated cells to 0.566 ± 0.016 in N-cadherin RNAi cells, and the protein reduced from 0.628 ± 0.010 to 0.282 ± 0.010. However, it seems that N-cadherin shRNA did not affect the levels of E-cadherin mRNA and protein (Figures 3 and 4).
N-cadherin level was elevated with the malignancy in the ESCC tissues in a previous experiment. When N-cadherin was down-regulated, the migration of the cells was also reduced, compared with the untreated cells. Compared with the untreated EC9706 cells (Figure 5A) and the cells with control vector (Figure 5B), the N-cadherin negative-cells (Figure 5C) migration across the membranes decreased dramatically.
Cadherins are a class of type-1 transmembrane proteins. They are calcium dependent cell-cell adhesion glycoproteins. They play important roles in tissue formation and maintenance during embryonic development, and in the induction and maintenance of normal architecture and function in adult tissues. The most researched proteins of the cadherin family are E-cadherin and N-cadherin[8].
In the present research with ESCC, we found down-regulation of E-cadherin and increased N-cadherin. Normal E-cadherin expression contributes to the maintenance of epithelial integrity and polarized function[910]. Mutations in E-cadherin gene are correlated with gastric, breast, colorectal, thyroid and ovarian cancer[1112]. Much lower levels were found to be present in the poorly differentiated lung cancer, indicating the worse prognosis[13]. Unlike E-cadherin, which is inversely correlated with invasiveness, N-cadherin may promote motility and invasion in carcinoma cells. N-cadherin has been shown to enhance cell migration during epithelial-mesenchymal transformation[14]. Aberrant N-cadherin expression was also found in breast carcinoma cells and prostate carcinoma cells[34]. In epithelial carcinoma, E-cadherin is down-regulated in most cases, sometimes accompanied by the up-regulation of another cadherin, for example, N-cadherin[1516]. For the present research with ESCC, less E-cadherin and more N-cadherin were expressed in the ESCC tissue with deep invasion, poor differentiation and lymph node metastasis than with superfacial invasion, well differentiated and negative metastasis tissues. The N-cadherin expression was increased in the advanced ESCC tissues where E-cadherin was down-regulated, suggesting that they undergo a switch from E- to N-cadherin expression. The shift in expression from E- to N-cadherin and their mutually exclusive expression pattern in invasive tumor cell lines strongly reflect that the dedifferentiation from an epithelial to a mesenchymal phenotype was often associated with an increased invasive state[17]. The exact underlying mechanism has not been clear, but in many carcinomas, this “cadherin switch” was observed, especially in those where mild and non-progressive cells transformed into a more invasive phenotype[31819]. Ras, Src, Rho, PI3K and Wnt signaling pathways were supposed to be involved in this switch[20–22].
We have proved that reduced E-cadherin and increased N-cadherin were present in advanced ESCC, and the following RNAi-mediated N-cadherin silence in EC9706 cell line further disclosed the correlation of E-cadherin and N-cadherin with ESCC progression. The down-regulation of N-cadherin did not change the expression of E-cadherin mRNA and its product. While N-cadherin and MMP-9 were reduced significantly in transcription level and translation level, less cells demonstrated invasiveness.
Local tumor invasion is characterized by at least two changes of function by the cancer cells. Firstly, these cells express higher levels of membrane-type and secreted proteolytic enzymes (e.g. the MMPs) in comparison with their normal epithelioid counterparts. Their contribution to invasion ranges from breakdown of the extracellular matrix, over-release of pro-invasive factors, to cleavage of cell-cell adhesion molecules[23]. Secondly, cancer cells are more motile than normal epithelial cells. Local tumor invasion is also made possible by disruption of epithelial cell junctions. E-cadherin, a part of the adherens junctions, plays an important role in maintaining the epithelioid cell organization and in preventing invasion[24]. Loss of function is thought to contribute to progression in cancer by increasing proliferation, invasion, and/or metastasis[5]. Forced expression of N-cadherin in well-differentiated breast cell lines did not change their E-cadherin expression as indicated, but stimulated marked increases in cell migration and invasion[25]. The ability of N-cadherin-expressing EC9706 cells to adhere to N-cadherin-expressing endothelial sheets may facilitate their transit through the vasculature and improve their ability to form metastasis. The present results also confirmed that the N-cadherin expression down-regulation did not affect E-cadherin mRNA and protein levels, but the invasiveness of all the EC9706 cells was weakened as compared with the untreated EC9706 cells. It might be postulated that N-cadherin, rather than E-cadherin, plays an important role in the cancer progression and metastasis.
Less invasiveness was shown in the N-cadherin-negative tumor cells. Therefore, knocking down N-cadherin can weaken the aggressiveness of cancer cells, by the mechanism involving more than a change in cellular adhesion. MMPs were thought to predominantly degrade structural components of the extracellular matrix (ECM), thereby facilitating cell migration. In addition to cleaving structural ECM components, collagen type IV and the cell-adhesive molecules are also MMP substrates, increasing the invasive behavior of cells. Type I collagen is the predominant constituent of the perivascular ECM, and as mentioned previously, a variety of MMPs are capable of degrading collagen, including interstitial collagenase and neutrophil collagenase. The metastatic cells thus use these proteases to invade basement membrane and its underlying connective tissues and then subsequently through the basement membrane of the small blood vessels and lymphatics[26–30]. With RT-PCR and Western blotting, the level of MMP-9 mRNA and protein in the RNAi-mediated N-cadherin silencing EC9706 cells was found to be reduced as compared with untreated cells. It was supposed that the lower invasiveness of EC9706 cells was the consequence of down-regulation of N-cadherin, by the mechanism of decreasing the MMP-9 expression. The MMP-9 reduction resulted in less degradation of ECM, and thereby, the cancer cells were less aggressive. But which signaling pathway was involved in the N-cadherin to MMP-9 should be studied in the future researches.
In this study, decreased E-cadherin expression and increased N-cadherin expression were found more frequently in advanced ESCC than in low grade ESCC, confirming that the down-regulation of E-cadherin expression and up-regulation of N-cadherin expression were closely associated with the infiltration, invasion and metastasis of ESCC. In vitro experiments also demonstrated that even the E-cadherin mRNA and protein did not change much in the N-cadherin knocking down EC9706 cells, but the invasiveness of cancer cells was dramatically reduced. The decreased MMP-9 mRNA and its product were observed in the N-cadherin-negative cells, the majority of which lost their ability of migration in vitro. It was supposed that the N-cadherin played a role of facilitating cell invasion by MMP-9. In summary, our data suggest that N-cadherin is an important factor in the invasiveness of esophageal squamous cell carcinoma and N-cadherin may serve as a potential molecular target for biotherapy of esophageal squamous cell carcinoma.
Among the members of cadherin family, E-cadherin and N-cadherin have been extensively studied for their biological activities and associations with cancer cell invasion. Latest research on prostate cancer and breast cancer has proved that the up-regulated N-cadherin plays even more important roles in cell progression and metastasis.
The shift in expression from E- to N-cadherin and their mutually exclusive expression pattern in invasive tumors strongly reflects dedifferentiation from an epithelial to a mesenchymal phenotype, often associated with an increased invasive state. This “cadherin switch” has been observed, especially in those where mild and non-progressive cells transformed into more invasive phenotypes. Therefore, many studies have focused on the exact underlying mechanism involved in this cadherin switch.
In the current study, the expression of N-cadherin and E-cadherin was first examined in esophageal squamous cell carcinoma (ESCC) specimens and the results revealed that increased expression of N-cadherin and decreased expression of E-cadherin were related to invasion, differentiation, and lymph node metastasis, the roles of N-cadherin in the invasiveness of ESCC were first investigated in the EC9706 cell line transfected by retroviral-mediated N-cadherin RNAi and the results revealed that N-cadherin knock-down significantly decreased the invasiveness of EC9706 cells.
This study has indicated that N-cadherin is an important factor in the invasiveness of ESCC and N-cadherin may serve as a potential molecular target for biotherapy of ESCC.
Cadherins are a class of type-1 transmembrane proteins. They are calcium-dependent cell-cell adhesion glycoproteins. They play important roles in tissue formation and maintenance during embryonic development, and in the induction and maintenance of normal architecture and function in adult tissues.
The authors examined the expression pattern of N-cadherin and E-cadherin. They demonstrated that N-cadherin is an important factor in the invasiveness of ESCC and it may serve as a potential molecular target for biotherapy of ESCC.
| 1. | Usami Y, Satake S, Nakayama F, Matsumoto M, Ohnuma K, Komori T, Semba S, Ito A, Yokozaki H. Snail-associated epithelial-mesenchymal transition promotes oesophageal squamous cell carcinoma motility and progression. J Pathol. 2008;215:330-339. [Cited in This Article: ] |
| 2. | Nair KS, Naidoo R, Chetty R. Microsatellite analysis of the APC gene and immunoexpression of E-cadherin, catenin, and tubulin in esophageal squamous cell carcinoma. Hum Pathol. 2006;37:125-134. [Cited in This Article: ] |
| 3. | Jaggi M, Nazemi T, Abrahams NA, Baker JJ, Galich A, Smith LM, Balaji KC. N-cadherin switching occurs in high Gleason grade prostate cancer. Prostate. 2006;66:193-199. [Cited in This Article: ] |
| 4. | Nagi C, Guttman M, Jaffer S, Qiao R, Keren R, Triana A, Li M, Godbold J, Bleiweiss IJ, Hazan RB. N-cadherin expression in breast cancer: correlation with an aggressive histologic variant--invasive micropapillary carcinoma. Breast Cancer Res Treat. 2005;94:225-235. [Cited in This Article: ] |
| 5. | Derycke L, Morbidelli L, Ziche M, De Wever O, Bracke M, Van Aken E. Soluble N-cadherin fragment promotes angiogenesis. Clin Exp Metastasis. 2006;23:187-201. [Cited in This Article: ] |
| 6. | Xu JZ, Yang WT. The criteria for judging the results of immunohistochemical method. Zhonghua Zhongliu Zazhi. 1996;6:229-231. [Cited in This Article: ] |
| 7. | Kwon YJ, Hung G, Anderson WF, Peng CA, Yu H. Determination of infectious retrovirus concentration from colony-forming assay with quantitative analysis. J Virol. 2003;77:5712-5720. [Cited in This Article: ] |
| 8. | Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319-8326. [Cited in This Article: ] |
| 9. | Larue L, Ohsugi M, Hirchenhain J, Kemler R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc Natl Acad Sci USA. 1994;91:8263-8267. [Cited in This Article: ] |
| 10. | Nelson WJ, Shore EM, Wang AZ, Hammerton RW. Identification of a membrane-cytoskeletal complex containing the cell adhesion molecule uvomorulin (E-cadherin), ankyrin, and fodrin in Madin-Darby canine kidney epithelial cells. J Cell Biol. 1990;110:349-357. [Cited in This Article: ] |
| 11. | Bosch FX, Andl C, Abel U, Kartenbeck J. E-cadherin is a selective and strongly dominant prognostic factor in squamous cell carcinoma: a comparison of E-cadherin with desmosomal components. Int J Cancer. 2005;114:779-790. [Cited in This Article: ] |
| 12. | Bellovin DI, Bates RC, Muzikansky A, Rimm DL, Mercurio AM. Altered localization of p120 catenin during epithelial to mesenchymal transition of colon carcinoma is prognostic for aggressive disease. Cancer Res. 2005;65:10938-10945. [Cited in This Article: ] |
| 13. | Moersig W, Horn S, Hilker M, Mayer E, Oelert H. Transfection of E-cadherin cDNA in human lung tumor cells reduces invasive potential of tumors. Thorac Cardiovasc Surg. 2002;50:45-48. [Cited in This Article: ] |
| 14. | Hazan RB, Kang L, Whooley BP, Borgen PI. N-cadherin promotes adhesion between invasive breast cancer cells and the stroma. Cell Adhes Commun. 1997;4:399-411. [Cited in This Article: ] |
| 15. | Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol. 2000;148:779-790. [Cited in This Article: ] |
| 16. | Utsuki S, Oka H, Sato Y, Tsutiya B, Kondo K, Tanizaki Y, Tanaka S, Fujii K. E, N-cadherins and beta-catenin expression in medulloblastoma and atypical teratoid/rhabdoid tumor. Neurol Med Chir (Tokyo). 2004;44:402-406; discussion 407. [Cited in This Article: ] |
| 17. | Hazan RB, Qiao R, Keren R, Badano I, Suyama K. Cadherin switch in tumor progression. Ann N Y Acad Sci. 2004;1014:155-163. [Cited in This Article: ] |
| 18. | Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin Cancer Res. 2007;13:7003-7011. [Cited in This Article: ] |
| 19. | Hotz B, Arndt M, Dullat S, Bhargava S, Buhr HJ, Hotz HG. Epithelial to mesenchymal transition: expression of the regulators snail, slug, and twist in pancreatic cancer. Clin Cancer Res. 2007;13:4769-4776. [Cited in This Article: ] |
| 20. | Nawshad A, Lagamba D, Polad A, Hay ED. Transforming growth factor-beta signaling during epithelial-mesenchymal transformation: implications for embryogenesis and tumor metastasis. Cells Tissues Organs. 2005;179:11-23. [Cited in This Article: ] |
| 21. | Alexander NR, Tran NL, Rekapally H, Summers CE, Glackin C, Heimark RL. N-cadherin gene expression in prostate carcinoma is modulated by integrin-dependent nuclear translocation of Twist1. Cancer Res. 2006;66:3365-3369. [Cited in This Article: ] |
| 22. | Gotzmann J, Mikula M, Eger A, Schulte-Hermann R, Foisner R, Beug H, Mikulits W. Molecular aspects of epithelial cell plasticity: implications for local tumor invasion and metastasis. Mutat Res. 2004;566:9-20. [Cited in This Article: ] |
| 23. | Stetler-Stevenson WG, Yu AE. Proteases in invasion: matrix metalloproteinases. Semin Cancer Biol. 2001;11:143-152. [Cited in This Article: ] |
| 24. | Behrens J, Mareel MM, Van Roy FM, Birchmeier W. Dissecting tumor cell invasion: epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion. J Cell Biol. 1989;108:2435-2447. [Cited in This Article: ] |
| 25. | Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J Cell Biol. 1999;147:631-644. [Cited in This Article: ] |
| 26. | Curran S, Murray GI. Matrix metalloproteinases in tumour invasion and metastasis. J Pathol. 1999;189:300-308. [Cited in This Article: ] |
| 27. | Rajapakse N, Kim MM, Mendis E, Huang R, Kim SK. Carboxylated chitooligosaccharides (CCOS) inhibit MMP-9 expression in human fibrosarcoma cells via down-regulation of AP-1. Biochim Biophys Acta. 2006;1760:1780-1788. [Cited in This Article: ] |
| 28. | Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161-174. [Cited in This Article: ] |
| 29. | Ortega N, Behonick DJ, Werb Z. Matrix remodeling during endochondral ossification. Trends Cell Biol. 2004;14:86-93. [Cited in This Article: ] |
| 30. | He Y, Liu XD, Chen ZY, Zhu J, Xiong Y, Li K, Dong JH, Li X. Interaction between cancer cells and stromal fibroblasts is required for activation of the uPAR-uPA-MMP-2 cascade in pancreatic cancer metastasis. Clin Cancer Res. 2007;13:3115-3124. [Cited in This Article: ] |