Published online Sep 7, 2014. doi: 10.3748/wjg.v20.i33.11753
Revised: January 15, 2014
Accepted: May 19, 2014
Published online: September 7, 2014
AIM: To evaluate the effects of osthol on intrahepatic fat synthesis, β-oxidation, inflammation, and insulin resistance by multifaceted analysis.
METHODS: Sprague-Dawley rats (n = 30) were randomly divided into control, non-alcoholic fatty liver disease (NAFLD), and osthol groups. NAFLD and osthol groups were fed with a high-fat diet for 14 wk. After 8 wk of the high-fat diet, the osthol group also received osthol 20 mg/kg orally 5 times/wk. To assess the insulin resistance, oral glucose tolerance was performed at the end of 14 wk. Immunohistochemical (4-HNE, F4/80) and hematoxylin and eosin (HE) staining were performed on liver tissue extracts after animal sacrifice at 14 wk. SREBP1c, FAS, SCD-1, PPAR-α, CROT, MCP-1, IRS-1, and IRS-2 mRNA expressions were assessed with reverse transcription-polymerase chain reaction.
RESULTS: HE staining revealed that, compared with the NAFLD group, the osthol group showed significantly decreased intrahepatic fat content (39.4% vs 21.0%; P = 0.021). SREBP1c expression in the NAFLD group increased compared to controls (P = 0.0001), while osthol treatment decreased SREBP1c expression compared with the NAFLD group (P = 0.0059). In the osthol group, intrahepatic FAS and SCD-1, which act downstream of SREBP1c, decreased significantly compared with the NAFLD group. Moreover, PPAR-α expression in the osthol group was also significantly higher than in the NAFLD group (P = 0.0147).
CONCLUSION: Osthol treatment attenuated liver steatosis by decreasing de novo liver triglyceride synthesis and had nominal effects on insulin resistance and liver inflammation.
Core tip: Nonalcoholic fatty liver disease is considered as a consequence of “multi-hit” processes. Osthol, a coumarin compound, has anti-inflammatory effects on various diseases. However, there is no multi-faceted and comprehensive evaluation of its effects. The current study evaluated effects of osthol on intrahepatic fat synthesis, β-oxidation, inflammation, and insulin resistance by multifaceted analysis.
-
Citation: Nam HH, Jun DW, Jeon HJ, Lee JS, Saeed WK, Kim EK. Osthol attenuates hepatic steatosis
via decreased triglyceride synthesis not by insulin resistance. World J Gastroenterol 2014; 20(33): 11753-11761 - URL: https://www.wjgnet.com/1007-9327/full/v20/i33/11753.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i33.11753
Nonalcoholic fatty liver disease (NAFLD) has a worldwide prevalence of about 20%-30% and is among the most common causes of chronic liver disease[1,2]. Approximately 50%-60% of non-alcoholic steatohepatitis (NASH) patients have accompanying complications such as diabetes mellitus, cardiovascular disease, and hyperlipidemia[1]. NAFLD and NASH are also strongly associated with insulin resistance and obesity[3]. In hepatocytes, increased fatty acid oxidation increases oxidative stress and leads to apoptosis, which is also thought to be involved in NASH pathophysiology. However, until recently, it is thought that a cell’s response to oxidative stress is more critical in determining its fate than the amount of oxidative stress. Furthermore, NAFLD is also regarded as a “multi-hit” disease[4,5] and could be the consequence of multiple highly entwined mechanisms such as insulin resistance, oxidative stress, mitochondrial insufficiency, endoplasmic reticulum stress, and apoptosis. Understanding these intricate mechanisms leading to NAFLD progression could provide a useful insight to understand NAFLD.
Insulin resistance together with oxidative stress has an important role in NAFLD pathophysiology[6-8]. An improvement in insulin resistance could decrease the incidence of NAFLD and NASH; however, hepatic insulin sensitizers have not provided significantly beneficial results in clinical trials[9-11]. Moreover, although antioxidant treatment improved NAFLD histology in clinical trials[6], the long-term effects of antioxidant treatments need further evaluation[12]. Cnidium monnieri fruits are used in traditional Chinese medicine. Osthol, the active constituent of Cnidium monnieri extracts, has anti-inflammatory and hepatic fat oxidizing properties. For instance, in a rat model of fatty liver, osthol not only decreased the fasting blood glucose and hepatic fat content, but also improved insulin resistance[13]. Zhang et al[14] also reported that osthol treatment decreased hepatic fat content by increasing the expression of hepatic peroxisome proliferator-activated receptor (PPAR)-α/γ. In the alcoholic fatty liver model, osthol treatment led to increased superoxide dismutase (SOD) activation and decreased oxidative stress[15]. These results suggest that osthol treatment not only reduces hepatic fat content and oxidative stress but also improves insulin resistance; however, the histological improvement in inflammation and fibrosis still requires further evaluation. Although the fat oxidizing effects of osthol have already been well studied, none of the previous studies evaluated the effects of osthol on liver inflammation and fibrosis simultaneously with the fat oxidizing effects. As multiple cellular mechanisms, such as hepatic fat synthesis, oxidative stress, inflammation, insulin resistance, and cellular adaptation, could all be involved in NAFLD pathophysiology, a comprehensive evaluation of osthol efficacy in NAFLD pathophysiology is needed. Therefore, the aim of the current study was to evaluate the precise mechanism and effects of osthol treatment on these multiple mechanism simultaneously.
A total of 30 Sprague-Dawley (SD) rats (4-wk-old) were purchased from Orient Animal Laboratory, Seoul, South Korea and were randomly divided into 3 groups: control, NAFLD and osthol. The control group was fed normal chow while a combination of 60% high-fat (HF) diet and 20% fructose was provided to NAFLD and osthol groups. The fructose was provided in drinking water to NAFLD and osthol groups. From the 9th to 14th wk the NAFLD and osthol groups were treated orally 5 times/wk with normal saline (200 μL) and osthol 20 mg/kg, respectively[14,16,17] (dissolved in sodium carboxymethyl cellulose and later in normal saline to make 200 μL volume). After 14 wk, anesthetized animals were euthanized by thoracotomy and blood samples were withdrawn by cardiac puncture. The liver tissues were extracted for polymerase chain reaction (PCR), hematoxylin and eosin (HE) staining and immunohistostaining analysis. The experimental protocol was approved by Hanyang Institutional Animal Care and Use Committee (HY-IACUC-11-064).
The body weight of the animals was measured weekly from the start of the experiment to just before sacrifice to evaluate the changes in body weight.
An oral glucose tolerance test (OGTT) was performed as follows[18]: briefly, after overnight fasting, the blood glucose level was measured through the tail vein at 0, 30, 60, 90, and 120 min after an oral glucose load of 2 g/kg body weight. After 14 wk, serum aspartate transaminase (AST) and alanine transaminase (ALT) were measured from the blood of sacrificed animals using a biochemical analytical system (Hitachi-747; Hitachi, Tokyo, Japan).
Formalin-fixed paraffin embedded sections of liver tissue samples were stained with HE for microscopic analysis. To assess hepatic steatosis, the tissue sections were scored for activity (degree of inflammation) and stage (degree of fibrosis) of disease according to the histological grading and staging systems, respectively. Hepatic steatosis was graded as follows: < 5% (score, 0); 5%-33% (score, 1), > 33%-66% (score, 2) and > 66% (score, 3); steatosis, 0-3; lobular inflammation, 0-4; portoperiportal activity, 0-4; and fibrosis, 0-4. For immunohistochemistry, the sections were stained with 4-hydroxynonenal (4-HNE) antibodies (Abcam, Cambridge, MA, USA) to assess lipid peroxidation. The macrophages were stained using F4/80 antibodies (Santa Cruz, CA, USA).
The total liver tissue RNA of each group was acquired using the Trizol Reagent (Invitrogen, United States). The RNA purity (1.9-2.0) was measured based on ration A260-280 with the Nano drop ND-2000 spectrophotometer (Thermo Fisher Scientific Inc., USA). The PCR had an incubation time of 10 min at 95 °C then 35 cycles (10 s at 95 °C, 59 °C, and 72 °C each) and 15 s at 65 °C for the final step. Image density was measured with Image J (http://rsb.info.nih.gov/ij/index.html). The primer sets used were as follows: GAPDH (Gene Bank ID: 24383) forward, 5’-TGC CAC TCA GAA GAC TGT GG-3’; reverse, 5’-TTC AGC TCT GGG ATG ACC TT-3’; SREBP1c (Gene Bank ID: 78968) forward, 5’-CGT TGT ACT GCA GCC ACA CT-3’; reverse, 5’-TGT GCT GTA AGA AGC GGA TG-3’; FAS (Gene Bank ID: 50671) forward, 5’-GAG TCT GTC TCC CGC TTG AC-3’; reverse, 5’-CCC TCC AGC ATG TAG ACC TT-3’; SCD-1 (Gene Bank ID: 246074) forward, 5’-ACC TTG CTC TGG GGG ATA TT-3’; reverse, 5’-GAT GAA GCA CAT GAG CAG GA-3’; PPAR-α (Gene Bank ID: 25747) forward, 5’-GAC AAG GCC TCA GGA TAC CA-3’; reverse, 5’-GTC TTC TCA GCC ATG CAC AA-3’; IRS-1 (Gene Bank ID: 25467) forward, 5’-ACA CAG CTG CAC AGA CCA AC-3’; reverse, 5’-CCC AAC TCA ACT CCA CCA CT-3’; IRS-2 (Gene Bank ID: 29376) forward, 5’-CAT CCA TGG CCT TCT CTC TC-3’; reverse, 5’-CCA TGA GAC TTA GCC GCT TC-3’; CROT (Gene Bank ID: 83842) forward, 5’-TCC GGA TGC TGT TTT CTA CC-3’; reverse 5’-GTT GCA TGT GGA CTG GTG TC-3’; ATF6 (Gene Bank ID: 304962) forward, 5’-CCC ACC AAA GGT CAG ACT GT-3’; reverse, 5’- CTT GGG ACT TTG AGC CTC TG-3’; MCP1 (Gene Bank ID: 24770) forward, 5’-TAG CAT CCA CGT GCT GTC TC-3’; reverse, 5’-GCT TGA GGT GGT TGT GGA AA-3’.
All experiments were independently repeated 3 times. The values are expressed as mean ± standard deviation. Statistical analysis was performed using SPSS for Windows version 18.0 (SPSS Inc., Chicago, IL, USA). One-way analysis of variance was performed to compare the means of different values, and a P-value < 0.05 was considered significant.
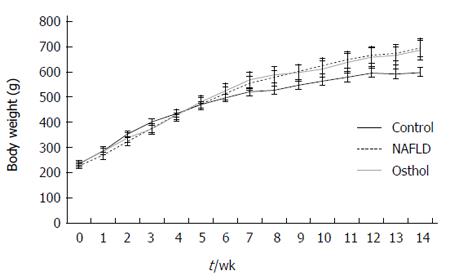
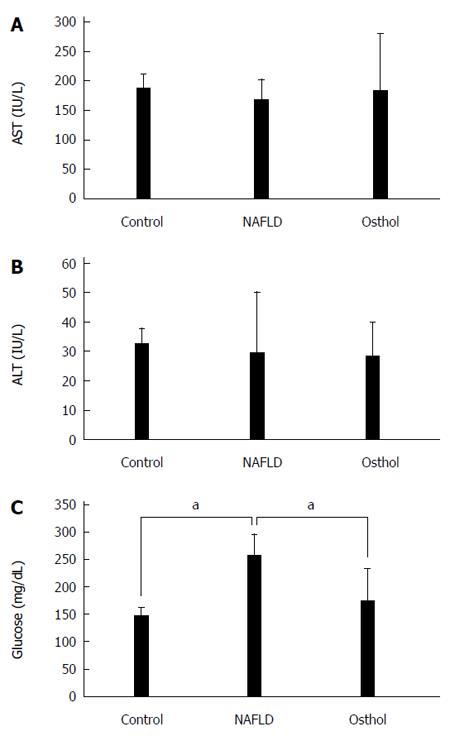
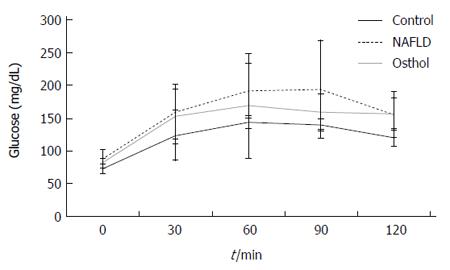
The average body weight of both NAFLD and osthol groups were higher than in the control group. However, there was no statistically significant difference in body weights of NAFLD and osthol groups (695.4 ± 61.2 g vs 685.7 ± 86.62 g, P = 0.78) (Figure 1). Moreover, the serum AST and ALT levels among the 3 groups also did not show any statistically significant difference (Figure 2A and B). The fasting blood glucose levels of both NAFLD and osthol groups were higher than controls. However, compared with the NAFLD group, fasting blood glucose was lower in the osthol group (258.3 mg/dL vs 175.8 mg/dL, P < 0.04) (Figure 2C). Glucose intolerance was assessed using the OGTT. In NAFLD and osthol treatment groups, the area under the receiver operating characteristic curve for the osthol group was lower than that for the NAFLD group, but this difference was not statistically significant (Figure 3).
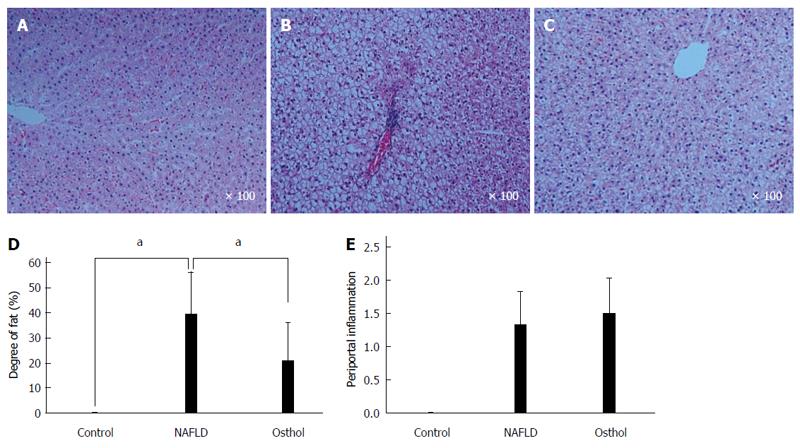
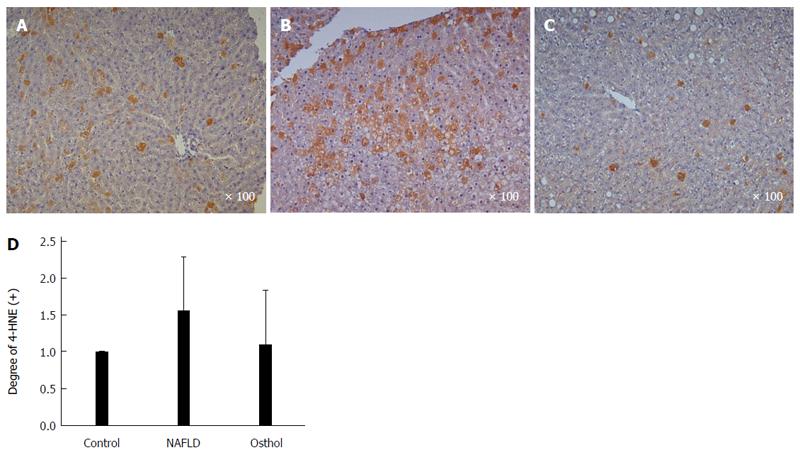
On HE staining the osthol group showed a statistically significant (P = 0.021) decrease in hepatic fat content compared with the NAFLD (21% and 39.44% respectively). However, there was no difference in periportal inflammation and degree of intrahepatic fibrosis between the NAFLD and osthol groups (Figure 4A-E). 4-HNE immunohistochemistry was performed to evaluate the extent of lipid peroxidation. 4-HNE staining revealed that the osthol group had a decrease in lipid peroxidation compared with the NAFLD group, but the difference was not statistically significant (Figures 5 and 6).
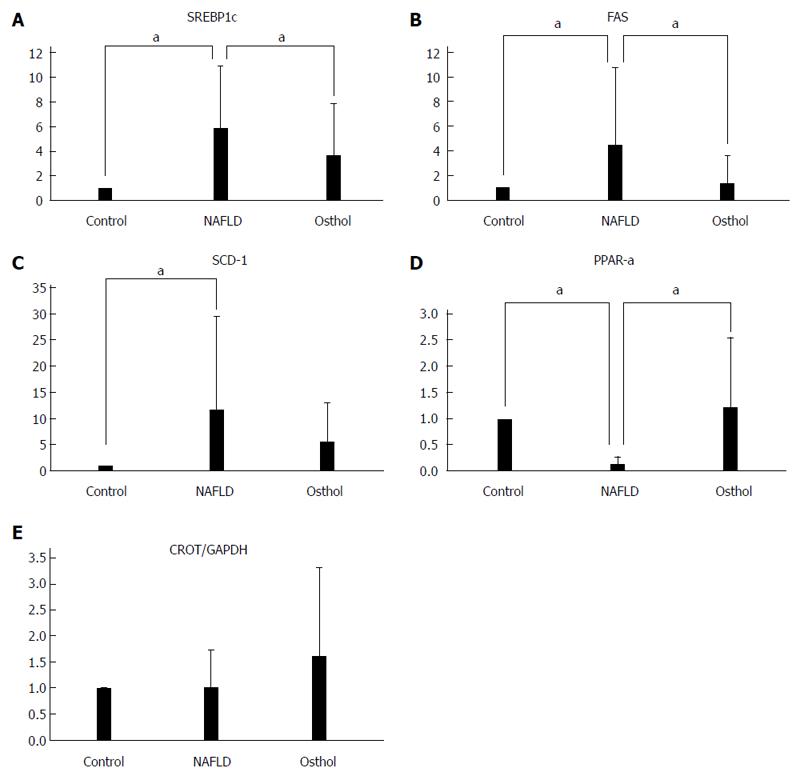
The transcription factor sterol regulatory element binding protein-1c (SREBP1c) regulates several lipogenic enzymes including acetyl-CoA carboxylase, pyruvate kinase, fatty acid synthase (FAS), and stearyl-CoA desaturase (SCD-1)[19-23]. SREBP1c, FAS and SCD-1 expression is increased in NAFLD[24,25]. We assessed the mRNA expressions of SREBP1c, FAS and SCD-1 to evaluate de novo intrahepatic fatty acid synthesis. SREBP1c expression increased in both NAFLD (P < 0.0001) and osthol groups compared with the control group. However, the expression of SREBP1c was lower in the osthol group compared with the NAFLD group (P = 0.0059) (Figure 7A). Similarly, the expression of both FAS and SCD-1 also increased in the osthol and NAFLD groups, but the expression was lower in the osthol compared with the NAFLD group (P = 0.001 and P = 0.059 respectively) (Figure 7B and C).
The mRNA expression of PPAR-α and carnitine octanoyltransferase (CROT) was assessed to evaluate intrahepatic lipids metabolism. The PPARs transcriptionally regulate certain genes including PPAR-α and PPAR-γ, which in turn regulate lipid metabolizing enzymes[14]. In the osthol group, PPAR-α expression significantly increased compared with that in the NAFLD group (P = 0.0147) (Figure 7D). Similarly, mRNA expression of CROT, which controls the transfer of fatty acids to mitochondria for β-oxidation, also increased in the osthol group, although it was not significantly different from the NAFLD group (Figure 7E).
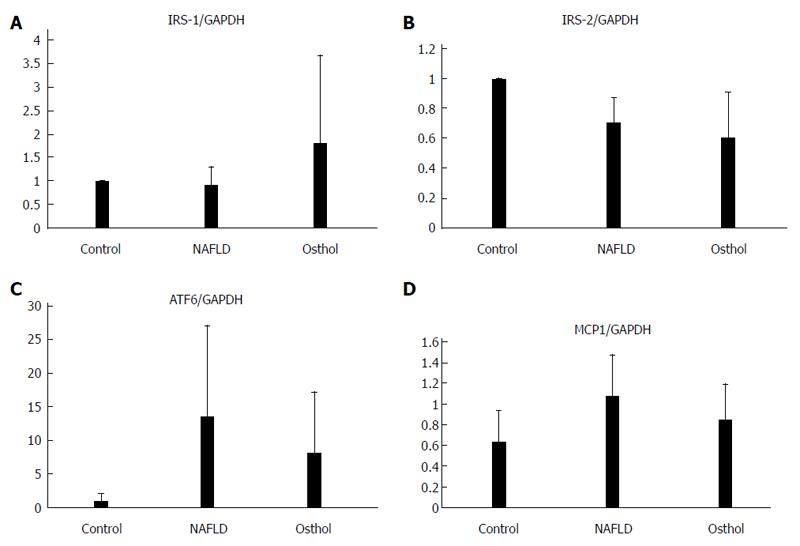
There was no statistically significant change in insulin receptor substrate-1 (IRS-1) and IRS-2 expressions (Figure 8A-B). Moreover, there was also no significant difference in expression of monocyte chemo-attractant protein-1 and activating transcription factor-6 (ATF6), an endoplasmic stress marker, between the osthol and NAFLD groups (Figure 8C and D).
NAFLD usually has a benign clinical course[26]; however, its inflammatory counterpart, the NASH can progress to chronic liver disease and fibrosis. Therefore, a reduction in hepatic inflammation and fibrosis is considered more important than a reduction in hepatic fat content. NASH is a “multi-hit” disease process which results from intrahepatic fat accumulation, increased oxidative stress, and abnormal hepatocyte adaptation[4]. The increased oxidative stress is an important risk factor leading to the progression of simple steatosis to steatohepatitis. In the current study, osthol treatment decreased the hepatic fat content mainly by decreasing de novo hepatic fat synthesis. Previous studies also reported that osthol treatment decreased hepatic fat content in NAFLD[15,27]. The decreased hepatic fat content is mainly due to improved insulin resistance and increased fat oxidation by PPAR-α activation.
Qi et al[13] reported that osthol treatment decreased HOMA-IR in NAFLD. In our study, osthol treatment lowered fasting blood glucose levels; however, there was no significant difference in OGTT between osthol and NAFLD groups, suggesting that osthol treatment did not affect the hepatic transcription factors involved in the insulin signaling pathway. SREBP1c is a key molecule in triglyceride synthesis. SCD-1 acts downstream of SREBP1 and FAS in the triglyceride synthesis pathway. In the current study, osthol treatment significantly decreased SREBP1c, FAS and SCD-1 expression. Moreover, compared with the NAFLD group, osthol treatment also increased PPAR-α and CROT expression, suggesting that osthol increases fatty acid oxidation. ATF6, an ER stress marker, was also decreased in the osthol group, but this was not statistically significant.
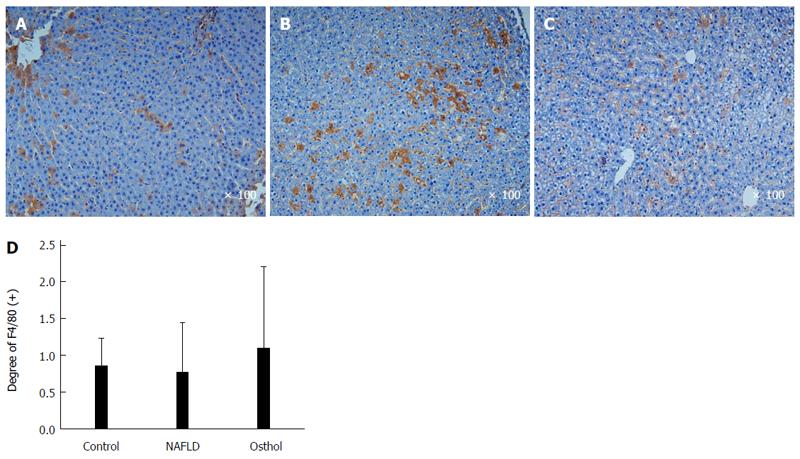
Zhang et al[16] reported a decrease in tumor necrosis factor-α, SOD and malondialdehyde following osthol administration. Moreover, previous studies also reported the possibility of decreased intrahepatic inflammation because of a reduction in surrogate markers of inflammation. However, none of the previous studies showed that osthol could attenuate intrahepatic inflammation as well as hepatic apoptosis. In our study, osthol administration did not decrease hepatic inflammation, fibrosis, and aminotransferases. Moreover, the MCP-1 and Kupffer cells, which have a crucial role in NASH development, were also not changed (Figures 6 and 8D, respectively). Furthermore, only Sun et al[27] showed that osthol decreased intrahepatic oxidative stress using an alcoholic fatty liver model. However, the oxidative stress has a different role in NAFLD and alcoholic fatty liver disease; therefore, these results cannot be compared directly.
Our study had the following limitations: First due to small size of the study, we used blood glucose, OGTT test, and total IRS to assess insulin resistance; however, the euglycemic clamp test is the gold standard for measuring insulin resistance[28]. Second, to induce liver inflammation and fibrosis, an HF diet was applied for 14 wk. However, to further evaluate the efficacy of osthol on liver inflammation and fibrosis, the duration of the HF diet should be extended, or genetically modified animals along with diet model should be used. Third, we did not evaluate the decrease in hepatic fat synthesis caused by decreased SREBP1c due to hepatocyte adaptation or apoptosis. Further studies are needed to evaluate the efficacy of osthol in improving liver inflammation and mitochondrial β-oxidation.
In conclusion, osthol administration for 6 wk decreased de novo hepatic fat synthesis and improved fatty liver by decreasing SREBP1c and increasing PPAR activation; however, the osthol treatment did not attenuate intrahepatic inflammation and fibrosis. Osthol may be used as a potential therapeutic agent to prevent NAFLD progression as a result of its ability to decrease de novo hepatic fat synthesis and to increase fatty acid oxidation.
The “two hit’’ theory is the widely accepted theory to explain non-alcoholic fatty liver disease pathophysiology. The first hit comprises accelerated lipidsaccumulation, while the second hit comprises increased lipid oxidation in the liver. Osthol, a coumarin compound, possesses anti-inflammatory and fat oxidizing properties. The authors aimed to evaluate the effects of osthol on hepatic fat content.
Osthol has anti-inflammatory and fat oxidization effects. In this study, the authors to simultaneously evaluated the effects of osthol on fat oxidation, inflammation, fibrosis and insulin resistance pathways.
The fat oxidizing effects of osthol have already been well documented. However, interestingly, none of previous studies evaluated the effect of osthol on liver inflammation and fibrosis in addition to the fat oxidizing effects. This is the first study to evaluate simultaneously the effects of osthol on inflammation, fibrosis, fat oxidation, and insulin resistance.
By understanding the mechanism of osthol effects on various pathways involved in NAFLD pathology, this study provides useful information for future studies and also highlights the potential use of osthol to slow progression of NAFLD.
Cnidium monnieri fruits are used in traditional Chinese medicine. Osthol is the active ingredient of Cnidium monnieri extracts.
In this study Ho Hyun Nam et al investigated the effects of osthol on intrahepatic fat synthesis, β-oxidation, inflammation and insulin resistance by multifaceted-analysis in a group of 30 rats randomly divided into control, NASH and osthol groups.
P- Reviewer: Mohn A, Sanal MG, Qu S S- Editor: Qi Y L- Editor: Cant MR E- Editor: Liu XM
| 1. | Lazo M, Hernaez R, Bonekamp S, Kamel IR, Brancati FL, Guallar E, Clark JM. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ. 2011;343:d6891. [PubMed] [Cited in This Article: ] |
| 2. | Wong VW, Chu WC, Wong GL, Chan RS, Chim AM, Ong A, Yeung DK, Yiu KK, Chu SH, Woo J. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut. 2012;61:409-415. [PubMed] [Cited in This Article: ] |
| 3. | Targher G, Byrne CD. Clinical Review: Nonalcoholic fatty liver disease: a novel cardiometabolic risk factor for type 2 diabetes and its complications. J Clin Endocrinol Metab. 2013;98:483-495. [PubMed] [Cited in This Article: ] |
| 4. | Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836-1846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1543] [Cited by in F6Publishing: 1629] [Article Influence: 116.4] [Reference Citation Analysis (0)] |
| 5. | Koteish A, Mae Diehl A. Animal models of steatohepatitis. Best Pract Res Clin Gastroenterol. 2002;16:679-690. [PubMed] [Cited in This Article: ] |
| 6. | Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685. [PubMed] [Cited in This Article: ] |
| 7. | Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231. [PubMed] [Cited in This Article: ] |
| 8. | Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology. 2005;42:987-1000. [PubMed] [Cited in This Article: ] |
| 9. | Ratziu V, Charlotte F, Bernhardt C, Giral P, Halbron M, Lenaour G, Hartmann-Heurtier A, Bruckert E, Poynard T. Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatology. 2010;51:445-453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 270] [Cited by in F6Publishing: 279] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 10. | Ratziu V, Giral P, Jacqueminet S, Charlotte F, Hartemann-Heurtier A, Serfaty L, Podevin P, Lacorte JM, Bernhardt C, Bruckert E. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology. 2008;135:100-110. [PubMed] [Cited in This Article: ] |
| 11. | Mehta K, Van Thiel DH, Shah N, Mobarhan S. Nonalcoholic fatty liver disease: pathogenesis and the role of antioxidants. Nutr Rev. 2002;60:289-293. [PubMed] [Cited in This Article: ] |
| 12. | Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37-46. [PubMed] [Cited in This Article: ] |
| 13. | Qi Z, Xue J, Zhang Y, Wang H, Xie M. Osthole ameliorates insulin resistance by increment of adiponectin release in high-fat and high-sucrose-induced fatty liver rats. Planta Med. 2011;77:231-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Zhang Y, Xie ML, Xue J, Gu ZL. Osthole regulates enzyme protein expression of CYP7A1 and DGAT2 via activation of PPARalpha/gamma in fat milk-induced fatty liver rats. J Asian Nat Prod Res. 2008;10:807-812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Zhang J, Xue J, Wang H, Zhang Y, Xie M. Osthole improves alcohol-induced fatty liver in mice by reduction of hepatic oxidative stress. Phytother Res. 2011;25:638-643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Zhang Y, Xie ML, Zhu LJ, Gu ZL. Therapeutic effect of osthole on hyperlipidemic fatty liver in rats. Acta Pharmacol Sin. 2007;28:398-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Zhang Y, Xie M, Xue J, Gu Z. Osthole improves fat milk-induced fatty liver in rats: modulation of hepatic PPAR-alpha/gamma-mediated lipogenic gene expression. Planta Med. 2007;73:718-724. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Ota T, Takamura T, Kurita S, Matsuzawa N, Kita Y, Uno M, Akahori H, Misu H, Sakurai M, Zen Y. Insulin resistance accelerates a dietary rat model of nonalcoholic steatohepatitis. Gastroenterology. 2007;132:282-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 189] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 19. | Tessari P, Coracina A, Cosma A, Tiengo A. Hepatic lipid metabolism and non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2009;19:291-302. [PubMed] [Cited in This Article: ] |
| 20. | Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331-340. [PubMed] [Cited in This Article: ] |
| 21. | Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147-152. [PubMed] [Cited in This Article: ] |
| 22. | Shimomura I, Shimano H, Horton JD, Goldstein JL, Brown MS. Differential expression of exons 1a and 1c in mRNAs for sterol regulatory element binding protein-1 in human and mouse organs and cultured cells. J Clin Invest. 1997;99:838. [Cited in This Article: ] |
| 23. | Adams LA, Angulo P, Lindor KD. Nonalcoholic fatty liver disease. CMAJ. 2005;172:899-905. [PubMed] [Cited in This Article: ] |
| 24. | Shimomura I, Bashmakov Y, Horton JD. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J Biol Chem. 1999;274:30028-30032. [PubMed] [Cited in This Article: ] |
| 25. | Kohjima M, Higuchi N, Kato M, Kotoh K, Yoshimoto T, Fujino T, Yada M, Yada R, Harada N, Enjoji M. SREBP-1c, regulated by the insulin and AMPK signaling pathways, plays a role in nonalcoholic fatty liver disease. Int J Mol Med. 2008;21:507-511. [PubMed] [Cited in This Article: ] |
| 26. | Teli MR, James OF, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995;22:1714-1719. [PubMed] [Cited in This Article: ] |
| 27. | Sun F, Xie ML, Xue J, Wang HB. Osthol regulates hepatic PPAR alpha-mediated lipogenic gene expression in alcoholic fatty liver murine. Phytomedicine. 2010;17:669-673. [PubMed] [Cited in This Article: ] |
| 28. | Skrha J, Haas T, Sindelka G, Prázný M, Widimský J, Cibula D, Svacina S. Comparison of the insulin action parameters from hyperinsulinemic clamps with homeostasis model assessment and QUICKI indexes in subjects with different endocrine disorders. J Clin Endocrinol Metab. 2004;89:135-141. [PubMed] [Cited in This Article: ] |