Published online Oct 21, 2005. doi: 10.3748/wjg.v11.i39.6125
Revised: January 23, 2005
Accepted: January 26, 2005
Published online: October 21, 2005
AIM: To determine the role of Fas/Fas ligand (FasL) in the immune escape of colon cancer cells.
METHODS: Immunohistochemistry was used to observe the expression of Fas and FasL in the tissues of colon cancer patients.In situ hybridization was used to detect the localization of FasL mRNA expression in cancer tissues. Terminal deoxynucleotide transferase-mediated dUTP nick end labeling (TUNEL) assay and CD45 staining were performed to detect the apoptosis of tumor-infiltrating lymphocytes (TILs). Co-culture assays of colon cancer cells (SW480) and Jurkat cells (Fas-sensitive cells) were performed to observe the counterattack of colon cancer cells to lymphocytes.
RESULTS: Of 53 cases of colon carcinomas, 23 cases (43.4%) expressed Fas which was significantly lower as compared to the normal colonic mucosa (73.3%, P<0.01), and 45 cases (84.9%) of colon carcinomas expressed FasL, whereas only two cases (3.75%) in normal mucosa expressed FasL. FasL expression in the colon cancer cells was found to be associated with increased cell death of TILs. The apoptotic rate of TIL in the FasL-positive staining regions of tumor cells was significantly higher than that in the FasL-negative staining region (54.84 ± 2.79% vs 25.73 ± 1.98%, P<0.01). The co-culture of SW480 cells and Jurkat cells confirmed the function of FasL on the SW480 cells. The apoptotic rates of Jurkat cells were found to be related with the amount of SW480 cells.
CONCLUSION: Colon cancer cells can escape the immune surveillance and killing via decreasing Fas expression, and can counterattack the immune system via increasing FasL expression. Fas/FasL can serve as potential targets for effective antitumor therapy.
- Citation: Zhu Q, Liu JY, Xu HW, Yang CM, Zhang AZ, Cui Y, Wang HB. Mechanism of counterattack of colorectal cancer cell by Fas/Fas ligand system. World J Gastroenterol 2005; 11(39): 6125-6129
- URL: https://www.wjgnet.com/1007-9327/full/v11/i39/6125.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i39.6125
Fas (Apo-1/CD95) is a 45-ku type I membrane protein, and Fas ligand (FasL) is a 37-40-ku type II membrane protein, which belong to the tumor necrosis factor receptor and ligand families. Activation of Fas by certain anti-Fas antibodies or by FasL results in apoptotic cell death in susceptible cells. Fas/FasL interaction constitutes one of the main systems mediating the cytotoxicity of T cells and regulating immune responses, tissue development, and homeostasis[1,2].
Fas system is one of the killing pathways by cytotoxic T lymphocytes (CTL) to tumor cells in human body; however, in recent reports, the most enticing interpretation of FasL expression by tumor cells is that it offers a further novel mechanism of immune evasion. Tumor cells could counterattack the immune system of human body by inducing apoptosis of immune effector cells through Fas/FasL system[3]. We used in vivo and in vitro methods to determine the role of Fas/FasL in the immune escape of colon cancer cells.
Fifty-three colon carcinomas were collected after surgical resection performed at Shandong Provincial Hospital. None of the patients had received chemo-, radio- or immuno-therapy before resection. Paraffin wax-embedded surgically resected tumor sections were deparaffinized in xylene and rehydrated before analysis. The primary antibodies were purified rabbit polyclonal anti-human Fas and FasL-specific IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 0.1 mg/mL in a wash buffer. Antibody binding was localized using a biotinylated secondary antibody, avidin conjugated horseradish peroxidase, and diamiobenzidine substrate, contained within the ABC detection kit. The immunizing peptide (Santa Cruz Biotechnology), to which the antibody was raised, was included at 1 mg/mL during primary antibody incubation as a direct internal competitive control for antibody specificity.
In situ hybridization was performed on paraffin-embedded human colonic tumor sections (4 mm thick), mounted on aminopropylethoxysilane-treated slides. A digoxigenin-labeled RNA hybridization probe (344 bp) was used, corresponding to codons 96-210 of the human FasL cDNA sequence. The nucleotide sequence of the FasL probe showed insignificant homology to any other sequence in the EMBL DNA sequence database. Prehybridization and hybridization were performed as previously described[3]. In order to confirm the specificity of hybridization, a 10-fold excess of unlabeled riboprobe was added to the digoxigenin-labeled probe prior to hybridization.
To identify tumor-infiltrating lymphocytes (TILs), CD45 staining was performed on consecutive sections with a mouse anti-human CD45 monoclonal IgG (clone 2B11+PD7/26, Dako Corp., Carpinteria, CA, USA, dilution 1/70) according to the standard immunohistochemical methods as described above. Alkaline phosphatase conjugated anti-alkaline phosphatase complex (DO651, Dako Corp.) was used to display the color of CD45 staining. The membrane of the CD45-positive cells was red. Apoptosis of TILs was detected in situ in frozen sections of resected tissues using Terminal deoxynucleotide transferase-mediated dUTP nick end labeling (TUNEL) assay (Boehringer Mannheim GmbH, Mannheim, Germany) applied to the histological sections. Visualization of the final reaction product was achieved by diaminobenzidine. TUNEL-positive TILs (with red membrane and brown nucleus) were counted at high power (×400) from fields of view chosen according to a systematic random sampling method. Approximately 1 000 TILs were counted and a TUNEL index (TI) was expressed as the percentage of TUNEL lymphocytes.
Colon cancer SW480 cells were grown in RPMI medium supplemented with penicillin G (100 U/mL), streptomycin (100 mg/mL) and 100 mL/L fetal bovine serum at 37 °C in humidified atmosphere containing 50 mL/L CO2 in air. Cell growth was determined by the 3-(4,5-methylthiazol-2yl)-2,5-diaphenyl-tertrazolium bromide (MTT) colorimetric assay. The assay was initiated by adding MTT solution to each well at a final concentration of 0.625 mg MTT/mL medium. After 4 h, the optical density was determined with an ELISA plate reader after removal of the medium and dissolving of the dye crystals in acidified isopropylalcohol.
Different concentrations of SW480 cells (0, 1 ×105/mL, 2×105/mL, 4×105/mL) were plated in triplicate on 24-multiwell tissue culture dishes. After 24 h, 2×104/mL Jurkat cells were seeded onto the plates. The wells with colon cancer cells alone (without overlying Jurkat cells) were maintained as additional controls. After 48 h, the non-adherent phase of the mixed culture was aspirated and assayed for viability using MTT assay. In experiments using recombinant FasFc (ZB4) as a competitor, similar experimental conditions were used with the following exception. MTT assay was used to evaluate the amount of Jurkat cells at the co-culture time of 24, 48, 72, and 96 h.
After being co-cultured with SW480 for 48 h, the Jurkat cells were collected and added with Hoechst 33258 (Sigma) on a slide glass. The specimens were examined under a Nikon fluorescence microscope with B2-type filter (magnification ×200). When apoptotic bodies or chromatin condensations were observed, the observation was defined as apoptosis. Apoptotic cells were assessed morphologically by staining with Hoechst 33258 using cells fixed with Clarke fixative (ethanol:acetic acid = 3:1). Apoptotic index (AI) was defined as follows: AI = 100×apoptotic cells/200 cells.
All experiments for cell cultures were performed at least in triplicate. Data were expressed as mean±SD. Statistical analysis was performed by one-way variance (ANOVA), and the comparisons between groups were performed by independent sample t-test. The regressive relation between two variables was performed by linear regression. A two-tailed P value less than 0.05 was considered statistically significant.
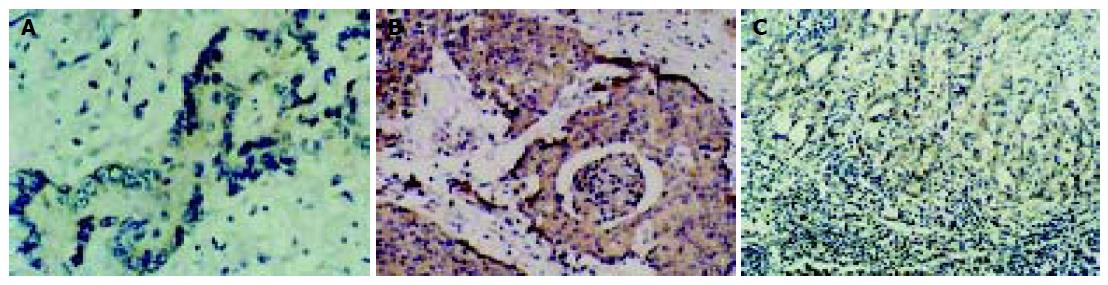
Of 53 cases of colon carcinomas, 23 cases (43.4%) expressed Fas which was significantly lower as compared to that in normal colonic mucosa (73.3%) (P<0.01), and 45 cases (84.9%) of colon carcinomas expressed FasL, whereas only 2 cases (3.75%) in normal mucosa expressed FasL. Intensity and extent of positive staining varied within individual tumors. Neoplastic areas with positive and negative staining for FasL frequently co-existed within the same tumor. The extent of FasL expression varied significantly between different cancer stages and differentiations (data not shown, Figures 1A and B). FasL expression also existed on TILs in all cases. TILs were smaller in volume than cancer cells, which could distinguish the two kinds of FasL-expressing cells.
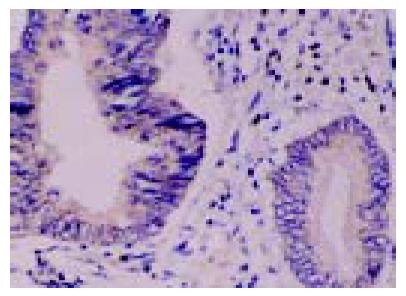
In situ hybridization was performed on paraffin-embedded human colonic tumor sections in order to detect the localization of FasL mRNA expression. FasL mRNA was detected in the colon cancer cells and in TILs (Figure 2). The location of FasL mRNA and expression of FasL protein coincided.
To further study the role of FasL expressed in cancer cells in tissues, 23 cases of lymph node metastases from colorectal carcinomas were obtained to detect the expression of FasL immunohistochemically. All cases of lymph node metastases showed FasL immunoreactivity in greater than 60% of the metastatic cells (Figure 1C).
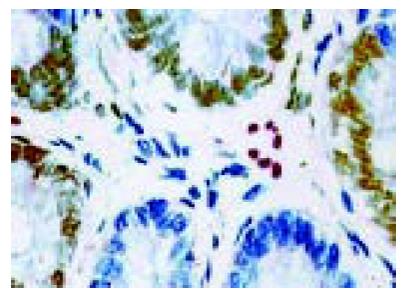
To evaluate apoptosis of TILs, we detected the presence of apoptotic cells in tissue sections using CD45 staining (WBC common antigen) and TUNEL techniques. Tonsil sections were included as positive controls, stained strongly, and omission of the primary antibody abolished staining. To quantify the extent of apoptosis of TILs, the percentage of TUNEL-positive and CD45-positive cells was calculated. The apoptotic rate of TILs in FasL-positive staining regions of tumor cells was significantly higher than that in FasL-negative region of tumor cells (54.84±2.79% vs 25.73±1.98%, P<0.01, Figure 3). In vivo FasL expression in colon cancer cells was found to be associated with increased cell death of TILs.
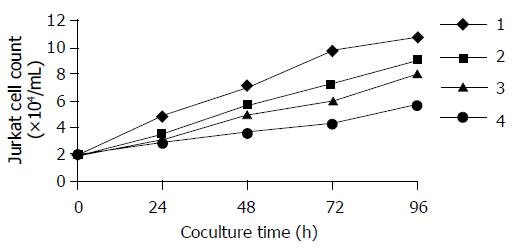
Jurkat cells grown in the absence of human colon cancer cells showed a marked proliferation after 48 h. However, when co-cultured over a mono-layer of the FasL-expressing colon cancer cell line SW480, we observed a marked decrease in Jurkat cells while using MTT assay (Figure 4). The Jurkat cell killing was largely inhibited by pre-incubating the colon cancer mono-layer with a low concentration of recombinant Fas neutral antibody ZB4, which is capable of binding to FasL and blocking Fas/FasL interaction.
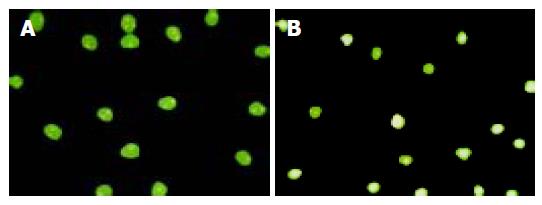
We used fluorescence microscopy to observe the shape of apoptotic Jurkat cells by the untreated SW480 cells. Condensation of chromatin, nuclear fragmentation and typical apoptotic bodies were observed in the tested group (Figure 5). The fluorescence microscopy results revealed that the apoptotic rates of Jurkat cells induced by SW480 cells after being co-cultured had a significant difference with the natural apoptotic rate of Jurkat cells (Table 1)
Fas molecule was identified for the first time in 1989 by two research groups of Germany and Japanese separately[4,5]. In the beginning days of the twentieth century, researchers worldwide paid attention to the role of Fas system in the process of CTL killing cancer cells. It has been concluded that CTL-induced apoptosis of cancer cells via Fas system is one of the two main ways of CTL killing cancer cells, namely, Fas-based cytotoxicity is one of the two molecular mechanisms of T cell-mediated cytotoxicity, the other is perforin-based[1].
In recent years, some reports found that caner cells could decrease Fas expression which could escape the immune surveillance and killing, but could express FasL which could induce apoptosis of CTL, especially TILs[3,6,7]. In these cases, Fas and FasL may contribute to tumor immune privilege by inducing FasL-mediated apoptosis of host CTL and NK cells. We found that Fas expression on the colon cancer cells decreased significantly compared to the normal colonic epithelial cells (P<0.05), which was in agreement with the previous reports by Houston et al[8] and Okada et al[9]. Colon cancer could escape the immune surveillance and killing by decreasing Fas expression.
In addition to decrease in Fas expression, functional FasL expression by certain cancer cell types has been implicated in tumor escape via destruction of infiltrating Fas-bearing lymphocytes. In this study, we also found that FasL expression was absent in normal colonic epithelial cells, whereas markedly increased in colon carcinomas (P<0.05), and it was obviously correlated with apoptosis of TILs in colon cancer tissues. To evaluate the functional significance of FasL expression in colon cancer cells, we performed co-culture experiments using the Fas-sensitive Jurkat cell line (of human T-cell origin) as a target cell. These cells have been demonstrated to be capable of apoptosis following exposure to FasL. In vitro, colon cancer cells without any treatment could mediate apoptosis of Jurkat cells by Fas and FasL. Expression of FasL potentially enables colon cancer cells to counterattack Fas-sensitive anti-tumor immune effector cells by delivering a Fas-mediated apoptotic death signal. Ohta et al[10] reported that 37 (82%) of the primary pancreatic cancers and both of the hepatic metastases expressed FasL, and the patients with FasL-positive tumor had significantly shorter survival times as compared with the FasL-negative, suggesting FasL as a significant prognostic factor of pancreatic cancer.
FasL expression may have effects on the immune system beyond the tumor site, and soluble FasL has been identified in certain types of FasL-positive leukemias and lymphomas[11]. Circulating forms of FasL (soluble FasL) were also found in blood from cancer patients, this could contribute to the generalized depression of cellular immunity. Thus, FasL expression might facilitate tumor invasion by inducing apoptosis in surrounding Fas-positive tissue, allowing the tumor to grow into the resulting space.
We found that large areas of most colonic tumors co-expressed FasL and Fas, indicating that these cells must have disabled the intracellular Fas apoptotic pathway due to intracellular defects in Fas signal transduction. Strand et al[12] reported that MMP-7 cleavage of Fas resulted in reduced Fas surface expression and decreased Fas-mediated apoptosis sensitivity of tumor cells. Moreover, overexpression of inhibitors of apoptosis or intracellular apoptotic signal transduction may further contribute to the observed resistance to Fas-dependent apoptosis in colon cancer[13]. Several additional mechanisms of the Fas resistance have been described. Expression of soluble Fas by liver cancer cells may be involved in the Fas resistance of liver cancer in vivo[14].
A "Fas counterattack"model of colon cancer immune escape can be proposed, in which colon cancer cells, by expressing FasL, may remove Fas-sensitive immune effector cells by apoptosis. Increased expression of FasL is associated with decreased Fas levels in colon cancer cells that can escape immune surveillance and facilitate tumor progression and metastasis. Fas/FasL can serve as potential targets for effective antitumor therapy[15].
Science Editor Kumar M and Guo SY Language Editor Elsevier HK
| 1. | Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449-1456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3053] [Cited by in F6Publishing: 2970] [Article Influence: 102.4] [Reference Citation Analysis (0)] |
| 2. | Maher S, Toomey D, Condron C, Bouchier-Hayes D. Activation-induced cell death: the controversial role of Fas and Fas ligand in immune privilege and tumour counterattack. Immunol Cell Biol. 2002;80:131-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 3. | Perabo FG, Kamp S, Schmidt D, Lindner H, Steiner G, Mattes RH, Wirger A, Pegelow K, Albers P, Kohn EC. Bladder cancer cells acquire competent mechanisms to escape Fas-mediated apoptosis and immune surveillance in the course of malignant transformation. Br J Cancer. 2001;84:1330-1338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Trauth BC, Klas C, Peters AM, Matzku S, Möller P, Falk W, Debatin KM, Krammer PH. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989;245:301-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1286] [Cited by in F6Publishing: 1269] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 5. | Yonehara S, Ishii A, Yonehara M. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989;169:1747-1756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1020] [Cited by in F6Publishing: 1044] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 6. | Krishnakumar S, Kandalam M, Mohan A, Iyer A, Venkatesan N, Biswas J, Shanmugam MP. Expression of Fas ligand in retinoblastoma. Cancer. 2004;101:1672-1676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Abrams SI. Positive and negative consequences of Fas/Fas ligand interactions in the antitumor response. Front Biosci. 2005;10:809-821. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Houston A, Bennett MW, O'Sullivan GC, Shanahan F, O'Connell J. Fas ligand mediates immune privilege and not inflammation in human colon cancer, irrespective of TGF-beta expression. Br J Cancer. 2003;89:1345-1351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Okada K, Komuta K, Hashimoto S, Matsuzaki S, Kanematsu T, Koji T. Frequency of apoptosis of tumor-infiltrating lymphocytes induced by fas counterattack in human colorectal carcinoma and its correlation with prognosis. Clin Cancer Res. 2000;6:3560-3564. [PubMed] [Cited in This Article: ] |
| 10. | Ohta T, Elnemr A, Kitagawa H, Kayahara M, Takamura H, Fujimura T, Nishimura G, Shimizu K, Yi SQ, Miwa K. Fas ligand expression in human pancreatic cancer. Oncol Rep. 2004;12:749-754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Hallermalm K, De Geer A, Kiessling R, Levitsky V, Levitskaya J. Autocrine secretion of Fas ligand shields tumor cells from Fas-mediated killing by cytotoxic lymphocytes. Cancer Res. 2004;64:6775-6782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Strand S, Vollmer P, van den Abeelen L, Gottfried D, Alla V, Heid H, Kuball J, Theobald M, Galle PR, Strand D. Cleavage of CD95 by matrix metalloproteinase-7 induces apoptosis resistance in tumour cells. Oncogene. 2004;23:3732-3736. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Kase S, Osaki M, Adachi H, Kaibara N, Ito H. Expression of Fas and Fas ligand in esophageal tissue mucosa and carcinomas. Int J Oncol. 2002;20:291-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 14. | Lee SH, Shin MS, Lee HS, Bae JH, Lee HK, Kim HS, Kim SY, Jang JJ, Joo M, Kang YK. Expression of Fas and Fas-related molecules in human hepatocellular carcinoma. Hum Pathol. 2001;32:250-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Kim R, Emi M, Tanabe K, Uchida Y, Toge T. The role of Fas ligand and transforming growth factor beta in tumor progression: molecular mechanisms of immune privilege via Fas-mediated apoptosis and potential targets for cancer therapy. Cancer. 2004;100:2281-2291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |