Published online Sep 7, 2014. doi: 10.3748/wjg.v20.i33.11525
Revised: February 9, 2014
Accepted: June 20, 2014
Published online: September 7, 2014
Inflammatory bowel disease (IBD) is a chronic, relapsing intestinal inflammatory disorder with unidentified causes. Both environmental factors and genetic aspects are believed to be crucial to the pathogenesis of IBD. The incidence and prevalence of IBD have recently been increasing throughout Asia, presumably secondary to environmental changes. This increasing trend in IBD epidemiology necessitates specific health care planning and education in Asia. To this end, we must gain a precise understanding of the distinctive clinical and therapeutic characteristics of Asian patients with IBD. The phenotypes of IBD reportedly differ considerably between Asians and Caucasians. Thus, use of the same management strategies for these different populations may not be appropriate. Moreover, investigation of the Asian-specific clinical aspects of IBD offers the possibility of identifying causative factors in the pathogenesis of IBD in this geographical area. Accordingly, this review summarizes current knowledge of the phenotypic manifestations and management practices of patients with IBD, with a special focus on a comparison of Eastern and Western perspectives.
Core tip: Over the past two decades, the incidence and prevalence of inflammatory bowel disease (IBD) have changed with a trend toward increasing across Asia, especially East Asia. This increasing trend in IBD epidemiology necessitates specific health care planning and education in Asia. To this end, we must gain a precise understanding of the distinctive clinical and therapeutic characteristics of Asian patients with IBD, compared to Caucasians patients with IBD. Accordingly, this review summarizes current knowledge of the phenotypic manifestations and management practices of patients with IBD, with a special focus on a comparison of Eastern and Western perspectives.
- Citation: Park SJ, Kim WH, Cheon JH. Clinical characteristics and treatment of inflammatory bowel disease: A comparison of Eastern and Western perspectives. World J Gastroenterol 2014; 20(33): 11525-11537
- URL: https://www.wjgnet.com/1007-9327/full/v20/i33/11525.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i33.11525
Inflammatory bowel disease (IBD) is a chronic, idiopathic inflammatory disorder of the gastrointestinal tract without identifiable causes. It comprises primarily Crohn’s disease (CD) and ulcerative colitis (UC). IBD was traditionally regarded as being prevalent in mainly Western countries. Over the past two decades, however, the incidence pattern of IBD has changed. The incidence in the West has remained relatively stable, while that in Asia has increased markedly[1]. This recent change in the incidence and prevalence of IBD is attributed to environmental changes.
The growing incidence of IBD in Asia has important implications for those who formulate health care policy plans; these individuals should provide specific health care planning, services, and education while balancing the health needs and social burdens of a given country. However, it also has important implications for clinicians and researchers. According to the published literature, the IBD phenotypes differ considerably between Asians and Caucasians. Nevertheless, most strategies for the prevention and treatment of IBD flare-up in Asia have followed Western guidelines. Gradual accumulation of data on IBD in Asians will facilitate the formulation of Asian-specific practice guidelines; however, direct comparisons of the clinical characteristics of IBD in Asian and Western countries are few. Formulation of appropriate strategies for Asian patients with IBD must begin with an accurate understanding of the different clinical characteristics of the two populations. These include clinical manifestations and therapeutic aspects, as well as epidemiology. To this end, this review summarizes and compares current knowledge of the phenotypic manifestations and management of patients with IBD in Eastern and Western countries. Furthermore, investigation of the Asian-specific clinical aspects of IBD offers the possibility of identifying causative factors in the pathogenesis of IBD, which will also be covered in this review.
Peak age of disease onset: Early studies from Western countries reported that CD was characteristically associated with a bimodal age distribution pattern, peaking at the ages of 20 to 39 years and showing a second smaller peak at the ages of 50 to 79 years[2-4]. Various peak ages of CD onset have been reported in Asia. A Korean study reported a smaller second peak in the incidence of CD[5]. There was also a trend toward a second peak in the Hong Kong population[6]. A recent prospective, population-based study from the Asia-Pacific region also indicated a smaller second peak in the incidence of CD, albeit at a younger age (40-44 years) than that in Western countries[7]. However, this bimodal presentation has not been uniformly identified in other Asian studies[8,9]. The reason for the bimodal distribution remains unclear, but some environmental factors are known to be associated with this phenomenon. One hypothesis is that it may be due to certain age-specific environmental factors, such as passive smoking in childhood and active smoking in adulthood. It may also be caused by the different sensitivities of different age groups to certain infectious factors[10]. We speculate that Asian people with genetic susceptibilities could develop CD at the early peak ages of 20-39 years; however, the later second peak might be smaller than that in the West because environmental factors are less prevalent in Asia. Accordingly, it is expected that the second peak in the incidence of CD in Asia will increase as the region becomes increasingly westernized over time (Table 1).
| Differences | Asian characteristics |
| Peak age at disease onset | Smaller second peak |
| Sex distribution of CD | Male predominance |
| Cigarette smoking in CD | Lower prevalence |
| CD distribution and behavior | Ileocolonic predominance |
| Similar behavior | |
| UC distribution and behavior | Similar distribution |
| Milder disease course | |
| Familial aggregation in IBD | Lower prevalence |
| Extraintestinal disease | Lower prevalence |
| Medical treatments | Lower use of thiopurine and anti-TNF therapy |
| Surgical treatments | Comparable in CD |
| Lower in UC | |
| UC-associated CRC | Comparable cumulative risk |
European and North American studies have consistently revealed that the incidence of CD in females is equal to or greater than that in males[2,4,11,12]. In striking contrast, a male predominance in Asian patients with CD has been reported. In all recent studies from Korea, China, and Japan, the male-to-female ratios ranged from 1.67:1 to 2.9:1[9,13,14]. A recent prospective, population-based study from the Asia-Pacific region also demonstrated a male predominance[7]. Interestingly, a recent European study indicated a slight male predominance (60%) in adult Eastern European patients with CD[15]. Smoking, vaccination, or other factors (e.g., geographic, ethnic, and social) might account for this sex difference[10]. Some Western researchers have suggested that the smoking rates, exposure of social life, or Westernized lifestyles were more prevalent in males than in females in Asia and that males might have more opportunities to receive medical services, including endoscopy, than females in Asia. However, if male predominance is in fact present in Asia, it is possible that changes in susceptible genes in sex chromosomes or changes in sex hormones might be involved in the pathogenesis of CD.
Smoking represents one of the most consistently observed environmental risk factors for CD. Studies in Western countries have shown that smoking is a strong risk factor for the development of CD, but that it protects against the development of UC[16-20].
Based on recent prospective, population-based cohort studies, the proportion of current or ex-smokers among Asian patients with CD (11.8%-28.0%)[7,21] is substantially lower than that among Western (57%) and Eastern European patients (62%)[15]. A study comparing patients between Melbourne and Hong Kong also reported that fewer patients were current or ex-smokers in Hong Kong (8%) than in Melbourne (50%)[22].
Western patients with CD who smoke have a worse disease course and are more likely to relapse after medically and surgically induced remission[23-25]. In terms of flare-up rates and therapeutic needs, disease severity is similar in patients who have never smoked and those who have stopped smoking, and both have a better course than continuing smokers[23]. Progression to stricturing or penetrating disease and subsequent surgery rates are reduced by smoking cessation[26]. However, smoking may not have the same effect in CD in different ethnic groups or geographic regions as in Western populations. For example, studies among Israeli Jews showed that smoking is not associated with the risk of CD[27-29]. More studies on Asian patients with CD are warranted to determine the impact of smoking on the development and progression of disease and its association with the disease phenotype in this population. Our hypothesis is that the lower rate of cigarette smoking among Asians may be one of the factors related to their better prognosis, as indicated by the lower rate of surgery in Asian than in Western patients with CD.
Previous studies have suggested that patients with IBD have less family clustering in East Asia. Studies from Asia have collectively reported that familial aggregation rates range from 0.0% to 3.0%[6,30-35], which is clearly lower than that in the Western population (13.4%)[36]. However, familial aggregation rates appear to be higher in West Asia, ranging from 12.9% to 19.0%[1,37]. Recent studies from Korea have suggested that the familial aggregation rate of IBD may increase with time, in parallel with the increase in the prevalence of IBD within the country[1,5,31]. Data on the familial clustering rates in other Asian countries must be validated by a longitudinal prospective cohort study. Moreover, it should be tested whether patients with family history of IBD would have a worse disease outcome than those without. Our own study showed no difference in clinical characteristics and outcomes between them[38]. We speculate that both environmental and genetic factors contribute to the low familial aggregation rates among Asian patients with CD. It might be valuable to elucidate the Asian-specific pathogenesis and establish prevention methods by investigating Asia-specific lifestyles or environmental factors, including breast feeding, tonsillectomy, childhood vaccination, infectious disease, or dietary intake of fiber and sugar.
In the West, CD has been found to occur in the ileum, colon, and both the ileum and colon in equal proportions of patients[15,39]. However, a number of studies from the West have reported isolated colonic disease to be the most common type of CD[11,40,41]. A study comparing patients in China and the US also reported that American white patients have more colorectal involvement[32]. However, ileocolonic disease appears to be the most common type of CD in Asia[5,7,21,33,34,42,43]. CD confined to the small bowel is also common in Asia. Western guidelines suggest a follow-up colonoscopy to assess mucosal healing or recurrence after biologic therapy or surgery. However, routine ileocolonoscopy for therapeutic monitoring or after surgery might be less useful in Asian patients. Instead, radiological evaluation might play a role in such cases because isolated ileal or ileocolonic disease is not entirely visible by ileocolonoscopy.
Upper gastrointestinal tract involvement, which is significantly associated with disease prognosis such as changes in behavior[44] or the need for early surgery or hospitalization[45], has been rarely reported in Asia. This might be due to the lack of consensus regarding the performance of routine gastroduodenoscopy in patients with newly diagnosed CD in real practice. A study from China found that 23.5% of patients had upper gastrointestinal tract involvement at the time of diagnosis[14], but other studies[3,7,11,46,47] from both the Asia-Pacific region and the West have reported lower proportions. In a recent Korean study, jejunal involvement was observed in 14.1% of patients with CD at the time of diagnosis[48]. This markedly different result compared to China might be an overestimation. Thus, further larger studies are needed to determine the proportion of upper gastrointestinal involvement in patients with CD in Asia.
Studies from the West have shown that the rates of inflammation, stricture, penetration, and perianal fistulas in CD at the time of diagnosis are 62%-81%, 5%-27%, 8%-14%, and 10%-27%, respectively[11,44,47,49,50]. Similarly, Asian studies have reported inflammatory disease in 40% to 69%, stricturing disease in 20%-29%, and penetrating disease in 10%-31% of patients with CD[13,21,33,34,45,51,52]. Interestingly, several studies documenting the disease behavior at the time of diagnosis have reported higher proportions of perianal fistula at the time of diagnosis in Hong Kong (33.3%)[33], Korea (36.7%)[34], and China (58.8%)[14] than in the West. Perianal fistula is known to be a poor prognostic factor in patients with CD[44,47]. In general, however, the prognosis of Asian patients with CD patients is reportedly better than that of Western patients with CD. Whether the presence of perianal fistula is actually a poor prognostic factor of CD remains to be determined and warrants further study. Actually, perianal fistula is described as independent from the penetrating type in the Montreal classification.
The evolution from inflammatory behavior to a more complicated disease behavior (stricturing or penetrating) is well demonstrated in the Western literature[46,47,53], and has also been shown in Asian studies from Hong Kong[33] and Korea[54].
Based on long-term follow-up studies from Japan, it appears that Japanese patients with CD have a long-term prognosis similar to that of their Western counterparts regarding cumulative operation rates[43]. However, compared with Western patients with CD[55], a recent study from Korea showed a better prognosis with respect to the incidence of surgery[13,34]. This can be partly explained by the conservative attitude regarding bowel surgery among Korean physicians and patients or the smaller numbers of patients with a severe phenotype because of the short history of CD in the Asian region, including Korea.
Extraintestinal manifestations (EIM) have been reported in Asian patients with CD with widely variable rates: 19.0%-58.8% in China[14,52,56] and 25.0% in Hong Kong[6]. Among EIM, a high rate of ankylosing spondylitis (9%) among patients with CD has been reported[6]. A study comparing patients between the US and China reported that American white patients developed more EIM (40% vs 20%; OR = 2.63; P = 0.013)[32], especially chronic arthralgia (32% vs 4%; OR = 13.07; P < 0.001). However, it is difficult to directly compare the prevalence of EIM between Asian and Western patients with CD because most Asian reports were hospital-based (not population-based) retrospective studies or prospective cohort studies involving small numbers of patients (n = 17, 58.8%)[14]. Moreover, they were performed under different diagnostic criteria for EIM. Generally, the prevalence of EIM in Asia is accepted to be similar or slightly lower than that in the West (19%-25% vs 21%-41%, respectively)[57-60]. Less frequent EIM may be associated with the relatively better prognosis among Asian patients than in Western patients because EIM, especially ankylosing spondylitis, is known to be associated with a poorer prognosis in patients with IBD.
Conventional therapy: The use of corticosteroids for CD is variable in studies from Asia. In one study, Asian specialists used corticosteroids as the first-line treatment in 50% of patients with mild CD and 84% of patients with moderate CD[61]. Moreover, they used corticosteroids as a maintenance therapy in approximately 25% of patients with CD[61], which might appear to be inappropriately high because corticosteroids have more side effects than placebo or low-dose 5-aminosalicylic acids (5-ASA)[62]. In a recent prospective European study, 54%-55% of patients with CD received corticosteroid therapy as an initial treatment during the first 3 mo of disease in Eastern and Western European centers, but did not during the maintenance phase[15].
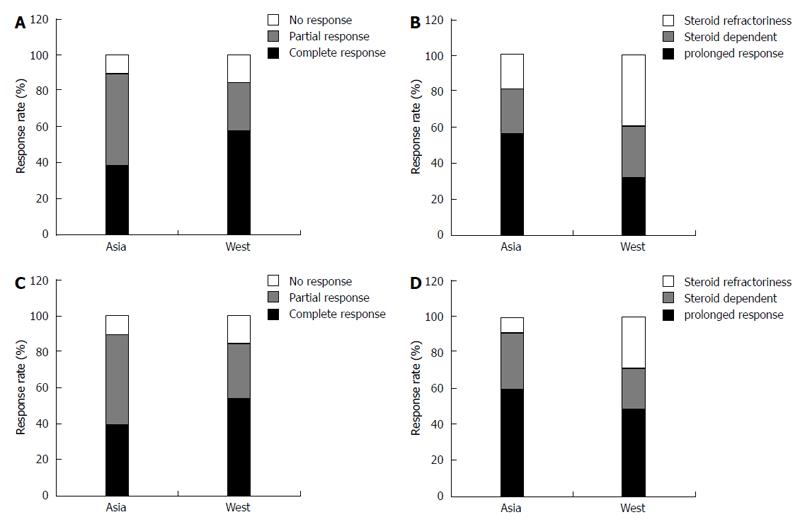
Among patients with CD, the overall response rates to corticosteroids in Asia were similar to or better than those in the West. The short-term response rates at 1 mo were > 80% in both Asia and the West (Figure 1A), and 56.6% and 32.0% of patients, respectively, were corticosteroid-free without the need for surgery at 1 year (Figure 1B)[63,64].
The use of thiopurines in Asia also varies among countries. A Korean single-center study reported that thiopurines were used in 63% of patients with CD[34]. A separate Korean study showed that 42% of patients received thiopurines and that the cumulative thiopurine requirement was 9.1% at 1 year, 32.2% at 5 years, and 51.6% at 10 years[65]. Another cross-sectional study from Hong Kong noted that thiopurines were used in 63.6% of patients[22]. The authors of this study stated that the use of thiopurines was significantly less frequent in Hong Kong patients than in Melbourne patients (63.6% vs 82.1%, respectively, P < 0.001). A single-center review from East China found that 61 of 227 (26.9%) patients had indications for immunomodulator use. However, such agents were prescribed to only 34.0% of the patients, and of these 34.0%, 38.0% received a subtherapeutic dose with no attempt to increase the dose[66]. A recent prospective, population-based study from the Asia-Pacific region reported that only 35% of patients with CD received immunomodulator therapy[7]. Taken together, these results indicate that Asian physicians have a tendency to inappropriately use large amounts of corticosteroids and insufficient amounts of thiopurines. It is important to educate physicians regarding these medications.
There appears to be a higher rate of adverse events, particularly leukopenia, in Asians than in Caucasians when taking thiopurines. Up to 40%-56% of Asian patients may reach the criterion for leucopenia, namely a white blood cell count of < 4000/mm3[67,68]. The cumulative incidence of myelotoxicity in the Western population was reported to be about 7%[69]. In Asia, however, thiopurine methyltransferase genotyping itself may not be as helpful in identifying patients who are expected to develop myelotoxicity as in Western countries[70], and the metabolites of thiopurines are not widely measured in real practice in many Asian countries. In Korea, therefore, physicians usually start a thiopurine at a smaller dose (e.g., 25 or 50 mg of azathioprine) and gradually increase the dose with regular evaluations of the white blood cell count instead of determining the metabolite concentration. Alternatively, they may maintain lower doses of thiopurines than recommended in the Western guidelines[71].
Biologics: In a recent prospective, population-based cohort study from Europe, the rates of biologics use as an initial treatment during the first 3 mo of disease were reportedly 7% and 2% in Western and Eastern European centers, respectively[15]. This approach was interpreted as top-down therapy or rapid accelerating therapy. However, the economic burden or the reimbursement system can be obstacles to the use of biologics in the very early phase after diagnosis of CD in many Asian countries. An Asian survey of IBD management practices in different countries found that no IBD specialists would consider anti-tumor necrosis factor (anti-TNF) agents as the first choice for the treatment of CD. Moreover, only 20% considered anti-TNF agents as the second choice[61]. In a cross-sectional study comparing the management of CD between Melbourne and Hong Kong, a significantly higher number of patients in Melbourne had been on anti-TNF agents than in Hong Kong (40% vs 11%)[22]. A retrospective study from Korea reported that 8.6% of patients with CD used infliximab[34].
The response rates to infliximab in Asia are similar to or higher than those in the West. Although it is difficult to directly compare the results of responses to infliximab in Asia and the West because of their different designs, the response rates at 2 wk after beginning induction therapy were 72% and 62% in Korea (unpublished retrospective data) and the West[72], respectively. Moreover, the response rates at 30 and 54 wk after beginning maintenance therapy were 91.7% vs 50.0% and 74.7% vs 39.0% in Korea (unpublished retrospective data) and the West[72], respectively. A similar pattern was reported in patients with fistulizing CD between Korea and Western countries. In Asia, anti-TNF agents are used less frequently because of the limited, strict indications under insurance coverage rules, and because of the social economic burden. Moreover, many Asian physicians have not accepted the latest treatment trends, such as rapid accelerated step-up or top-down therapeutic approaches. Again, it is necessary to educate Asian physicians regarding the adequate use of the latest medical treatments for IBD.
Complementary and alternative medicines: There are diverse rates of use of complementary and alternative medicines (CAMs) across Asia. One study from China reported that 90% of patients used concomitant traditional Chinese medications[73]. Moreover, various proportions of Western patients with IBD use CAM, ranging from 23% to 49% in recent studies[74-77]. In seven randomized controlled trials of patients with CD, Artemisia absinthium (wormwood) and Tripterygium wilfordii were superior to placebo in terms of inducing remission and preventing clinical recurrence of postoperative CD, respectively[78]. In two systematic reviews, omega-3 fatty acids did not appear to be effective for the maintenance of CD remission[79,80]. Effective anti-inflammatory moieties have yet to be defined.
Another problem is the indiscriminate use of various CAMs without sufficient evidence in patients with IBD. More studies are needed to determine the efficacy and safety of CAM in such patients. In addition, the development of new CAMs from natural products would be helpful in situations in which few drugs are available for patients with IBD.
The cumulative operation rates for CD in Japanese studies are comparable to those of Western cohorts, ranging from 25.9% to 44.4% at 5 years and 46.3% to 80.1% at 10 years, respectively[43,81]. The surgical resection rate for patients with CD in Hong Kong (29% at 10 years)[6] is similar to that in a population-based study from Norway, which reported a 10-year cumulative surgical rate of 37.9%[55]. In a cross-sectional study, there was also no significant difference in the proportion of patients with CD who underwent surgery in Melbourne compared with in Hong Kong (55.1% vs 46.0%, respectively, P = 0.065)[22]. Similarly, the cumulative probability of intestinal resection in Korea was reported to be 15.5% after 1 year, 25.0% after 5 years, and 32.8% after 10-15 years. Asian patients with CD are currently considered to have a similar or slightly lower rate of surgery than that among their Western counterparts[34]. The lack of long-term follow-up studies in Asia makes it difficult to draw a concrete conclusion regarding the cumulative surgical resection rates between Asia and the West.
Age and sex: The median age at the time of diagnosis of UC among patients in Asia is similar to or slightly older than that among patients in the West (35-44 years in Asia[5,9,51,82-85] and 30-40 years in the West[39,86,87]). In a cross-sectional study, patients with UC in Hong Kong were diagnosed at an older age than Caucasians in Melbourne (median age, 38 years vs 30 years, respectively). The authors suggested that this may be partly explained by a delay in diagnosis in Hong Kong[22]. Another possible explanation would be a weaker influence of genetic factors in Asian patients, which delays disease occurrence (Table 1).
The majority of studies from the West have shown an equal sex distribution for UC, although some reported a male predominance[86,88]. A growing number of studies in Asia have shown an equal sex distribution[5,84,85,87,89] or slight male predominance[9,35,52,82,90]. Collectively, the age and sex distributions of patients with UC are not largely different between the East and West.
Family history: Studies in Asia have reported a family history in 0.0%-3.4% of patients with UC[35,82,85]. This figure is lower than the 10%-25% reported in Western countries[37]. A recent population-based cohort study conducted in the Asia-Pacific area showed a family history in 3% of patients in Asia and in 17% of patients in Australia (P < 0.001)[7]. Interestingly, in Korea, an increase in the prevalence of a positive family history from 1.3% in 2001 to 2.7% in 2005[5] paralleled the increased incidence of IBD. This suggests that the low prevalence of a family history may be a reflection of the low population prevalence and will probably change with time.
In terms of genetic associations, a previous Japanese genome-wide association study (GWAS) and a recent Korean GWAS showed considerable overlap of genetic associations for UC between Asia and the West. Despite the overlap of genetic associations of Asian and Western patients with UC, as can be seen by the lower proportion of patients with a family history of UC in Asia compared with the West, we realize that UC is one of the principal forms of IBD with complex manifestations, and genetic factors that account for only a portion of the overall disease development. This indicates a need to better explore gene-environment interactions or Asia-specific environmental factors of etiological importance in the development of IBD.
Disease extent: In UC, the extent of disease is classified into three types: proctitis, left-sided colitis, and extensive colitis. In Western population-based studies, these three types comprise 30%-60% of cases, 16%-40% of cases, and 18%-35% of cases, respectively[91-93]. In Asian population-based studies, they comprise 25.0%-43.7%, 31.0%-31.4%, and 24.9%-39.0%, respectively[5,42]. Most hospital-based studies in Asia have shown a trend toward a lower proportion of proctitis (8.5%-38.4%), higher proportion of left-sided colitis (29.7%-70.2%), and similar proportion of extensive colitis (21.3%-42.4%) compared with population-based studies[35,52,84,85,94], indicating that more severe cases were recruited into the hospital-based studies.
In terms of age and disease extent, several studies have reported that the extent of UC at diagnosis differs significantly according to age at diagnosis[83,95]. In one Korean study, proctitis was more common in elderly patients (28.9% in the young group vs 33.8% in the elderly group) and extensive colitis was more common in younger patients (35.1% in the young group vs 22.5% in the elderly group), suggesting a poorer clinical outcome in younger patients (P < 0.05)[83].
In a recent comparative epidemiological study of IBD across Asia and the Pacific, the extent of UC was classified as proctitis in 37%, left-sided colitis in 32%, and extensive colitis in 31%. These results are not significantly different from those in a study from Australia, which classified UC as proctitis in 32%, left-sided colitis in 27%, and extensive colitis in 41% (all P > 0.05)[7]. A recent population-based cohort study from the West and East Europe showed that the ratios of disease extent for UC from Western and Eastern European centers were proctitis in 20% and 22%, left-sided colitis in 41% and 46%, and extensive colitis in 38% and 32%, respectively[15]. Collectively, a slightly higher proportion of extensive colitis is observed in Western countries than in Asia, suggesting a more favorable prognosis of UC in Asia.
Disease course: Although definitions of clinical relapse and measurements of disease severity vary among studies, most suggest that Asian patients with UC have a milder disease course than do patients from Western countries[82,90,96]. In Asian studies, most patients with UC had a chronic relapsing disease course rather than continuous active disease[84,97], similar to Western data[98]. In a Malaysian study, the rate of maintaining remission was reported as 64.3% of patients with UC at 10 years after diagnosis, the rate of a chronic relapsing disease course as 25%, and the rate of chronic persistent disease with low or high activity as 3% after 10 years[97]. In a Norwegian study, the rate of maintaining remission was reported to be 55% of patients with UC at 10 years after diagnosis, which are slightly lower than the Malaysian data[98], and the rate of a chronic relapsing disease course to be 37%. In a Korean study, the cumulative relapse rate at 10 years after diagnosis (88.4%) was similar to that of a Western study (83%). However, the cumulative probabilities of colectomy (2.0% after 1 year, 2.8% after 3 years, and 3.3% after 5-15 years)[96] were lower than those in a Western study (3.5% after 1 year, 7.6% after 5 years, and 9.8% after 10 years)[98].
Based on the larger numbers of patients with a remission status, lower numbers of patients with a chronic relapsing or persistent active disease course, and lower cumulative operation rates, Asian patients with UC appear to have a milder disease course than that of Western patients with UC. However, the above-mentioned lower cumulative surgical rates may also be associated with diversity in management strategies or different levels of acceptance of colectomy by physicians and/or patients between Asia and the West in addition to disease severity.
The overall prevalence of colorectal cancer (CRC) associated with UC is reportedly 3%-5% in the West[99] and 0.0% to 2.2% in Asia[35,52,85,90,96,100-103]. In a previous meta-analysis from the West, the cumulative risk of CRC associated with UC was reportedly 1.6%, 8.3%, and 18.4% at 10, 20, and 30 years, respectively[104]. In Asian studies, the cumulative risk of CRC in patients with UC was reportedly 0.70% to 1.15% at 10 years, 3.56%-7.90% at 20 years, and 14.4%-33.2% at 30 years[100,103], which is comparable with Western cohorts. However, considering the hospital-based design of most Asian studies, the actual corresponding risks might be lower than estimated.
Recently, however, a Western report showed that the risk of CRC decreased from 1979 to 2008 (RR = 1.34 in 1979-1988 to 0.57 in 1999-2008) and that the overall risk of CRC among patients with UC was comparable with that of the general population (RR = 1.07; 95%CI: 0.95-1.21). These findings suggest that a diagnosis of UC no longer seems to increase patients’ risk of CRC. However, subgroups of patients with UC, including those diagnosed with UC in childhood or as adolescents, those with a long duration of disease, and those with concomitant primary sclerosing cholangitis, remain at increased risk[105].
Current evidence indicates that the risk of CRC in Asian patients with UC is slightly lower than that in Western patients. There is a chance that the prevalence of CRC will increase with the rising incidence and increasing proportion of patients with a longer follow-up in Asian countries. A very recent single-center study in Korea showed a substantial increase in CRC among patients with long-standing UC, which is comparable to Western data (unpublished data). Long-term prospective follow-up studies are warranted to estimate the actual risk of CRC in Asian patients with UC.
Although there are limited numbers of long-term prospective cohort studies and variations in the definition of EIM in Asian studies, the prevalence of EIM in UC seems to be lower in Asian than in Western countries[58,59,73]. The most commonly involved sites of EIM differ between Asia and the West. The most commonly involved site of EIM is the joints in Asian patients with UC (2.0%-19.5%). Next, eye and skin involvement accounts for 0.0%-4.2% and 0.0%-4.2%, respectively[35,52,82,90,97]. In contrast, eye involvement (iritis/uveitis) in females (3.8%) and primary sclerosing cholangitis (PSC) in males (3.0%) were the most commonly involved sites in a Western population-based study[58].
PSC associated with UC is less prevalent in Asia (0.0%-1.7%)[52,84,96,97,106] than in the West (1.6-7.0%)[59,107]. Because PSC is associated with a risk of CRC in patients with UC, a diagnosis of PSC should not be neglected in real practice despite the low prevalence of PSC in Asia.
Conventional therapy: In a recent population-based cohort study in the Asia-Pacific area that compared UC treatments in the first year between Asia and Australia[7], treatment with antibiotics (22% vs 14%, P = 0.15) and immunomodulators (thiopurine or methotrexate) (18% vs 9%, P = 0.05) did not differ between Asia and Australia. Mesalazine (79% vs 62%, P = 0.012) and corticosteroids (62% vs 28%, P < 0.0001) were more commonly prescribed for IBD at the time of diagnosis in Australia than in Asia. Topical therapy (mesalazine or corticosteroids) for UC was also more frequently prescribed in Australia than in Asia (55% vs 28%, P = 0.04).
In a recent population-based European study, 69 (44%) patients with left-sided colitis and 19 (23%) with extensive colitis in Western Europe received more frequently receive combination therapy with oral and topical 5-ASA compared with 21 (42%) and 6 (22%), respectively, in Eastern Europe[15]. In this study, 26%-33% of patients with UC received corticosteroid therapy as initial treatment during the first 3 mo of disease in Eastern and Western European centers.
In an Asian survey of management practices for IBD in different countries[61], 83%, 75%, and 61% of respondents preferred 5-ASA, a combination of topical and oral 5-ASA, and corticosteroids, respectively, as induction treatment of mild-to-moderate UC. Almost all respondents agreed that maintenance therapy should be recommended for patients with IBD in remission, with most recommending the use of 5-ASA to maintain remission in UC (91%). However, they also replied that thiopurines and corticosteroids were needed to maintain the remission in approximately 30% and 13% of patients with UC, respectively.
In one cross-sectional study, there was less use of corticosteroids (15.3% vs 46.5%, P < 0.001) and thiopurines (19.7% vs 55.3%, P < 0.001) for UC in Hong Kong than in Melbourne, which also reflects differences in practice according to region[22]. The authors suggested that Asian physicians prefer to manage UC with less intense medical treatments despite more extensive UC and that they use less thiopurines for maintenance therapy compared with the physicians in Melbourne. Again, it is important to educate Asian physicians in terms of following the adequate use of medical treatments according to the practice guidelines for IBD.
Among the UC patients who receive 5-ASA or sulfasalazine therapy, 49.6%[108] and 72.0% to 75.0%[109,110] experienced disease relapse in Asia and the West, respectively. The cumulative relapse rate was 21.5% after 1 year, 36.5% after 2 years, 46.9% after 3 years, and 59.8% after 5 years during maintenance therapy with 5-ASA/sulfasalazine, and both the disease extent at diagnosis and anemia were major predictive factors for clinical relapse after 5-ASA/sulfasalazine therapy for Korean patients with mild to moderate UC[108].
The overall response rates of Asian patients with UC to corticosteroids are similar to or better than those of patients in the West. Short-term response rates at 1 mo were more than 89.2% and 84.0% in Asia and the West (Figure 1C), and 59.4% and 49.0% showed a prolonged response at 1 year, respectively (Figure 1D)[64,111]
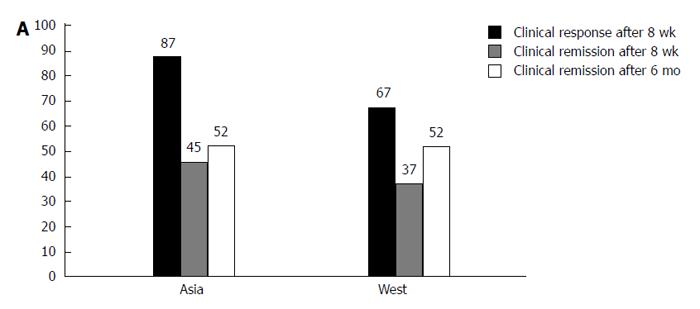
A significant difference in the use of anti-TNF inhibitors between Hong Kong and Melbourne has been shown (2/203 vs 12/159, P = 0.001)[22]. An Asian survey of IBD management practices in different countries found that < 15% of Asian physicians would use anti-TNF therapy in the management of UC[61]. In many countries in Asia, the use of biologic agents is self-financed, making the high cost an obstacle to their wider use. However, a short-term population-based study showed that treatment with biological therapy in the first year after diagnosis (2.0% vs 0.0%, P = 0.21) did not differ between Asia and Australia[7]. Long-term follow-up studies are needed to show the chronological trends in the use of anti-TNF therapy in Asia. Emerging studies suggest that anti-TNF therapies are effective and safe in Asian patients with UC. The rates of clinical response and remission to infliximab were 87% and 45% in patients with UC at week 8[112], which is slightly higher than the rate of clinical response in the West (69.4%-64.5% at week 8)[113] (Figure 2). Data on long-term efficacy were obtained from 85 of 134 Korean patients who were followed up for more than 6 mo after the first dose of infliximab, and 44 of them (52%) were in remission, which is compatible with UC data in the West[114]. An another recent Korea study reported that 66.3% of patients demonstrated a clinical response at week 8, 32.6% of whom were determined to be in clinical remission[115]; this is similar to the response rates in previous Western reports. Collectively, a similar or slightly higher response rate of infliximab in patients with UC is observed in Asia than in Western countries. In the future, it is expected that other anti-TNF agents, such as adalimumab and golimumab, will be used more widely in addition to infliximab in patients with UC in Asian countries.
In 14 randomized controlled trials of patients with UC, aloe vera gel, Triticum aestivum (wheat grass juice), Andrographis paniculata extract (HMPL-004), and topical Xilei San were superior to placebo in inducing remission or response, and curcumin was superior to placebo in maintaining remission. Boswellia serrata gum resin and Plantago ovata seeds were as effective as mesalazine, whereas Oenothera biennis (evening primrose oil) was not effective and had relapse rates similar to those of omega-3 fatty acids in the treatment of UC[78]. Larger controlled studies with stricter endpoints and better-defined patient groups are required to obtain more conclusive findings regarding the use of CAM in IBD.
Over the past two decades, the incidence and prevalence of IBD have changed with a trend toward increasing across Asia, especially East Asia. A younger second peak age of disease onset has been shown in Asian populations with CD compared to in Western populations. There is a predominance of male sex and ileocolonic involvement among Asian patients with CD. In patients with UC, the age and sex distribution are not different between Asia and the West. The proportion of current or ex-smokers among Asian patients with CD is lower than that among Western patients. The familial aggregation rates of patients with CD and UC are lower in Asia, but appear to be higher in the West. The disease extent in Asian patients with UC is not significantly different from, and may be slightly less severe than, that in Western populations. Asian patients with UC seem to have a milder disease course than do patients in Western countries. PSC associated with UC is less prevalent in Asia than in the West. Cumulative surgical resection rates in patients with CD do not appear to be different between Asia and the West despite the lack of large-scale, long-term follow-up studies in Asia. Whereas the cumulative surgical resection rates in Asian patients with UC are lower than those in Western patients, the cumulative risk of CRC associated with UC among Asian patients with UC is reportedly comparable with that among Western patients. The use of thiopurine or biologics in patients with IBD remains less frequent in Asia than in the West. There appears to be a higher rate of adverse events, particularly myelotoxicity, in Asians than in Caucasians prescribed thiopurines. The treatment responses for corticosteroids, thiopurines, and biologics of Asian patients with IBD are slightly better than or comparable to those of Western patients.
Several recent prospective, population-based cohort studies were conducted in Asia. Long-term follow-up results from these cohort studies are warranted to help clinicians and researchers further objectively compare the disease prognosis between Asian and Western countries, provide specific health care planning and education, and offer the possibility of identifying causative factors in a population with a rapidly increasing incidence in Asia.
P- Reviewer: Green J, Tovey FI S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Thia KT, Loftus EV, Sandborn WJ, Yang SK. An update on the epidemiology of inflammatory bowel disease in Asia. Am J Gastroenterol. 2008;103:3167-3182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 371] [Cited by in F6Publishing: 393] [Article Influence: 24.6] [Reference Citation Analysis (1)] |
| 2. | Loftus CG, Loftus EV, Harmsen WS, Zinsmeister AR, Tremaine WJ, Melton LJ, Sandborn WJ. Update on the incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota, 1940-2000. Inflamm Bowel Dis. 2007;13:254-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 473] [Cited by in F6Publishing: 464] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 3. | Ekbom A, Helmick C, Zack M, Adami HO. The epidemiology of inflammatory bowel disease: a large, population-based study in Sweden. Gastroenterology. 1991;100:350-358. [PubMed] [Cited in This Article: ] |
| 4. | Loftus EV, Silverstein MD, Sandborn WJ, Tremaine WJ, Harmsen WS, Zinsmeister AR. Crohn’s disease in Olmsted County, Minnesota, 1940-1993: incidence, prevalence, and survival. Gastroenterology. 1998;114:1161-1168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 458] [Cited by in F6Publishing: 427] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 5. | Yang SK, Yun S, Kim JH, Park JY, Kim HY, Kim YH, Chang DK, Kim JS, Song IS, Park JB. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986-2005: a KASID study. Inflamm Bowel Dis. 2008;14:542-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 379] [Cited by in F6Publishing: 313] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 6. | Leong RW, Lau JY, Sung JJ. The epidemiology and phenotype of Crohn’s disease in the Chinese population. Inflamm Bowel Dis. 2004;10:646-651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 166] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Ng SC, Tang W, Ching JY, Wong M, Chow CM, Hui AJ, Wong TC, Leung VK, Tsang SW, Yu HH. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn’s and colitis epidemiology study. Gastroenterology. 2013;145:158-165.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 515] [Cited by in F6Publishing: 535] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 8. | Ouyang Q, Tandon R, Goh KL, Ooi CJ, Ogata H, Fiocchi C. The emergence of inflammatory bowel disease in the Asian Pacific region. Curr Opin Gastroenterol. 2005;21:408-413. [PubMed] [Cited in This Article: ] |
| 9. | Asakura K, Nishiwaki Y, Inoue N, Hibi T, Watanabe M, Takebayashi T. Prevalence of ulcerative colitis and Crohn’s disease in Japan. J Gastroenterol. 2009;44:659-665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 197] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 10. | Wang YF, Zhang H, Ouyang Q. Clinical manifestations of inflammatory bowel disease: East and West differences. J Dig Dis. 2007;8:121-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Sjöberg D, Holmström T, Larsson M, Nielsen AL, Holmquist L, Ekbom A, Rönnblom A. Incidence and clinical course of Crohn’s disease during the first year - results from the IBD Cohort of the Uppsala Region (ICURE) of Sweden 2005-2009. J Crohns Colitis. 2014;8:215-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 12. | Loftus EV, Schoenfeld P, Sandborn WJ. The epidemiology and natural history of Crohn’s disease in population-based patient cohorts from North America: a systematic review. Aliment Pharmacol Ther. 2002;16:51-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 327] [Cited by in F6Publishing: 350] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 13. | Moon CM, Park DI, Kim ER, Kim YH, Lee CK, Lee SH, Kim JH, Huh KC, Jung SA, Yoon SM. Clinical features and predictors of clinical outcomes in Korean patients with Crohn’s disease: a Korean association for the study of intestinal diseases multicenter study. J Gastroenterol Hepatol. 2014;29:74-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Zeng Z, Zhu Z, Yang Y, Ruan W, Peng X, Su Y, Peng L, Chen J, Yin Q, Zhao C. Incidence and clinical characteristics of inflammatory bowel disease in a developed region of Guangdong Province, China: a prospective population-based study. J Gastroenterol Hepatol. 2013;28:1148-1153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 15. | Burisch J, Pedersen N, Čuković-Čavka S, Brinar M, Kaimakliotis I, Duricova D, Shonová O, Vind I, Avnstrøm S, Thorsgaard N. East-West gradient in the incidence of inflammatory bowel disease in Europe: the ECCO-EpiCom inception cohort. Gut. 2014;63:588-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 263] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 16. | van der Heide F, Dijkstra A, Weersma RK, Albersnagel FA, van der Logt EM, Faber KN, Sluiter WJ, Kleibeuker JH, Dijkstra G. Effects of active and passive smoking on disease course of Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis. 2009;15:1199-1207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Calkins BM. A meta-analysis of the role of smoking in inflammatory bowel disease. Dig Dis Sci. 1989;34:1841-1854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 424] [Cited by in F6Publishing: 364] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 18. | Silverstein MD, Lashner BA, Hanauer SB, Evans AA, Kirsner JB. Cigarette smoking in Crohn’s disease. Am J Gastroenterol. 1989;84:31-33. [PubMed] [Cited in This Article: ] |
| 19. | Sutherland LR, Ramcharan S, Bryant H, Fick G. Effect of cigarette smoking on recurrence of Crohn’s disease. Gastroenterology. 1990;98:1123-1128. [PubMed] [Cited in This Article: ] |
| 20. | Mahid SS, Minor KS, Soto RE, Hornung CA, Galandiuk S. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clin Proc. 2006;81:1462-1471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 479] [Cited by in F6Publishing: 469] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 21. | Zhao J, Ng SC, Lei Y, Yi F, Li J, Yu L, Zou K, Dan Z, Dai M, Ding Y. First prospective, population-based inflammatory bowel disease incidence study in mainland of China: the emergence of “western” disease. Inflamm Bowel Dis. 2013;19:1839-1845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Prideaux L, Kamm MA, De Cruz P, Williams J, Bell SJ, Connell WR, Brown SJ, Lust M, Desmond PV, Chan H. Comparison of clinical characteristics and management of inflammatory bowel disease in Hong Kong versus Melbourne. J Gastroenterol Hepatol. 2012;27:919-927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Cosnes J, Beaugerie L, Carbonnel F, Gendre JP. Smoking cessation and the course of Crohn’s disease: an intervention study. Gastroenterology. 2001;120:1093-1099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 290] [Cited by in F6Publishing: 266] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 24. | Lakatos PL, Szamosi T, Lakatos L. Smoking in inflammatory bowel diseases: good, bad or ugly? World J Gastroenterol. 2007;13:6134-6139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 57] [Cited by in F6Publishing: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Nunes T, Etchevers MJ, Domènech E, García-Sánchez V, Ber Y, Peñalva M, Merino O, Nos P, Garcia-Planella E, Casbas AG, Esteve M, Taxonera Samsó C, Montoro Huguet M, Gisbert JP, Martín Arranz MD, García-Sepulcre MF, Barreiro-de Acosta M, Beltrán B, Alcaide Suárez N, Saro Gismera C, Cabriada JL, Cañas-Ventura A, Gomollón F, Panés J, Tobacco-Eneida Study Group of G. Smoking does influence disease behaviour and impacts the need for therapy in Crohn’s disease in the biologic era. Aliment Pharmacol Ther. 2013;38:752-760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 26. | Lawrance IC, Murray K, Batman B, Gearry RB, Grafton R, Krishnaprasad K, Andrews JM, Prosser R, Bampton PA, Cooke SE. Crohn’s disease and smoking: is it ever too late to quit? J Crohns Colitis. 2013;7:e665-e671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Reif S, Klein I, Arber N, Gilat T. Lack of association between smoking and inflammatory bowel disease in Jewish patients in Israel. Gastroenterology. 1995;108:1683-1687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Fich A, Eliakim R, Sperber AD, Carel RS, Rachmilewitz D. The association between smoking and inflammatory bowel disease among israeli jewish patients. Inflamm Bowel Dis. 1997;3:6-9. [PubMed] [Cited in This Article: ] |
| 29. | Reif S, Lavy A, Keter D, Fich A, Eliakim R, Halak A, Broide E, Niv Y, Ron Y, Patz J. Lack of association between smoking and Crohn’s disease but the usual association with ulcerative colitis in Jewish patients in Israel: a multicenter study. Am J Gastroenterol. 2000;95:474-478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Thia KT, Luman W, Jin OC. Crohn’s disease runs a more aggressive course in young Asian patients. Inflamm Bowel Dis. 2006;12:57-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Park JB, Yang SK, Byeon JS, Park ER, Moon G, Myung SJ, Park WK, Yoon SG, Kim HS, Lee JG. Familial occurrence of inflammatory bowel disease in Korea. Inflamm Bowel Dis. 2006;12:1146-1151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Luo CH, Wexner SD, Liu QS, Li L, Weiss E, Zhao RH. The differences between American and Chinese patients with Crohn’s disease. Colorectal Dis. 2011;13:166-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Chow DK, Leong RW, Lai LH, Wong GL, Leung WK, Chan FK, Sung JJ. Changes in Crohn’s disease phenotype over time in the Chinese population: validation of the Montreal classification system. Inflamm Bowel Dis. 2008;14:536-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Ye BD, Yang SK, Cho YK, Park SH, Yang DH, Yoon SM, Kim KJ, Byeon JS, Myung SJ, Yu CS. Clinical features and long-term prognosis of Crohn’s disease in Korea. Scand J Gastroenterol. 2010;45:1178-1185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 35. | Wang Y, Ouyang Q. Ulcerative colitis in China: retrospective analysis of 3100 hospitalized patients. J Gastroenterol Hepatol. 2007;22:1450-1455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 36. | Monsén U, Bernell O, Johansson C, Hellers G. Prevalence of inflammatory bowel disease among relatives of patients with Crohn’s disease. Scand J Gastroenterol. 1991;26:302-306. [PubMed] [Cited in This Article: ] |
| 37. | Orholm M, Munkholm P, Langholz E, Nielsen OH, Sørensen TI, Binder V. Familial occurrence of inflammatory bowel disease. N Engl J Med. 1991;324:84-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 462] [Cited by in F6Publishing: 401] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 38. | Chung SH, Park SJ, Lee HS, Cheon JH, Hong SP, Kim TI, Kim WH. Similar clinical characteristics of familial and sporadic inflammatory bowel disease in Korea. World J Gastroenterol. 2014;In press. [Cited in This Article: ] |
| 39. | Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785-1794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1390] [Cited by in F6Publishing: 1417] [Article Influence: 109.0] [Reference Citation Analysis (0)] |
| 40. | Lapidus A. Crohn’s disease in Stockholm County during 1990-2001: an epidemiological update. World J Gastroenterol. 2006;12:75-81. [PubMed] [Cited in This Article: ] |
| 41. | Wolters FL, Russel MG, Sijbrandij J, Ambergen T, Odes S, Riis L, Langholz E, Politi P, Qasim A, Koutroubakis I. Phenotype at diagnosis predicts recurrence rates in Crohn’s disease. Gut. 2006;55:1124-1130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 165] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 42. | Shin DH, Sinn DH, Kim YH, Kim JY, Chang DK, Kim EJ, Ryu HY, Song HU, Kim IY, Kim do H. Increasing incidence of inflammatory bowel disease among young men in Korea between 2003 and 2008. Dig Dis Sci. 2011;56:1154-1159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Oriuchi T, Hiwatashi N, Kinouchi Y, Takahashi S, Takagi S, Negoro K, Shimosegawa T. Clinical course and longterm prognosis of Japanese patients with Crohn’s disease: predictive factors, rates of operation, and mortality. J Gastroenterol. 2003;38:942-953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR, Loftus EV. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology. 2010;139:1147-1155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 481] [Cited by in F6Publishing: 502] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 45. | Chow DK, Sung JJ, Wu JC, Tsoi KK, Leong RW, Chan FK. Upper gastrointestinal tract phenotype of Crohn’s disease is associated with early surgery and further hospitalization. Inflamm Bowel Dis. 2009;15:551-557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 46. | Louis E, Collard A, Oger AF, Degroote E, Aboul Nasr El Yafi FA, Belaiche J. Behaviour of Crohn’s disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49:777-782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 684] [Cited by in F6Publishing: 664] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 47. | Tarrant KM, Barclay ML, Frampton CM, Gearry RB. Perianal disease predicts changes in Crohn’s disease phenotype-results of a population-based study of inflammatory bowel disease phenotype. Am J Gastroenterol. 2008;103:3082-3093. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 172] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 48. | Park SK, Yang SK, Park SH, Park SH, Kim JW, Yang DH, Jung KW, Kim KJ, Ye BD, Byeon JS. Long-term prognosis of the jejunal involvement of Crohn’s disease. J Clin Gastroenterol. 2012;47:400-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | Solberg IC, Vatn MH, Høie O, Stray N, Sauar J, Jahnsen J, Moum B, Lygren I. Clinical course in Crohn’s disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol. 2007;5:1430-1438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 502] [Cited by in F6Publishing: 488] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 50. | Schwartz DA, Loftus EV, Tremaine WJ, Panaccione R, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of fistulizing Crohn’s disease in Olmsted County, Minnesota. Gastroenterology. 2002;122:875-880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 739] [Cited by in F6Publishing: 634] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 51. | Subasinghe D, Nawarathna NM, Samarasekera DN. Disease characteristics of inflammatory bowel disease (IBD): findings from a tertiary care centre in South Asia. J Gastrointest Surg. 2011;15:1562-1567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 52. | Jiang L, Xia B, Li J, Ye M, Yan W, Deng C, Ding Y, Luo H, Hou W, Zhao Q. Retrospective survey of 452 patients with inflammatory bowel disease in Wuhan city, central China. Inflamm Bowel Dis. 2006;12:212-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 53. | Cosnes J, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, Gendre JP. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis. 2002;8:244-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 981] [Cited by in F6Publishing: 913] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 54. | Baik SH, Park KJ, Lee KY, Cho YB, Choi GS, Lee KY, Yoon SN, Yu CS. Characteristic phenotypes in Korean Crohn’s disease patients who underwent intestinal surgery for the treatment. J Korean Med Sci. 2013;28:575-579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 55. | Solberg IC, Lygren I, Cvancarova M, Jahnsen J, Stray N, Sauar J, Schreiber S, Moum B, Vatn MH; IBSEN Study Group. Predictive value of serologic markers in a population-based Norwegian cohort with inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:406-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 56. | APDW2004 Chinese IBD Working Group. Retrospective analysis of 515 cases of Crohn’s disease hospitalization in China: nationwide study from 1990 to 2003. J Gastroenterol Hepatol. 2006;21:1009-1015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 57. | Orchard T. Extraintestinal complications of inflammatory bowel disease. Curr Gastroenterol Rep. 2003;5:512-517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 58. | Bernstein CN, Blanchard JF, Rawsthorne P, Yu N. The prevalence of extraintestinal diseases in inflammatory bowel disease: a population-based study. Am J Gastroenterol. 2001;96:1116-1122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 495] [Cited by in F6Publishing: 475] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 59. | Lakatos L, Pandur T, David G, Balogh Z, Kuronya P, Tollas A, Lakatos PL. Association of extraintestinal manifestations of inflammatory bowel disease in a province of western Hungary with disease phenotype: results of a 25-year follow-up study. World J Gastroenterol. 2003;9:2300-2307. [PubMed] [Cited in This Article: ] |
| 60. | Su CG, Judge TA, Lichtenstein GR. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Clin North Am. 2002;31:307-327. [PubMed] [Cited in This Article: ] |
| 61. | Sung JJ, Kamm MA, Marteau P. Asian perspectives in the management of inflammatory bowel disease: findings from a recent survey. J Gastroenterol Hepatol. 2010;25:183-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 62. | Benchimol EI, Seow CH, Steinhart AH, Griffiths AM. Traditional corticosteroids for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2008;CD006792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 63. | Kim DH, Cheon JH, Park JJ, Yoon JY, Moon CM, Hong SP, Kim TI, Kim WH. Clinical outcomes and predictive factors for response after the first course of corticosteroid therapy in patients with Crohn’s disease. Gut Liver. 2013;7:58-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 64. | Faubion WA, Loftus EV, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 833] [Cited by in F6Publishing: 759] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 65. | Park JJ, Cheon JH, Hong SP, Kim TI, Kim WH. Outcome predictors for thiopurine maintenance therapy in patients with Crohn’s disease. Dig Dis Sci. 2012;57:133-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 66. | Huang LJ, Zhu Q, Lei M, Cao Q. Current use of immunosuppressive agents in inflammatory bowel disease patients in East China. World J Gastroenterol. 2009;15:3055-3059. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 13] [Cited by in F6Publishing: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 67. | Tajiri H, Tomomasa T, Yoden A, Konno M, Sasaki M, Maisawa S, Sumazaki R, Shimizu T, Toyoda S, Etani Y. Efficacy and safety of azathioprine and 6-mercaptopurine in Japanese pediatric patients with ulcerative colitis: a survey of the Japanese Society for Pediatric Inflammatory Bowel Disease. Digestion. 2008;77:150-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 68. | Kim JH, Cheon JH, Kim WH. [The frequency and the course of the adverse effects of azathioprine/6-mercaptopurine treatment in patients with inflammatory bowel disease]. Korean J Gastroenterol. 2008;51:291-297. [PubMed] [Cited in This Article: ] |
| 69. | Gisbert JP, Gomollón F. Thiopurine-induced myelotoxicity in patients with inflammatory bowel disease: a review. Am J Gastroenterol. 2008;103:1783-1800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 177] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 70. | Jung YS, Cheon JH, Park JJ, Moon CM, Kim ES, Lee JH, Kim SW, Kim JH, Hong SP, Kim TI. Correlation of genotypes for thiopurine methyltransferase and inosine triphosphate pyrophosphatase with long-term clinical outcomes in Korean patients with inflammatory bowel diseases during treatment with thiopurine drugs. J Hum Genet. 2010;55:121-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 71. | Kim DU, Kim YH, Kim BJ, Chang DK, Son HJ, Rhee PL, Kim JJ, Rhee JC. The efficacy of low dose azathioprine/6-mercaptopurine in patients with inflammatory bowel disease. Hepatogastroenterology. 2009;56:1395-1402. [PubMed] [Cited in This Article: ] |
| 72. | Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, DeWoody KL, Schaible TF, Rutgeerts PJ. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med. 1997;337:1029-1035. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2328] [Cited by in F6Publishing: 2214] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 73. | Wang YF, Ouyang Q, Hu RW. Progression of inflammatory bowel disease in China. J Dig Dis. 2010;11:76-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 74. | Rawsthorne P, Clara I, Graff LA, Bernstein KI, Carr R, Walker JR, Ediger J, Rogala L, Miller N, Bernstein CN. The Manitoba Inflammatory Bowel Disease Cohort Study: a prospective longitudinal evaluation of the use of complementary and alternative medicine services and products. Gut. 2012;61:521-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 75. | Opheim R, Hoivik ML, Solberg IC, Moum B. Complementary and alternative medicine in patients with inflammatory bowel disease: the results of a population-based inception cohort study (IBSEN). J Crohns Colitis. 2012;6:345-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 76. | Opheim R, Bernklev T, Fagermoen MS, Cvancarova M, Moum B. Use of complementary and alternative medicine in patients with inflammatory bowel disease: results of a cross-sectional study in Norway. Scand J Gastroenterol. 2012;47:1436-1447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 77. | Fernández A, Barreiro-de Acosta M, Vallejo N, Iglesias M, Carmona A, González-Portela C, Lorenzo A, Domínguez-Muñoz JE. Complementary and alternative medicine in inflammatory bowel disease patients: frequency and risk factors. Dig Liver Dis. 2012;44:904-908. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 78. | Ng SC, Lam YT, Tsoi KK, Chan FK, Sung JJ, Wu JC. Systematic review: the efficacy of herbal therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38:854-863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 79. | MacLean CH, Mojica WA, Newberry SJ, Pencharz J, Garland RH, Tu W, Hilton LG, Gralnek IM, Rhodes S, Khanna P. Systematic review of the effects of n-3 fatty acids in inflammatory bowel disease. Am J Clin Nutr. 2005;82:611-619. [PubMed] [Cited in This Article: ] |
| 80. | Turner D, Shah PS, Steinhart AH, Zlotkin S, Griffiths AM. Maintenance of remission in inflammatory bowel disease using omega-3 fatty acids (fish oil): a systematic review and meta-analyses. Inflamm Bowel Dis. 2011;17:336-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 81. | Okada M, Sakurai T, Yao T, Iida M, Okabe N, Maeda K, Matsui T, Fuchigami T, Yoshinaga K, Imamura K. Clinical course and long-term prognosis of Crohn’s disease in Japan. J Gastroenterol. 1994;29:406-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 82. | Lok KH, Hung HG, Ng CH, Kwong KC, Yip WM, Lau SF, Li KK, Li KF, Szeto ML. Epidemiology and clinical characteristics of ulcerative colitis in Chinese population: experience from a single center in Hong Kong. J Gastroenterol Hepatol. 2008;23:406-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 83. | Lee JH, Cheon JH, Moon CM, Park JJ, Hong SP, Kim TI, Kim WH. Do patients with ulcerative colitis diagnosed at a young age have more severe disease activity than patients diagnosed when older? Digestion. 2010;81:237-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 84. | Fujimoto T, Kato J, Nasu J, Kuriyama M, Okada H, Yamamoto H, Mizuno M, Shiratori Y. Change of clinical characteristics of ulcerative colitis in Japan: analysis of 844 hospital-based patients from 1981 to 2000. Eur J Gastroenterol Hepatol. 2007;19:229-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 85. | Chow DK, Leong RW, Tsoi KK, Ng SS, Leung WK, Wu JC, Wong VW, Chan FK, Sung JJ. Long-term follow-up of ulcerative colitis in the Chinese population. Am J Gastroenterol. 2009;104:647-654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 86. | Loftus EV. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504-1517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2085] [Cited by in F6Publishing: 2046] [Article Influence: 102.3] [Reference Citation Analysis (1)] |
| 87. | Vind I, Riis L, Jess T, Knudsen E, Pedersen N, Elkjaer M, Bak Andersen I, Wewer V, Nørregaard P, Moesgaard F. Increasing incidences of inflammatory bowel disease and decreasing surgery rates in Copenhagen City and County, 2003-2005: a population-based study from the Danish Crohn colitis database. Am J Gastroenterol. 2006;101:1274-1282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 379] [Cited by in F6Publishing: 379] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 88. | Bernstein CN, Wajda A, Svenson LW, MacKenzie A, Koehoorn M, Jackson M, Fedorak R, Israel D, Blanchard JF. The epidemiology of inflammatory bowel disease in Canada: a population-based study. Am J Gastroenterol. 2006;101:1559-1568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 402] [Cited by in F6Publishing: 422] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 89. | Ishige T, Tomomasa T, Takebayashi T, Asakura K, Watanabe M, Suzuki T, Miyazawa R, Arakawa H. Inflammatory bowel disease in children: epidemiological analysis of the nationwide IBD registry in Japan. J Gastroenterol. 2010;45:911-917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 90. | Ling KL, Ooi CJ, Luman W, Cheong WK, Choen FS, Ng HS. Clinical characteristics of ulcerative colitis in Singapore, a multiracial city-state. J Clin Gastroenterol. 2002;35:144-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 91. | Jess T, Riis L, Vind I, Winther KV, Borg S, Binder V, Langholz E, Thomsen OØ, Munkholm P. Changes in clinical characteristics, course, and prognosis of inflammatory bowel disease during the last 5 decades: a population-based study from Copenhagen, Denmark. Inflamm Bowel Dis. 2007;13:481-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 287] [Cited by in F6Publishing: 282] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 92. | Henriksen M, Jahnsen J, Lygren I, Sauar J, Kjellevold Ø, Schulz T, Vatn MH, Moum B. Ulcerative colitis and clinical course: results of a 5-year population-based follow-up study (the IBSEN study). Inflamm Bowel Dis. 2006;12:543-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 189] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 93. | Sjöberg D, Holmström T, Larsson M, Nielsen AL, Holmquist L, Ekbom A, Rönnblom A. Incidence and natural history of ulcerative colitis in the Uppsala Region of Sweden 2005-2009 - results from the IBD cohort of the Uppsala Region (ICURE). J Crohns Colitis. 2013;7:e351-e357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 94. | Shiga H, Takagi S, Inoue R, Kinouchi Y, Ohkubo T, Takahashi S, Negoro K, Yokoyama H, Kato S, Fukushima K. What determines the later clinical course of patients who do not undergo colectomy at the first attack? A Japanese cohort study on ulcerative colitis. Digestion. 2010;81:104-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 95. | Quezada SM, Cross RK. Association of age at diagnosis and ulcerative colitis phenotype. Dig Dis Sci. 2012;57:2402-2407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 96. | Park SH, Kim YM, Yang SK, Kim SH, Byeon JS, Myung SJ, Cho YK, Yu CS, Choi KS, Chung JW. Clinical features and natural history of ulcerative colitis in Korea. Inflamm Bowel Dis. 2007;13:278-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 97. | Hilmi I, Singh R, Ganesananthan S, Yatim I, Radzi M, Chua AB, Tan HJ, Huang S, Chin KS, Menon J. Demography and clinical course of ulcerative colitis in a multiracial Asian population: a nationwide study from Malaysia. J Dig Dis. 2009;10:15-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 98. | Solberg IC, Lygren I, Jahnsen J, Aadland E, Høie O, Cvancarova M, Bernklev T, Henriksen M, Sauar J, Vatn MH. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN Study). Scand J Gastroenterol. 2009;44:431-440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 498] [Cited by in F6Publishing: 502] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 99. | Dobbins WO. Dysplasia and malignancy in inflammatory bowel disease. Annu Rev Med. 1984;35:33-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 51] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 100. | Kim BJ, Yang SK, Kim JS, Jeen YT, Choi H, Han DS, Kim HJ, Kim WH, Kim JY, Chang DK. Trends of ulcerative colitis-associated colorectal cancer in Korea: A KASID study. J Gastroenterol Hepatol. 2009;24:667-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 101. | Fujita T, Ando T, Watanabe O, Hasegawa M, Miyake N, Kondo S, Kato T, Ishiguro K, Nakamura M, Miyahara R. Clinicopathological study of colorectal cancer occurring in patients with ulcerative colitis: results from a single hospital in Japan. Hepatogastroenterology. 2010;57:487-492. [PubMed] [Cited in This Article: ] |
| 102. | Senanayake SM, Fernandopulle AN, Niriella MA, Wijesinghe NT, Ranaweera A, Mufeena MN, Pathmeswaran A, Nawarathne NM, de Silva AP, de Silva HJ. The long-term outcomes of a cohort of Sri Lankan patients with ulcerative colitis: a retrospective study at two national referral centers and review of literature. Clin Exp Gastroenterol. 2013;6:195-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 103. | Gong W, Lv N, Wang B, Chen Y, Huang Y, Pan W, Jiang B. Risk of ulcerative colitis-associated colorectal cancer in China: a multi-center retrospective study. Dig Dis Sci. 2012;57:503-507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 104. | Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1985] [Cited by in F6Publishing: 1942] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 105. | Jess T, Simonsen J, Jørgensen KT, Pedersen BV, Nielsen NM, Frisch M. Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years. Gastroenterology. 2012;143:375-381.e1; quiz e13-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 369] [Cited by in F6Publishing: 355] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 106. | Ye BD, Yang SK, Boo SJ, Cho YK, Yang DH, Yoon SM, Kim KJ, Byeon JS, Myung SJ, Yu CS. Clinical characteristics of ulcerative colitis associated with primary sclerosing cholangitis in Korea. Inflamm Bowel Dis. 2011;17:1901-1906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 107. | Loftus EV. Management of extraintestinal manifestations and other complications of inflammatory bowel disease. Curr Gastroenterol Rep. 2004;6:506-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 108. | Lee HJ, Jung ES, Lee JH, Hong SP, Kim TI, Kim WH, Cheon JH. Long-term clinical outcomes and factors predictive of relapse after 5-aminosalicylate or sulfasalazine therapy in patients with mild-to-moderate ulcerative colitis. Hepatogastroenterology. 2012;59:1415-1420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 14] [Reference Citation Analysis (0)] |
| 109. | Bello C, Belaiche J, Louis E, Reenaers C. Evolution and predictive factors of relapse in ulcerative colitis patients treated with mesalazine after a first course of corticosteroids. J Crohns Colitis. 2011;5:196-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 110. | Garcia-Planella E, Mañosa M, Van Domselaar M, Gordillo J, Zabana Y, Cabré E, López San Román A, Domènech E. Long-term outcome of ulcerative colitis in patients who achieve clinical remission with a first course of corticosteroids. Dig Liver Dis. 2012;44:206-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 111. | Yoon JY, Cheon JH, Park JJ, Hong SP, Kim TI, Kim WH. Clinical outcomes and factors for response prediction after the first course of corticosteroid therapy in patients with active ulcerative colitis. J Gastroenterol Hepatol. 2011;26:1114-1122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 112. | Lee KM, Jeen YT, Cho JY, Lee CK, Koo JS, Park DI, Im JP, Park SJ, Kim YS, Kim TO. Efficacy, safety, and predictors of response to infliximab therapy for ulcerative colitis: a Korean multicenter retrospective study. J Gastroenterol Hepatol. 2013;28:1829-1833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 113. | Wilhelm SM, McKenney KA, Rivait KN, Kale-Pradhan PB. A review of infliximab use in ulcerative colitis. Clin Ther. 2008;30:223-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 114. | Armuzzi A, Pugliese D, Danese S, Rizzo G, Felice C, Marzo M, Andrisani G, Fiorino G, Sociale O, Papa A. Infliximab in steroid-dependent ulcerative colitis: effectiveness and predictors of clinical and endoscopic remission. Inflamm Bowel Dis. 2013;19:1065-1072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 115. | Park SH, Yang SK, Hong SM, Park SK, Kim JW, Lee HJ, Yang DH, Jung KW, Kim KJ, Ye BD. Severe disease activity and cytomegalovirus colitis are predictive of a nonresponse to infliximab in patients with ulcerative colitis. Dig Dis Sci. 2013;58:3592-3599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |