Published online Jul 28, 2013. doi: 10.3748/wjg.v19.i28.4475
Revised: February 13, 2013
Accepted: March 28, 2013
Published online: July 28, 2013
AIM: To describe the role of resistin in liver fibrosis.
METHODS: For the in vivo animal study, Sprague Dawley rats were subjected to bile duct ligation (BDL) for 4 wk. Rat liver, adipose tissue (epididymal fat) and serum were analyzed for resistin expression. For the in vitro experiment, rat primary hepatic stellate cells (HSCs) and Kupffer cells (KCs) were used. HSCs were exposed to recombinant resistin, and collagen I, transforming growth factor β1, α smooth muscle actin, tissue inhibitor of metalloproteinase 1 and connective tissue growth factor expression were analyzed. Resistin gene and protein expression was quantified as was the expression of pro-inflammatory cytokines including tumor necrosis factor α (TNFα), interleukin (IL)-1, IL-6, IL-8 and monocyte chemotactic protein-1 (MCP-1). The effects of resistin on HSC proliferation, migration and apoptosis were determined. The effects of resistin on KCs were also investigated.
RESULTS: Following BDL, rat epididymal fat and serum rather than liver showed higher resistin expression compared to control rats. In liver, resistin was expressed in quiescent HSCs and KCs. Resistin treatment resulted in enhancement of TNFα, IL-6, IL-8 and MCP-1 gene expression and increased IL-6 and MCP-1 protein in HSCs. Resistin activated HSC phospho-MAPK/p38, and p38 inhibition diminished IL-6 and MCP-1 expression. Furthermore, resistin facilitated HSC proliferation and migration, but decreased apoptosis which was via an IL-6 and MCP-1 mechanism. Finally, resistin-induced transforming growth factor β1 from KCs enhanced HSC collagen Iexpression.
CONCLUSION: Resistin directly and indirectly modulates HSC behavior towards a more pro-fibrogenic phenotype.
Core tip: Resistin activated hepatic stellate cells (HSCs) phospho-MAPK/p38, and p38 inhibition diminished interleukin 6 (IL-6) and monocyte chemotactic protein-1 (MCP-1) expression. Furthermore, resistin facilitated HSC proliferation and migration, but decreased apoptosis which was via an IL-6 and MCP-1 mechanism. Finally, resistin-induced transforming growth factor β1 from Kupffer cells enhanced HSC collagen I expression. Resistin directly and indirectly modulates HSC behavior towards a more pro-fibrogenic phenotype.
- Citation: Dong ZX, Su L, Brymora J, Bird C, Xie Q, George J, Wang JH. Resistin mediates the hepatic stellate cell phenotype. World J Gastroenterol 2013; 19(28): 4475-4485
- URL: https://www.wjgnet.com/1007-9327/full/v19/i28/4475.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i28.4475
Metabolic alterations such as glucose intolerance, increased energy expenditure, and negative nitrogen balance with depletion of fat and skeletal muscle mass are frequently encountered in patients with cirrhosis[1,2]. In particular, glucose intolerance and insulin resistance are almost universal[3,4] and, in part, mediate the progression of fibrosis[5]. However, the mechanisms whereby metabolic alterations mediate disease progression are unclear. Adipose tissue secreted proteins (adipokines) such as leptin and adiponectin modulate metabolic homeostasis and have direct effects on the hepatic fibrogenic cascade. For example, leptin promotes liver fibrosis, while adiponectin is anti-inflammatory and anti-fibrotic[6-9]. Resistin, another adipokine, has been reported to be associated with impaired insulin sensitivity and glucose intolerance[10-13], but its role in hepatic fibrosis has not been adequately delineated[14-17].
Resistin is almost exclusively expressed in the white adipose tissue of rodents, but is expressed in humans predominantly by monocytes/macrophages[18]. Several reports indicate that the serum levels of resistin are elevated in cirrhosis[14-16], increasing progressively with worsening liver function as determined by the Child-Pugh class[17]. Furthermore, in patients with liver disease, resistin levels are correlated with the extent of insulin resistance and with clinical complications and prognosis[16]. In a recent animal study[19], hyperinsulinemia and increased tumor necrosis factor α (TNFα) secretion following bile duct ligation (BDL) were shown to up-regulate adipose tissue resistin gene expression which could subsequently contribute to liver fibrosis. A recent human study noted that resistin expression was low in normal liver, but was increased in severe fibrosis, suggesting that intra-hepatic resistin derived from monocytes/macrophages might contribute to fibrosis[15,20,21].
In the present study, we undertook in vivo and in vitro studies to elucidate the role of resistin in liver fibrosis. We show that resistin has increased expression in the epididymal fat and serum of cirrhotic rats. Resistin has a pro-inflammatory role in mediating the release of TNFα, interleukin (IL)-6, IL-8 and monocyte chemotactic protein-1 (MCP-1) in hepatic stellate cells (HSCs). Importantly, we demonstrate that resistin directly and indirectly mediates HSC activated phenotype in IL-6/MCP-1 and transforming growth factor (TGF) β1 dependent mechanisms, respectively, indicating that resistin contributes to the pro-inflammatory and pro-fibrotic phenotype of activated HSCs.
Recombinant mouse resistin protein, recombinant IL-6, and MCP-1 ELISA kits, IL-6, MCP-1 and TGFβ1 antibodies were purchased from RD Systems (Minneapolis, MN, United States). Nycodenz, α smooth muscle actin (αSMA) mouse antibody was purchased from Sigma-Aldrich (St. Louis, MO, United States). Pronase E, DNase I and collagenase B were purchased from Roche Applied Sciences (Indianapolis, IN, United States). Resistin rabbit polyclonal antibody was purchased from Abbiotec TM (San Diego, CA, United States). p-p38, pERK1/2, pJNK, nuclear factor κB (NF-κB), p-p65 and p-p50 mouse monoclonal antibodies, p-p38 inhibitor (SB203580) and pJNK inhibitor (SP600125) were purchased from Cell Signaling Technology, Inc (Beverly, MA, United States). The BrdU ELISA kit was purchased from Roche Diagnostics (Castle Hills, NSW, Australia). Anti-mouse IgG conjugated to horseradish peroxidase was purchased from GE Healthcare Life Sciences (Piscataway, NJ, United States). DMEM medium was obtained from Invitrogen (Carlsbad, CA, United States).
Male Sprague Dawley (SD) rats were obtained from the Animal Resources Centre (Perth, Australia). All animals were maintained under 12-h light/dark cycles with food and water ad libitum. For the in vivo experiment, BDL or a sham surgical procedure was performed on rats. After 4 wk, rat liver, epididymal fat and serum were collected for resistin quantification. All experimental protocols were approved by the Sydney West Area Health Service Animal Research Ethics Committee.
Rat HSCs were isolated by a two-step (collagenase B and pronase E) perfusion method under ketamine and xylazine anesthesia as reported previously[6]. Briefly, rat liver was perfused through the portal vein using Ca2+- and Mg2+-free Gey’s Balanced Salt Solution (GBSS, Sigma, United States) and then sequentially with pronase E followed by collagenase B (Roche Applied Science, Castle Hills, NSW, Australia). The liver was excised, gently dispersed in GBSS containing 0.01% DNase I and the cell suspension filtered through a sterile nylon mesh and subjected to low-speed centrifugation. The resultant cell pellet was mixed with 30% Nycodenz to obtain an 11% final Nycodenz/cell suspension. After centrifugation at 1400 g for 20 min, HSCs were collected, resuspended in culture medium, and plated on 6 well plates with 10% FCS/DMEM at a density of 0.8 × 106 cells/well. Cell viability was assessed by trypan blue exclusion and was routinely more than 95%. Purity was 95% as determined by morphology, vitamin A autofluorescence and desmin positivity. HSCs were maintained in 95% air and 5% CO2 in DMEM (Gibco, United States) with 10% FCS and 1% penicillin/streptomycin. KCs were further obtained and purified by elutriation[6]. KCs were identified by their ability to phagocytose latex beads; viability was > 96% and purity > 98%. KCs were cultured in 10% FCS/DMEM/1% penicillin-streptomycin.
Treatments: For recombinant mouse resistin (RD Systems, Minneapolis, MN, United States), we undertook a dose ranging study based on previous reports[20-23] using 10, 50, 250 and 500 ng/mL. We found that 500 ng/mL was the optimal dose which was used in all subsequent experiments. Primary rat HSCs and KCs were cultured for the time periods indicated and serum starved (0.2%) for 4 h prior to treatment. Subsequently, control (vehicle) and resistin (500 ng/mL) were added to the culture wells. After 24 h or extended culture as indicated, total RNA and protein were extracted. For the KC-HSC co-culture experiment, control (vehicle) and resistin (500 ng/mL) were added to cultured KCs at day 2 for 24 h, then KCs were washed three times with PBS and fresh medium was added and cultured for another 24 h. Afterwards, KC conditioned medium (KM) was transferred to HSCs at day 4 for 24 h co-culture. In one experiment, lipopolysaccharide (LPS, 50 ng/mL) was used to further activate cultured KCs.
Total cellular RNA was prepared from HSCs using TRI@ REAGENT (Molecular Research Center, INC., Cincinnati, OH, United States). Complementary DNA (cDNA) was synthesized from 1 μg RNA using SuperScript III reverse transcriptase and 0.5 nmol of random primers (Invitrogen, Carlsbad, CA, United States). Real-time quantitative reverse-transcription polymerase chain reaction (qRT-PCR) was performed using SYBR Green Platinum SYBR Green SuperMix (Invitrogen, United States). The synthesized cDNA was amplified using the following sequence specific primers: resistin 5’-CAAGACTTCAGCTCCCTACTGC-3’ (forward) and 5’-GACGGTTGTGCCTTCTGG-3’ (reverse); collagenα1 (I) 5’-TTCACCTACAGCACGCTTGTG-3’ (forward) and 5’-TCTTGGTGGTTTTGTATTCGATGA-3’ (reverse); TGFβ1 5’-TCGACATGGAGCTGG TGAAA-3’ (forward) and 5’-GAGCCTTAGTTTGGACAGGATCTG-3’ (reverse); αSMA5’-CGATAGAACACGGCATCATC-3’ (forward) and 5’-CATCAGGCAGTTCGTAGCTC-3’ (reverse); tissue inhibitor of metalloproteinase 1 (TIMP1) 5’-AAGGGCTACCAGAGCGATCA-3’ (forward) and 5’-GGTATTGCCAGGTGCACAAAT-3’ (reverse); connective tissue growth factor (CTGF) 5’-CGCCAACCGCAAGATTG-3’ (forward) and 5’-ACACGGACCCACCGAAGAC-3’ (reverse); IL-6 5’- CCCTTCAGGAACAGCTATGAA-3’ (forward) and 5’-ACAACATCAGTCCCAAGAAGG-3’ (reverse); IL-1 α 5’-ACATCCGTGGAGCTCTCTTTACA-3’ (forward) and 5’-TTAAATGAACGAAGTGAACAGTACAGATT-3’ (reverse); IL-1β 5’-TACCTATGTC TTGCCCGTGGAG-3’ (forward) and 5’-ATCATCCCACGAGTCACAGAGG-3’ (reverse); TNFα 5’-GCCCAGACCCTCACACTC-3’ (forward) and 5’-CCACTCCAGCTGCTCCTCT -3’(reverse); IL-8 5’-TCTGCAGCTCTGTGTGAAGG-3’ (forward) and 5’-AATTTCTGGTT TGGCGCAGT-3’ (reverse); MCP-1 5’-AGCATCCACGTGCTGTCTC-3’ (forward) and 5’-GATCATCTTGCCAGTGAATGAG-3’ (reverse). The relative amount of mRNA was calculated by reference to a calibration curve. The final result for each sample was normalized to the respective β actin value.
Immunoblotting: Cell culture media were removed and the cells washed with PBS and lysed on ice in a buffer containing 20 mmol/L Tris, 0.5 mmol/L MgCl2, 1 mmol/L Dithiothreitol (DTT), 3 mmol/L NaN3, and a mixture of protease and phosphatase-inhibitors. Cell lysates were disrupted using a sonicator on ice. After centrifugation at 13000 g for 15 min, the supernatant was collected as cytoplasmic protein. Nuclear protein was extracted as described previously[6]. The protein concentration was determined using the Bradford Protein Assay (Bio-Rad, Sydney, Australia). Immunoblotting was performed as previously described with some modifications[6,24]. Total protein (20 μg per lane) was resolved by electrophoresis on 12% sodium dodecyl sulfate polyacrylamide gels (SDS-PAGE) under reducing conditions. The electrophoresed proteins were electrotransferred onto Polyvinylidene difluoride membranes (Immobilin-P, Millipore, Bedford, MA, United States). The membranes were blocked with 5% skim milk (Resistin, TGFβ1, p-p38, pERK1/2, pJNK, NF-κB p-p65, NF-κB p-p50 and αSMA) for 60 min and then incubated overnight with primary antibody (Resistin 1:200, p-p38 1:1000, pERK1/2 1:1000, pJNK 1:1000, p-p65 1:1000, p-p50 1:1000, TGFβ1 1:500, αSMA 1:2000) at 4 °C. After 3 washes with 0.05% Tween-20/TBS, anti-mouse IgG (peroxidase conjugate) secondary antibody was applied. Blots were visualized by enhanced chemiluminescence (Pierce Perbio, Rockford, IL, United States). All images were quantified by densitometry.
Sirius red staining and collagen quantification were performed according to a previously published protocol[6,25]. Briefly, Sirius red F3BA solution (0.1% in saturated picric acid) was added to cell layers fixed in Bouin’s solution. After 1 h the cell layers were washed in tap water and again in 0.01 mol/L HCl to remove unbound dye. For collagen quantification, the dye was dissolved in 0.1 mol/L NaOH and absorbance determined at 450 nm. The amount of collagen was normalized to the protein concentration using the Bradford Reagent. Assays were performed in duplicate.
The media were collected from cultured HSCs following 24 h stimulation with resistin (500 ng/mL). Levels of IL-6 and MCP-1 in the media were determined according to the manufacturer’s instructions (RD Systems). Rat serum was collected and resistin concentration detected using a resistin rat ELISA kit (Boivendor) according to manufacturer’s instructions.
HSC proliferation: Cell proliferation was analyzed using a BrdU-based enzyme-linked immunosorbent assay (Roche Diagnostics) according to the manufacturer’s instructions. HSCs at day 4 were treated with resistin (500 ng/mL) or other agents as indicated for 24 h. The cells were subsequently labeled with BrdU for 2 h at 37 °C. Cells were then fixed and incubated with a peroxidase-conjugated anti-BrdU antibody for 90 min at room temperature. After adding the peroxidase substrate, 3,3’,5,5’-tetramethylbenzidine, BrdU incorporation was determined by measuring optical densities at 450 nm (background 620 nm).
HSC migration: HSC migration was assessed both with the wound scratch assay and a modified Boyden chamber. For the wound scratch assay, using a sterile 200 μL pipette tip, three separate wounds were generated through the cell monolayer. HSCs (90% confluence) at day 6 cultured in 12-well plates were treated with resistin (500 ng/mL) or other agents as indicated. The scratch area was photographed immediately and 6 h after scratching and cell migration into the scratch area calculated as the area covered by cells in the percentage of the initial scratch area. For the second method, a cell culture insert (12 well, BD) was used and the porous membrane (pore size 8 μm) of the filter was coated with 30 μg/mL collagen I at 37 °C for 30-60 min. HSCs at day 6 were trypsinized and placed into the upper chamber (105 cells/mL). The lower wells were filled with resistin (500 ng/mL) or other agents as indicated. After 6 h of incubation at 37 °C, cells adhering to the upper side of the filter were removed with a cotton swab. The filters were then fixed with 100% methanol and stained with HEMA-3. The numbers of HSCs on the lower side of the filter were counted in five randomly chosen microscopic fields at a magnification of × 400 by changing the focus.
HSC apoptosis: Annexin-V/PI labeling was used to detect HSC apoptosis. Briefly, trypsinized HSCs were washed twice in PBS, stained with annexin-V (10 µL) and PI (5 μL) for 10 min, and the apoptotic rate quantified by FACS Calibur flow cytometry (Becton Dickinson Inc.) at 488 nm. More than 1 × 104 cells were detected, and the results were analyzed with FlowJo software (Treestar, United States). The population of apoptotic cells was identified as annexin V+/PI-. The percentage of apoptotic cells was calculated according to total annexin V+/PI- divided by total cells.
The results are expressed as mean ± SD. Comparisons between 2 groups were analyzed using the Student t test. For the comparison of more than two groups, we used two-way ANOVA. P values < 0.05 were considered statistically significant. All calculations were performed using Statistical Program for Social Sciences (SPSS) software 13.0 (SPSS Inc., Chicago, IL, United States).
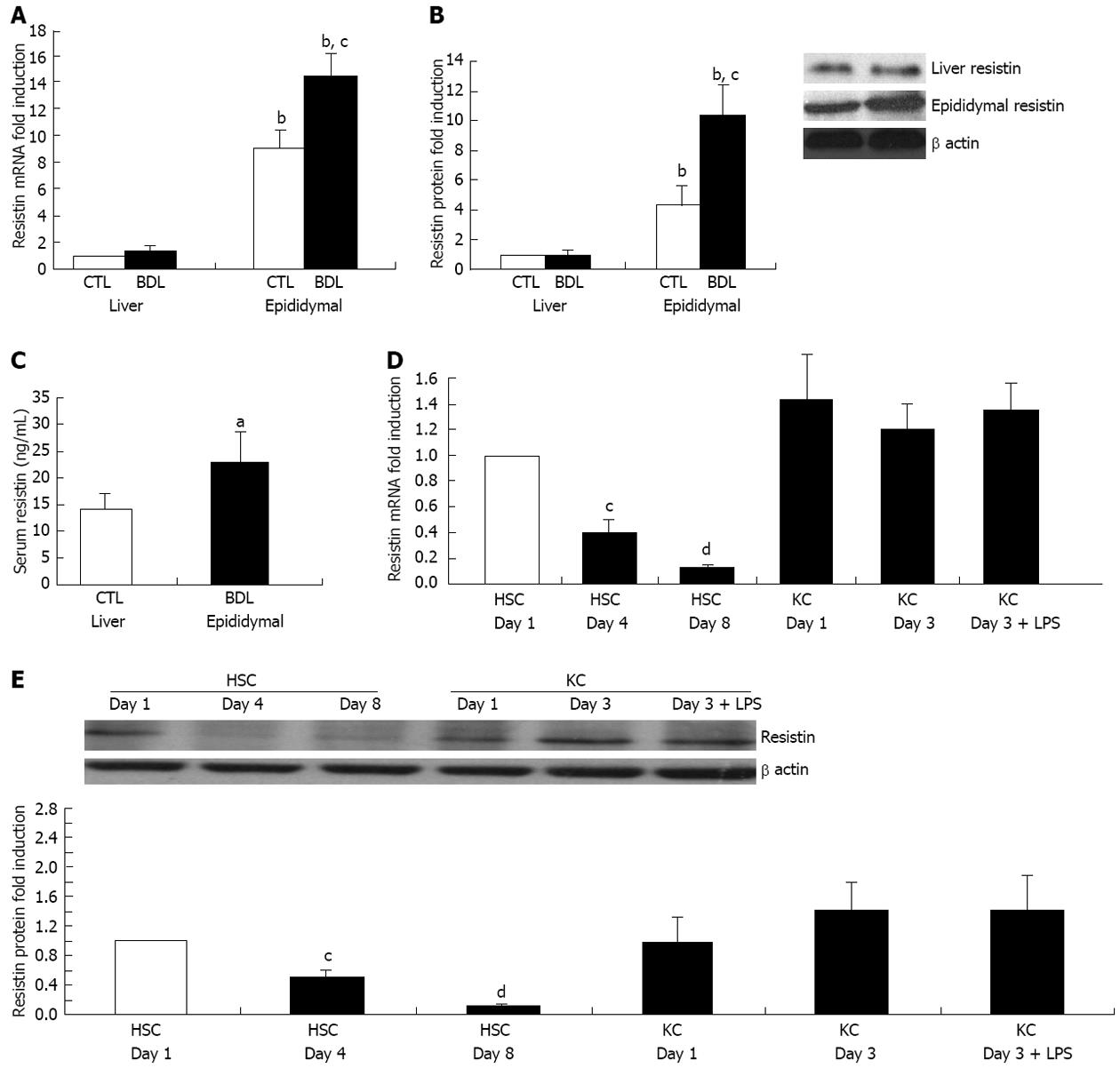
Resistin expression in liver, epididymal fat and serum in BDL and sham rats was examined. We noted that resistin expression in epididymal fat was considerably higher than that in liver in the BDL or sham rats (Figure 1A and B, all P < 0.01). BDL rat epididymal fat mRNA and protein level were further up-regulated compared to sham rats (Figure 1A and B, both P < 0.05). Similarly, BDL rat serum resistin level was also elevated (Figure 1C, P < 0.05). However, liver resistin mRNA and protein were unchanged in the BDL and sham groups (Figure 1A and B). These findings suggest that increased adipose resistin rather than liver resistin may play a vital role in resistin-mediated liver injury in rodents. Therefore, we undertook detailed in vitro experiments in order to explore the impact of exogenous resistin on HSC activated phenotype.
Resistin mRNA was detected in quiescent rat HSCs (Figure 1D) at day 1 and was reduced by 90% (P < 0.01) following activation for 8 d on plastic. Resistin mRNA was expressed in quiescent KCs (day 1) and activated KCs (3 d), without significant changes over time. LPS (50 ng/mL for 24 h) stimulation of KCs at day 3 did not enhance resistin expression (Figure 1D). Consistent with the mRNA data, resistin protein expression declined 6-fold in HSCs at day 8 with no change in KCs at day 3 and after LPS stimulation (Figure 1E). These data indicated that autocrine HSC resistin and paracrine KC resistin are unlikely to be of major importance in mediating any effects on activated HSCs.
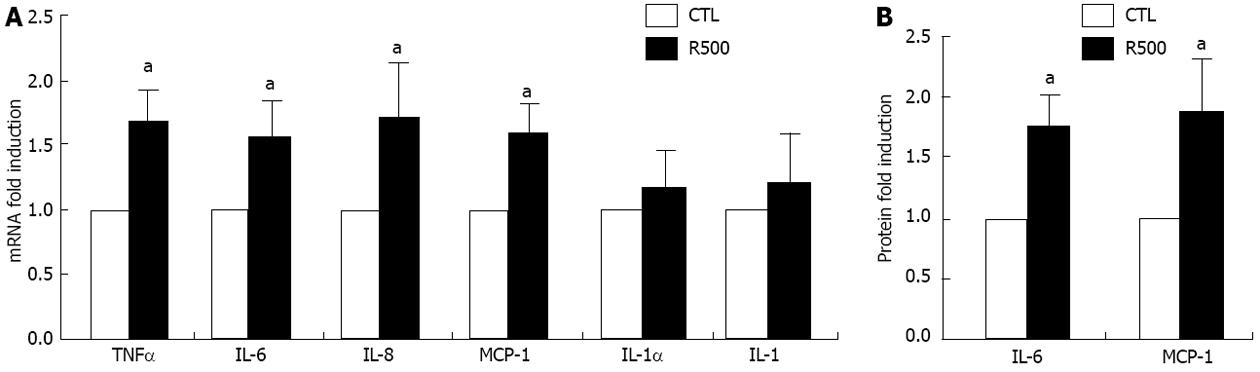
Pro-inflammatory cytokines and chemokines play a permissive role in liver fibrosis[26-29] and previous reports suggest that resistin increases MCP-1 secretion. We evaluated the expression of TNFα, IL-1α, IL-1β, IL-6, IL-8 and MCP-1 in rat HSCs after stimulation with resistin. As demonstrated, resistin (500 ng/mL) stimulation for 24 h markedly up-regulated the expression of TNFα, IL-6, IL-8 and MCP-1 mRNA (Figure 2A, all P < 0.05), but not that of IL-1α and IL-1β. To rule out any potential effects of inadvertent endotoxin contamination, we repeated these studies in the presence of Polymyxin B and noted no difference in the gene expression profile (data not shown). Finally, using trypan blue staining and LDH assays at 24, 48 and 72 h, we excluded the possibility of direct cellular toxicity due to the resistin dose used (data not shown). Since IL-6 and MCP-1 are well documented to play a role in mediating hepatic fibrosis, their protein concentrations were estimated in conditioned medium. As shown in Figure 2B, resistin administration increased IL-6 and MCP-1 concentrations 1.7 and 1.8 fold after 24-h of treatment (both P < 0.05).
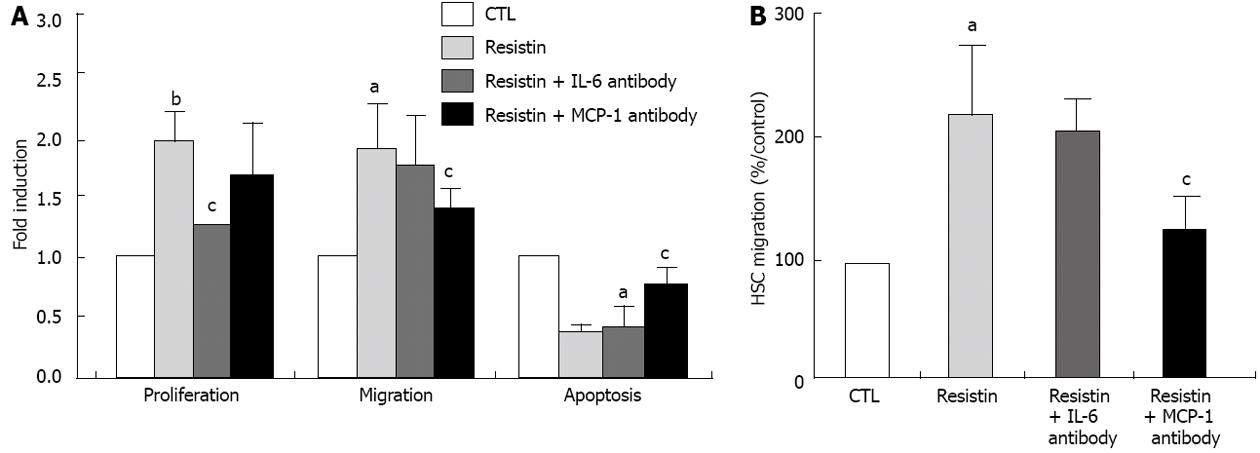
During the process of chronic liver injury, activated HSCs proliferate and migrate to sites of inflammation and have reduced apoptosis. This phenotype is part of the expected adaptive wound healing response to injury. Hence, we sought to determine the role of resistin in mediating activated HSC behavior. As demonstrated in Figure 3A, resistin enhanced HSC proliferation by approximately 90% compared to the control (P < 0.01). Using the wound scratch assay and a modified Boyden chamber, compared to the control, resistin treatment resulted in an approximately 80% and approximately 220% increase in HSC migration, respectively (P < 0.05, Figure 3A and B). We next examined the role of resistin on HSC apoptosis. In contrast, resistin significantly reduced HSC apoptosis (56%, P < 0.05, Figure 3A), as shown by annexin V/IP flow cytometry. Finally, we determined whether up-regulation of IL-6 and MCP-1 was responsible for the changed HSC phenotype by resistin. As expected, resistin-mediated HSC proliferation, migration and apoptosis were partially, but significantly reversed (all P < 0.05, Figure 3A and B) by IL-6 (5 μg/mL) and MCP-1 (10 μg/mL) neutralization, respectively. These data suggest that resistin triggered HSC IL-6 and MCP-1 production, thereby modulating HSC phenotype.
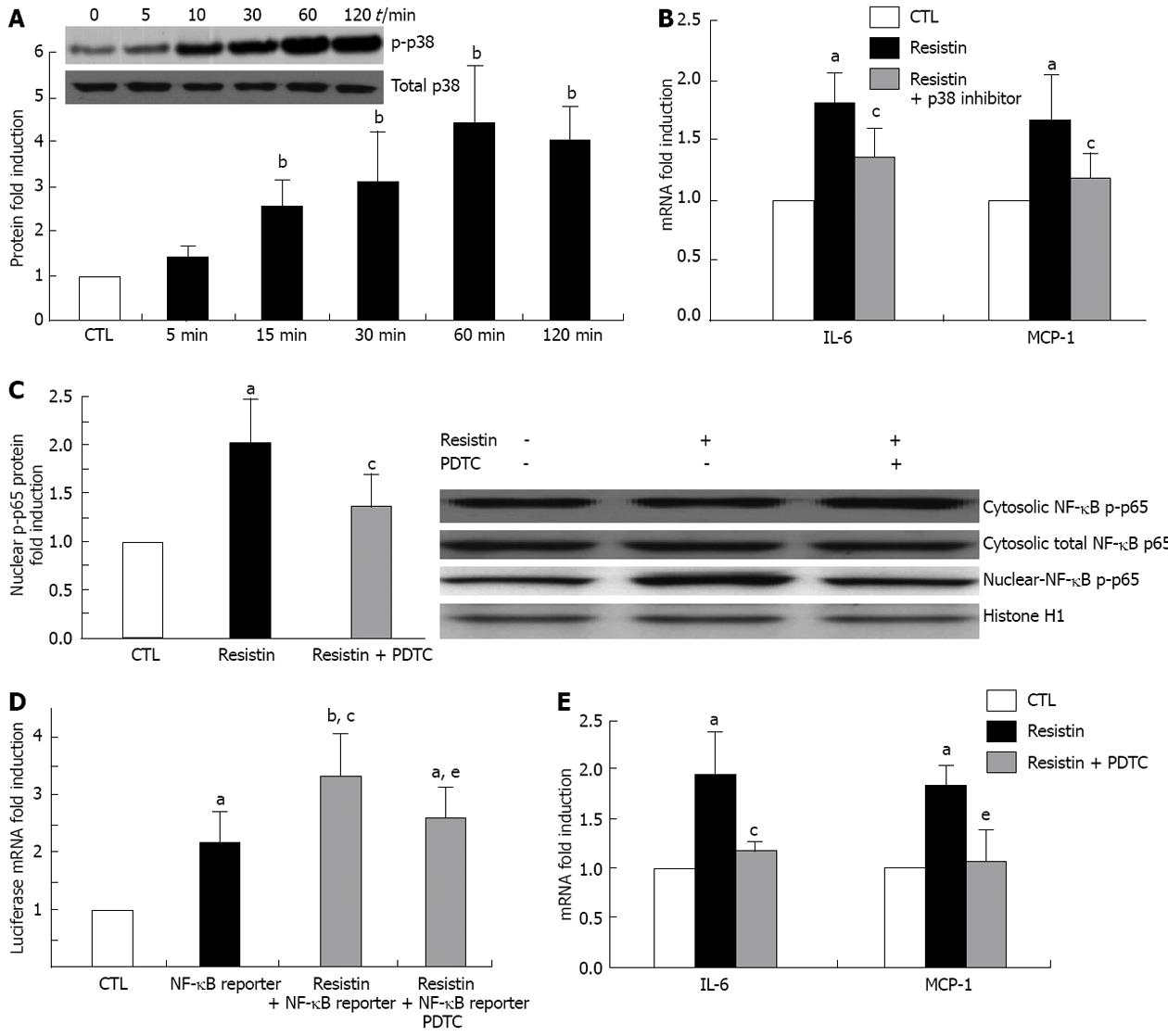
Mitogen-activated protein kinases (MAPK) and NF-κB play critical roles in the induction of pro-inflammatory cytokines and chemokines, and regulate cell biological behaviors. Therefore, we determined whether resistin activates HSC MAPK (p38, ERK1/2 and JNK) and NF-κB. Phoshor-p38, ERK1/2 and JNK in the cytoplasm as well as NF-κB p65 and p50 in cytosolic and nuclear extracts were analyzed by immunoblotting. The results showed that cytoplasmic p-p38 and nuclear p-p65 were up-regulated (both P < 0.05, Figure 4A and C). Changes in cytosolic pERK1/2, pJNK (data not shown), p65 and cytosolic and nuclear p-p50 (data not shown) were not observed. In the p-p38 inhibition experiment using SB203580, we found that p-p38 activation was responsible for IL-6 and MCP-1 induction in HSCs (P < 0.05, Figure 4B). Furthermore, resistin (500 ng/mL) enhanced NF-κB DNA binding ability (luciferase mRNA. P < 0.05, Figure 4D). As expected, NF-κB inhibition by pyrrolidine dithiocarbamate (PDTC) (100 µmol/L) attenuated the resistin-induced increase in nuclear p-p65 and NF-κB DNA binding ability (P < 0.05, Figure 4C and D). Similarly, PDTC reversed resistin-induced up-regulation of IL-6 and MCP-1 (Figure 4E).
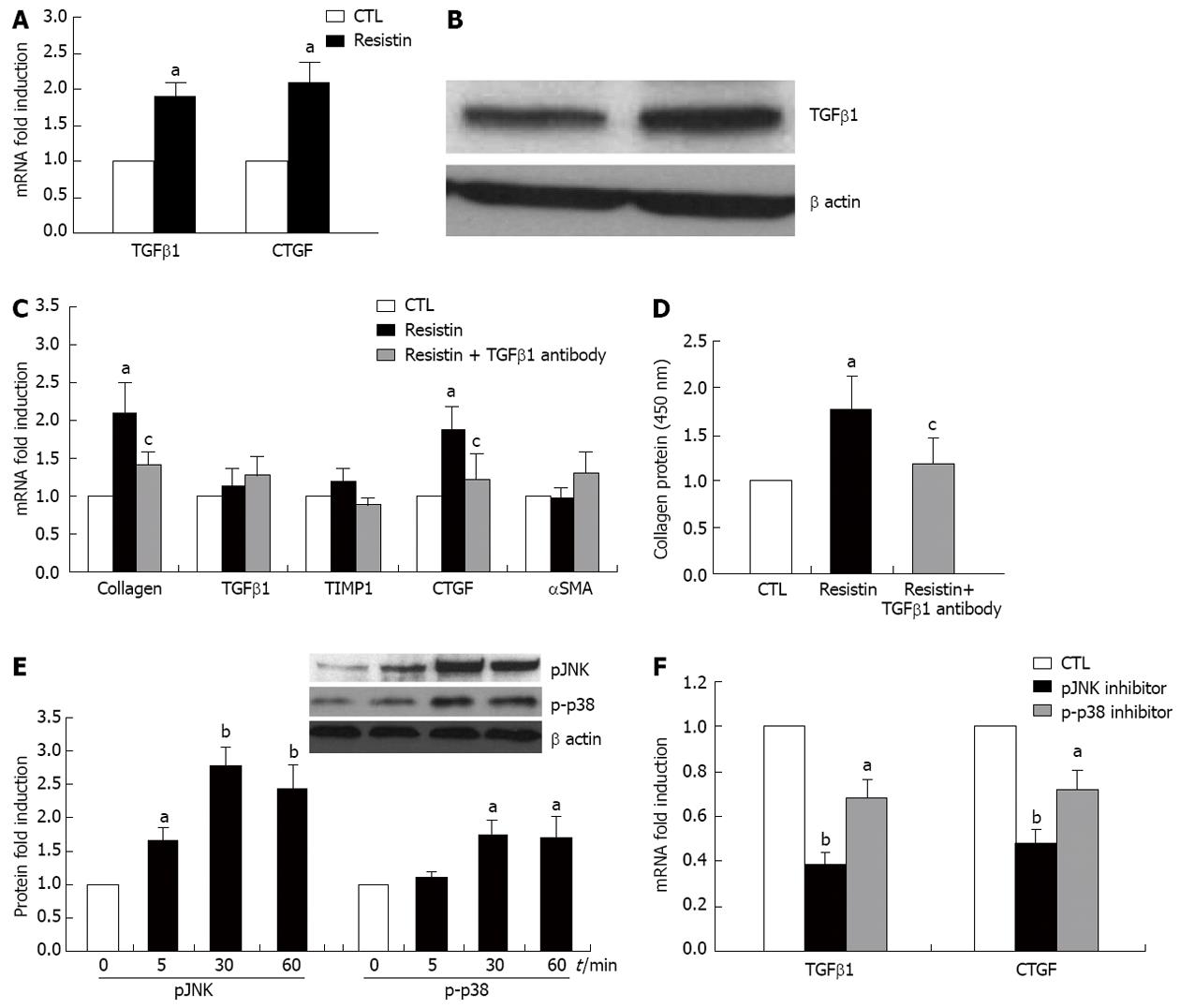
To determine whether resistin affects KCs and whether KCs participate in the process of resistin-mediated HSC phenotype, the appropriate experiments were undertaken. As shown in Figure 5A and B, resistin up-regulated TGFβ1 and CTGF mRNA in KCs and enhanced TGFβ1 protein in KC medium (both P < 0.05). Co-culture of HSCs and KC conditioned medium resulted in a significant increase in HSC collagen I and CTGF expression (Figure 5C and D, all P < 0.05), however, TGFβ1 (10 μg/mL) neutralization diminished this increase (Figure 5C and D, all P < 0.05). Downstream signaling responsible for increased KC TGFβ1 and CTGF expression by resistin were further analyzed. We found that pJNK and p-p38 were activated following exposure to resistin. Furthermore, pJNK and p-p38 inhibition partially, but significantly reversed resistin-induced TGFβ1 and CTGF enhancement (Figure 5E and F, P < 0.05 and 0.01). These data suggest that resistin affected HSC activated phenotype by increased TGFβ1 from KCs.
Resistin is suggested to play a pathogenic role in insulin resistance and altered glucose metabolism in rodents[3,4]. For example, lowering plasma resistin in insulin-resistant mice decreases blood glucose levels and improves insulin sensitivity[10,30,31], while treatment of normal mice with resistin impairs glucose tolerance and insulin actions[10,30]. Resistin may also play a pivotal role in inflammation since it up-regulates IL-6 and TNFα expression in human peripheral blood mononuclear cells via NF-κB activation[22]. Furthermore, the addition of resistin protein from both mice and humans to macrophages results in enhanced secretion of pro-inflammatory cytokines including TNFα and IL-12[32]. In human cirrhosis, resistin levels in the liver and plasma are elevated and increase further with the severity of liver disease[14-17,33]. This suggests that the pro-inflammatory activities of resistin may modulate liver inflammation and drive disease progression in cirrhosis.
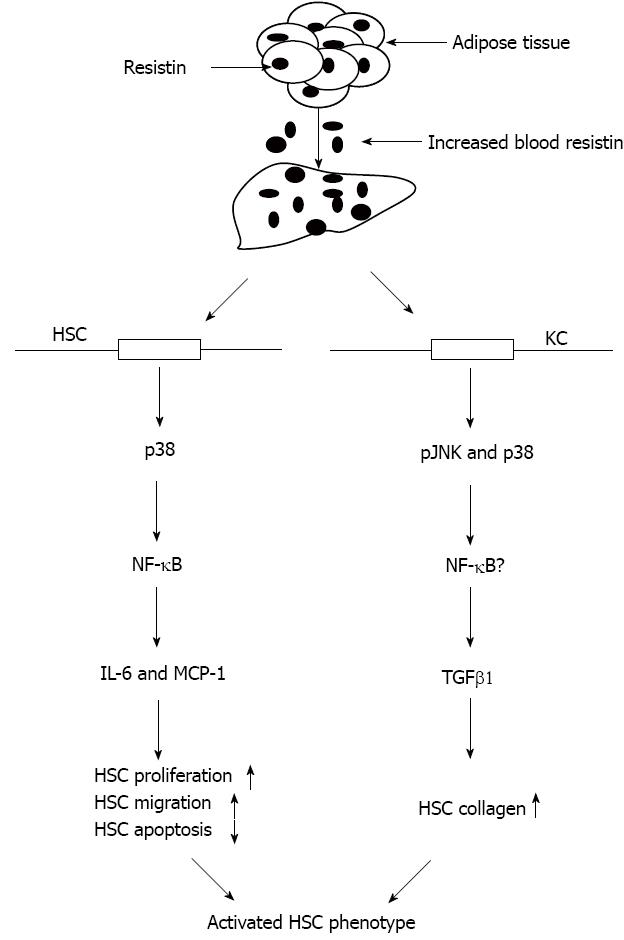
This study provides evidence that in cirrhotic rats, adipose tissue (epididymal fat) and blood resistin are up-regulated, and adipose tissue may be the main source of resistin secretion. Rat liver, HSCs and KCs express resistin, but are unlikely to be important sources of resistin secretion in cirrhosis. Resistin exerts pro-inflammatory activities on HSCs with enhanced secretion of pro-inflammatory cytokines (TNFα, IL-6, IL-8 and MCP-1). Most importantly, resistin promotes HSC proliferation and migration, while inhibiting their apoptosis via an IL-6 and MCP-1 mechanism. KCs participate in this process by up-regulating HSC collagen I through increased TGFβ1. Taken together, our data suggest that resistin promotes the progression of liver injury.
Resistin is almost exclusively expressed by white adipose tissue in rodents, but is expressed by monocytes and macrophages in humans[18,34,35]. Liver infiltrating CD43 cells and KCs have been suggested as key sources of resistin in the liver of cirrhotic patients[20,21], thus resistin was more abundant in adipose tissue than in human liver[20,21]. In this study, although rat quiescent HSCs expressed resistin, it declined markedly on activation. The relevant mechanism is unclear, however, adipogenic transcriptional regulation may be required for maintenance of the quiescent HSC phenotype[26]. KCs also expressed resistin but no change was found on activation or LPS stimulation. Therefore, it is unlikely that resistin derived from HSCs and KCs contributed to the increase in serum resistin in BDL rats, thus HSCs and KCs are non-critical sources of resistin. However, other liver cell types may not represent a likely source of resistin production as hepatocytes and endothelial cells do not express resistin[15]. Thus, adipose tissue, including epididymal fat, could be the predominant source of resistin in liver injured rodents. It has been demonstrated in in vivo and ex vivo studies, that increased TNFα and insulin in BDL cirrhotic rats stimulate adipose resistin expression[14,15,19].
Why LPS was unable to trigger resistin secretion by KCs is unknown. KCs belongs to the macrophage family, and many studies have shown that LPS exposure induced resistin production in human and rodent macrophages[36,37]. The mechanisms involved require further clarification.
As expected, HSC expression of TNFα, IL-6, IL-8 and MCP-1 mRNAs was increased on resistin exposure, as was IL-6 and MCP-1 protein. Bertolani et al[20] reported similar findings in human HSCs and noted that resistin up-regulated human HSC MCP-1 that was dependent on a Ca2+/NF-κB-dependent pathway[20]. We further demonstrated that resistin directly augmented HSC proliferation and migration, but reduced HSC apoptosis via an IL-6 and MCP-1 mechanism. These novel data imply that IL-6 and MCP-1 inhibition may prevent resistin-induced liver fibrogenesis. The pro-fibrogenic effects of IL-6 and MCP-1 are well documented in the literature[38-40]. Moreover, we found that resistin was able to promote KC activation as it stimulated enhancement of KC TGFβ1 expression. Thus, increased TGFβ1 led to up-regulation of HSC collagen I and HSC activation. This is an important finding, as TGFβ1 is a potent profibrogenic cytokine. Interestingly, this phenomenon is similar to our previous report[6]. We observed that the profibrogenic role of leptin could be achieved at least through TGFβ1 from KCs[6]. Collectively, these data indicate that resistin is able to modulate HSC behaviors towards a more pro-fibrogenic phenotype.
Although many functions of resistin in inflammation and inflammation-related diseases have been described, the relevant intracellular signaling pathway of resistin is not yet completely understood. We further demonstrated that resistin mediated HSC IL-6 and MCP-1 via p38 and KC TGFβ1 via pJNK and p-p38 (Figure 6). These results may provide evidence to prevent resistin-mediated liver injury/fibrosis using relevant signaling inhibitors.
In summary, this study demonstrates that in rodents, resistin production in the context of liver injury is principally non-hepatic in origin. Extrahepatic resistin could contribute to liver fibrosis by its direct and indirect profibrogenic effects on HSCs. Further studies on resistin knockout and transgenic animals are needed.
We thank Dr. Nanthakumar Subramaniam and Mehdi Ramezani-Moghadam for excellent technical assistance.
Metabolic abnormalities usually cause the progression of liver fibrosis. To date, the mechanism whereby metabolic alterations mediate disease progression are unclear. Resistin, an adipokine, has been reported to be associated with metabolic alterations, however, its role in hepatic fibrosis has not been clearly investigated.
Although many functions of resistin in inflammation and inflammation-related diseases have been described, the relevant intracellular signaling pathways of resistin in liver fibrosis are not yet completely understood.
To date, there have been a limited number of studies regarding the impact of resistin on the phenotype of hepatic stellate cells and how it functions in liver fibrosis. In this study, the authors employed a direct analysis to identify the significant correlation between resistin and hepatic stellate cells (HSCs). The authors confirmed that resistin mediated-HSCs move towards a more pro-fibrotic phenotype which is dependent on interleukin 6/monocyte chemotactic protein and/or transforming growth factor β1.
By understanding the mechanism whereby resistin mediates HSC activation, this study may provide evidence to prevent resistin-mediated liver injury/fibrosis using relevant signaling inhibitors.
The serum levels of resistin are elevated in cirrhosis, and the changed phenotype of HSCs play an important role in the pathogenesis of liver fibrosis. Resistin is involved in this process.
The paper reported the effects of the adipokine resistin on the biology of hepatic stellate cells and Kupffer cells. It is well presented.
P- Reviewer Marra F S- Editor Huang XZ L- Editor Webster JR E- Editor Ma S
| 1. | Peng S, Plank LD, McCall JL, Gillanders LK, McIlroy K, Gane EJ. Body composition, muscle function, and energy expenditure in patients with liver cirrhosis: a comprehensive study. Am J Clin Nutr. 2007;85:1257-1266. [PubMed] [Cited in This Article: ] |
| 2. | Lin SY, Chen WY, Lee FY, Huang CJ, Sheu WH. Activation of ubiquitin-proteasome pathway is involved in skeletal muscle wasting in a rat model with biliary cirrhosis: potential role of TNF-alpha. Am J Physiol Endocrinol Metab. 2005;288:E493-E501. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Petrides AS. Liver disease and diabetes mellitus. Diabetes Rev. 1994;2:2–18. [Cited in This Article: ] |
| 4. | Hui JM, Sud A, Farrell GC, Bandara P, Byth K, Kench JG, McCaughan GW, George J. Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression [corrected]. Gastroenterology. 2003;125:1695-1704. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology. 2005;42:987-1000. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Wang J, Leclercq I, Brymora JM, Xu N, Ramezani-Moghadam M, London RM, Brigstock D, George J. Kupffer cells mediate leptin-induced liver fibrosis. Gastroenterology. 2009;137:713-723. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Wang J, Brymora J, George J. Roles of adipokines in liver injury and fibrosis. Expert Rev Gastroenterol Hepatol. 2008;2:47-57. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Ikejima K, Takei Y, Honda H, Hirose M, Yoshikawa M, Zhang YJ, Lang T, Fukuda T, Yamashina S, Kitamura T. Leptin receptor-mediated signaling regulates hepatic fibrogenesis and remodeling of extracellular matrix in the rat. Gastroenterology. 2002;122:1399-1410. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Kamada Y, Tamura S, Kiso S, Matsumoto H, Saji Y, Yoshida Y, Fukui K, Maeda N, Nishizawa H, Nagaretani H. Enhanced carbon tetrachloride-induced liver fibrosis in mice lacking adiponectin. Gastroenterology. 2003;125:1796-1807. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307-312. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Qatanani M, Szwergold NR, Greaves DR, Ahima RS, Lazar MA. Macrophage-derived human resistin exacerbates adipose tissue inflammation and insulin resistance in mice. J Clin Invest. 2009;119:531-539. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Muse ED, Lam TK, Scherer PE, Rossetti L. Hypothalamic resistin induces hepatic insulin resistance. J Clin Invest. 2007;117:1670-1678. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Pravenec M, Kazdová L, Landa V, Zidek V, Mlejnek P, Jansa P, Wang J, Qi N, Kurtz TW. Transgenic and recombinant resistin impair skeletal muscle glucose metabolism in the spontaneously hypertensive rat. J Biol Chem. 2003;278:45209-45215. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Yagmur E, Trautwein C, Gressner AM, Tacke F. Resistin serum levels are associated with insulin resistance, disease severity, clinical complications, and prognosis in patients with chronic liver diseases. Am J Gastroenterol. 2006;101:1244-1252. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Bahr MJ, Ockenga J, Böker KH, Manns MP, Tietge UJ. Elevated resistin levels in cirrhosis are associated with the proinflammatory state and altered hepatic glucose metabolism but not with insulin resistance. Am J Physiol Endocrinol Metab. 2006;291:E199-E206. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Tsochatzis E, Papatheodoridis GV, Hadziyannis E, Georgiou A, Kafiri G, Tiniakos DG, Manesis EK, Archimandritis AJ. Serum adipokine levels in chronic liver diseases: association of resistin levels with fibrosis severity. Scand J Gastroenterol. 2008;43:1128-1136. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Kakizaki S, Sohara N, Yamazaki Y, Horiguchi N, Kanda D, Kabeya K, Katakai K, Sato K, Takagi H, Mori M. Elevated plasma resistin concentrations in patients with liver cirrhosis. J Gastroenterol Hepatol. 2008;23:73-77. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Steppan CM, Lazar MA. The current biology of resistin. J Intern Med. 2004;255:439-447. [PubMed] [Cited in This Article: ] |
| 19. | Lin SY, Sheu WH, Chen WY, Lee FY, Huang CJ. Stimulated resistin expression in white adipose of rats with bile duct ligation-induced liver cirrhosis: relationship to cirrhotic hyperinsulinemia and increased tumor necrosis factor-alpha. Mol Cell Endocrinol. 2005;232:1-8. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Bertolani C, Sancho-Bru P, Failli P, Bataller R, Aleffi S, DeFranco R, Mazzinghi B, Romagnani P, Milani S, Ginés P. Resistin as an intrahepatic cytokine: overexpression during chronic injury and induction of proinflammatory actions in hepatic stellate cells. Am J Pathol. 2006;169:2042-2053. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Szalowska E, Elferink MG, Hoek A, Groothuis GM, Vonk RJ. Resistin is more abundant in liver than adipose tissue and is not up-regulated by lipopolysaccharide. J Clin Endocrinol Metab. 2009;94:3051-3057. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005;174:5789-5795. [PubMed] [Cited in This Article: ] |
| 23. | Son YM, Ahn SM, Kim GR, Moon YS, Kim SH, Park YM, Lee WK, Min TS, Han SH, Yun CH. Resistin enhances the expansion of regulatory T cells through modulation of dendritic cells. BMC Immunol. 2010;11:33. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Teoh N, Dela Pena A, Farrell G. Hepatic ischemic preconditioning in mice is associated with activation of NF-kappaB, p38 kinase, and cell cycle entry. Hepatology. 2002;36:94-102. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Liu Y, Brymora J, Zhang H, Smith B, Ramezani-Moghadam M, George J, Wang J. Leptin and acetaldehyde synergistically promotes αSMA expression in hepatic stellate cells by an interleukin 6-dependent mechanism. Alcohol Clin Exp Res. 2011;35:921-928. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Tsukamoto H. Cytokine regulation of hepatic stellate cells in liver fibrosis. Alcohol Clin Exp Res. 1999;23:911-916. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Farajzadeh-Sheikh A, Jolodar A, Ghaemmaghami S. Sequence characterization of cDNA sequence of encoding of an antimicrobial Peptide with no disulfide bridge from the Iranian mesobuthus eupeus venomous glands. Iran Red Crescent Med J. 2013;15:36-41. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Seki E, de Minicis S, Inokuchi S, Taura K, Miyai K, van Rooijen N, Schwabe RF, Brenner DA. CCR2 promotes hepatic fibrosis in mice. Hepatology. 2009;50:185-197. [PubMed] [DOI] [Cited in This Article: ] |
| 29. | Tharp K, Mattingly C. Whistleblowing. Plast Surg Nurs. 1991;11:33-34. [PubMed] [DOI] [Cited in This Article: ] |
| 30. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [PubMed] [DOI] [Cited in This Article: ] |
| 31. | Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655-1669. [PubMed] [DOI] [Cited in This Article: ] |
| 32. | Bissell DM, Roulot D, George J. Transforming growth factor beta and the liver. Hepatology. 2001;34:859-867. [PubMed] [DOI] [Cited in This Article: ] |
| 33. | Kusminski CM, McTernan PG, Kumar S. Role of resistin in obesity, insulin resistance and Type II diabetes. Clin Sci (Lond). 2005;109:243-256. [PubMed] [DOI] [Cited in This Article: ] |
| 34. | Muse ED, Obici S, Bhanot S, Monia BP, McKay RA, Rajala MW, Scherer PE, Rossetti L. Role of resistin in diet-induced hepatic insulin resistance. J Clin Invest. 2004;114:232-239. [PubMed] [DOI] [Cited in This Article: ] |
| 35. | Silswal N, Singh AK, Aruna B, Mukhopadhyay S, Ghosh S, Ehtesham NZ. Human resistin stimulates the pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by NF-kappaB-dependent pathway. Biochem Biophys Res Commun. 2005;334:1092-1101. [PubMed] [DOI] [Cited in This Article: ] |
| 36. | Edwards CR, Hindle AK, Latham PS, Fu SW, Brody FJ. Resistin expression correlates with steatohepatitis in morbidly obese patients. Surg Endosc. 2013;27:1310-1314. [PubMed] [DOI] [Cited in This Article: ] |
| 37. | Lehrke M, Reilly MP, Millington SC, Iqbal N, Rader DJ, Lazar MA. An inflammatory cascade leading to hyperresistinemia in humans. PLoS Med. 2004;1:e45. [PubMed] [DOI] [Cited in This Article: ] |
| 38. | Savage DB, Sewter CP, Klenk ES, Segal DG, Vidal-Puig A, Considine RV, O’Rahilly S. Resistin / Fizz3 expression in relation to obesity and peroxisome proliferator-activated receptor-gamma action in humans. Diabetes. 2001;50:2199-2202. [PubMed] [Cited in This Article: ] |
| 39. | Lu SC, Shieh WY, Chen CY, Hsu SC, Chen HL. Lipopolysaccharide increases resistin gene expression in vivo and in vitro. FEBS Lett. 2002;530:158-162. [PubMed] [DOI] [Cited in This Article: ] |
| 40. | Kaser S, Kaser A, Sandhofer A, Ebenbichler CF, Tilg H, Patsch JR. Resistin messenger-RNA expression is increased by proinflammatory cytokines in vitro. Biochem Biophys Res Commun. 2003;309:286-290. [PubMed] [DOI] [Cited in This Article: ] |