Published online Sep 7, 2015. doi: 10.3748/wjg.v21.i33.9727
Peer-review started: March 15, 2015
First decision: April 13, 2015
Revised: June 6, 2015
Accepted: June 26, 2015
Article in press: June 26, 2015
Published online: September 7, 2015
AIM: To confirm the anti-invasion and anti-migration effects of down-regulation of Notch1 combined with interleukin (IL)-24 in hepatocellular carcinoma (HCC) cells.
METHODS: γ-secretase inhibitors (GSIs) were used to down-regulate Notch1. HepG2 and SMMC7721 cells were seeded in 96-well plates and treated with GSI-I or/and IL-24 for 48 h. Cell viability was measured by MTT assay. The cellular and nuclear morphology was observed under a fluorescence microscope. To further verify the apoptotic phenotype, cell cultures were also analyzed by flow cytometry with Annexin V-FITC/propidium iodide staining. The expression of Notch1, SNAIL1, SNAIL2, E-cadherin, IL-24, XIAP and VEGF was detected by Western blot. The invasion and migration capacities of HCC cells were detected by wound healing assays. Notch1 and Snail were down-regulated by RNA interference, and the target proteins were analyzed by Western blot. To investigate the mechanism of apoptosis, we analyzed HepG2 cells treated with siNotch1 or siCON plus IL-24 or not for 48 h by caspase-3/7 activity luminescent assay.
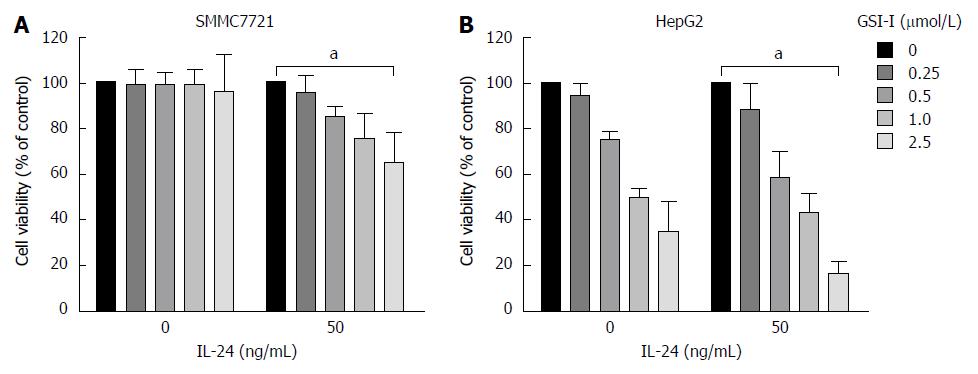
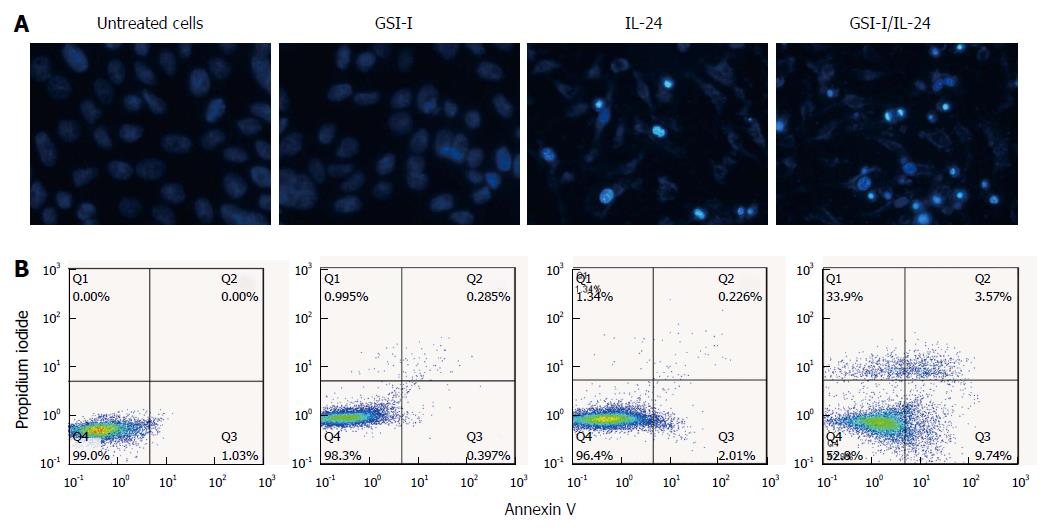
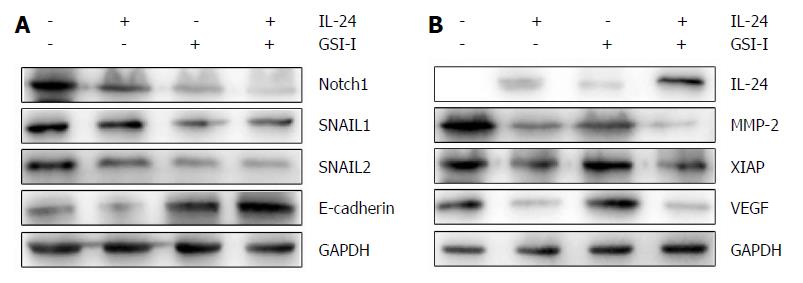
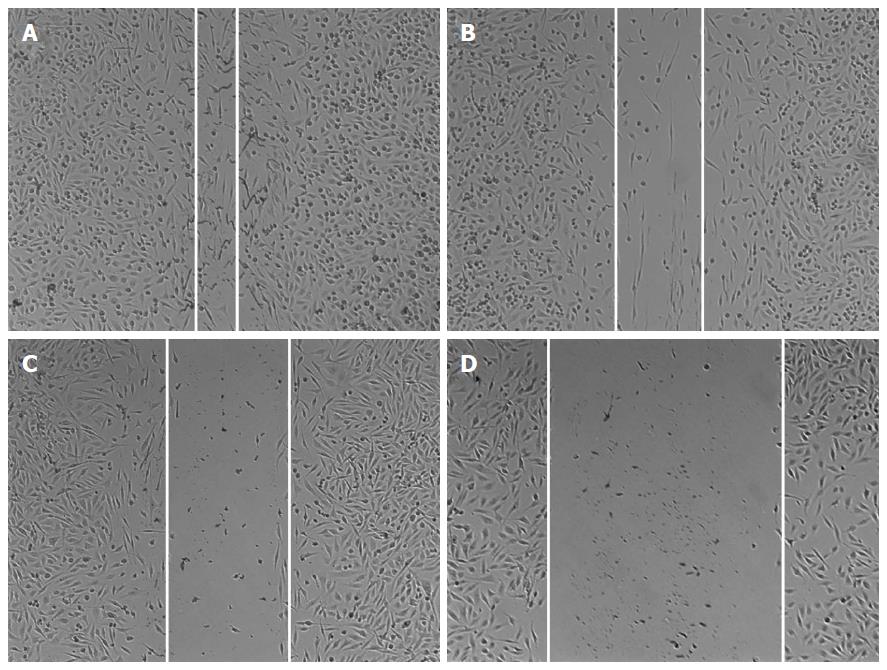
RESULTS: GSI-I at a dose of 2.5 μmol/L for 24 h caused a reduction in cell viability of about 38% in HepG2 cells. The addition of 50 ng/mL IL-24 in combination with 1 or 2.5 μmol/L GSI-I reduced cell viability of about 30% and 15%, respectively. Treatment with IL-24 alone did not induce any cytotoxic effect. In SMMC7721 cells with the addition of IL-24 to GSI-I (2.5 μmol/L), the reduction of cell viability was only about 25%. Following GSI-I/IL-24 combined treatment for 6 h, the apoptotic rate of HepG2 cells was 47.2%, while no significant effect was observed in cells treated with the compounds employed separately. Decreased expression of Notch1 and its associated proteins SNAIL1 and SNAIL2 was detected in HepG2 cells. Increased E-cadherin protein expression was noted in the presence of IL-24 and GSI-I. Furthermore, the increased GSI-I and IL-24 in HepG2 cell was associated with downregulation of MMP-2, XIAP and VEGF. In the absence of treatment, HepG2 cells could migrate into the scratched space in 24 h. With IL-24 or GSI-I treatment, the wound was still open after 24 h. And the distance of the wound closure strongly correlated with the concentrations of IL-24 and GSI-I. Treatment of Notch-1 silenced HepG2 cells with 50 ng/mL IL-24 alone for 48 h induced cytotoxic effects very similar to those observed in non-silenced cells treated with GSI-I/IL-24 combination. Caspase-3/7 activity was increased in the presence of siNotch1 plus IL-24 treatment.
CONCLUSION: Down-regulation of Notch1 by GSI-I or siRNA combined with IL-24 can sensitize apoptosis and decrease the invasion and migration capabilities of HepG2 cells.
Core tip: The down-regulation of Notch1 by γ-secretase inhibitor (GSI-I) or siRNA combined with interleukin (IL)-24, could sensitize apoptosis, increase expression of E-cadherin and decrease the invasion and migration capabilities of HepG2 cells. These results indicate for the first time that GSI-I/IL-24 combination might represent a novel and potentially effective tool for hepatocellular carcinoma treatment.
- Citation: Han B, Liu SH, Guo WD, Zhang B, Wang JP, Cao YK, Liu J. Notch1 downregulation combined with interleukin-24 inhibits invasion and migration of hepatocellular carcinoma cells. World J Gastroenterol 2015; 21(33): 9727-9735
- URL: https://www.wjgnet.com/1007-9327/full/v21/i33/9727.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i33.9727
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide[1] and approximately half of these cases occurred in China[2]. Despite the development of various therapies, the outcome for HCC patients is still poor. The major reason is that HCC often relapses due to intrahepatic and distant metastases after curative surgical resection or transplantation[3]. Thus, the discovery and research of new molecular targets of blocking metastasis are the primary goal for HCC therapy.
Notch signaling is not only involved in the regulation of cell proliferation and differentiation but also plays an important role in cancer[4]. The Notch signaling pathway includes Notch ligands, negative and positive regulators, and transcription factors. The mRNA and protein expression of Notch1, which is one of the Notch signaling pathway receptors, is significantly higher in HCC than in adjacent non-tumor liver tissue in the previous report. Deregulated Notch receptor expression in human HCC was reported by Gao et al[5]. Snail, which is one of the zinc-finger transcription factors, has the function of repressing the transcription of the E-cadherin gene through binding to the E-boxes of the CDH1 promoter[6]. The up-regulation of Snail is also correlated with metastasis and poor prognosis, whereas decrease of Snail is critical for reducing growth and invasiveness of cancer[7,8]. Many studies have shown that E-cadherin is a cell-cell adhesion protein fulfilling a prominent role in epithelial differentiation, which is relevant to metastasis, tumor invasion, motility, and unfavorable prognosis[9-11]. E-cadherin expression is beneficial for intraepithelial expansion and invasiveness in a variety of solid tumors, as well as for the intrahepatic metastasis of HCC[12-15]. In the MHCC97L (HCC cell lines), abnormal Notch1 expression has been shown to be strongly associated with HCC metastasis, which may be regulated through the Notch1/Snail1/E-cadherin pathway[16].
Melanoma differentiation associated gene-7 (MDA-7)/interleukin-24 (IL-24) is a member of the IL-10 family, and previous reports have showed that overexpression of MDA-7/IL-24 causes tumor growth suppression and tumor cell apoptosis in lung cancer, mesotheliomas, melanoma, breast cancer, osteosarcoma, pancreatic cancer, glioblastoma, prostate cancer and so on[17-19], indicating that MDA-7/IL-24 may prove to be a potential drug for cancer therapy. MDA-7/IL-24 inhibits HepG2 and BEL-7402 cell adhesion and invasion by increasing the expression of E-cadherin and p-ERK[20].
Since Notch receptors are activated by γ-secretase, γ-secretase inhibitors (GSIs) have attracted increasing interest[21]. GSIs have been used for the treatment of Alzheimer’s disease to prevent amyloid precursor protein cleavage and the consequent release of amyloid β-peptide[22]. Recently, it has been reported that GSIs also have the ability to induce growth arrest and/or apoptosis in some tumor cell lines, while other tumor cells were resistant to the molecules[23].
In this study, we found that the IL-24 mediated apoptosis of HCC cells was sensitized by GSI-I. The down-regulation of Notch1 by siRNA or GSI-I could increase the expression of E-cadherin and decrease the invasion and migration capabilities of HepG2 cells. These in vitro results indicate, for the first time, that GSI-I/IL-24 combination might be used as a novel and potentially effective tool for HCC treatment.
The human HCC cell lines (HepG2 and SMMC-7721 were obtained from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences) were cultivated in DMEM medium supplemented with 10% FCS (fetal calf serum, Hyclone laboratories, Logan, UT, United States). All experiments were carried out using a confluent monolayer of HCC cell cultures. Cells were maintained at 37 °C in a humidified atmosphere containing 5% CO2. The primary antibodies for Notch1 (120 kDa), E-cadherin (120 kDa), SNAIL1 (29 kDa), SNAIL2 (29 kDa), MMP-2 (74 kDa), XIAP (55 kDa), VEGF (31 kDa) and GAPDH (37 kDa) were purchased from Santa Cruz Biotechnology (SantaCruz, CA, United States). All secondary antibodies were obtained from Pierce (Rockford, IL, United States). Small interfering RNA (siRNA) targeting Notch1 and control siRNA (siCON) were obtained from Santa Cruz Biotechnology. LipofectinTM2000 was purchased from Life Technologies (Carlsbad, CA, United States). All other chemicals and solutions were purchased from Sigma-Aldrich unless otherwise indicated.
HepG2 and SMMC7721 cells were seeded in 96-well plates and treated with GSI-I or/and IL-24 for 48 h, separately. Then, 10 μL of 3-(4,5-dimethylthiazolyl-2) 2,5-diphenyltetrazolium bromide (MTT, 5 mg/mL, Sigma-Aldrich) was added to each well and incubated for 4 h at 37 °C. The formazan granules were dissolved in 150 μL dimethyl sulfoxide (DMSO) for 10 min. Optical density (OD) was then measured at a wavelength of 490 nm. Each MTT assay was performed in quadruplicate and repeated three times.
In order to observe the presence of condensed chromatin and apoptotic bodies, cells were stained with Hoechst 33258 dye. Cells seeded in 96-well plates were fixed in 3:1 methanol/acetic acid for 10 min at room temperature, washed in PBS (phosphate buffered saline) and stained for 30 min in PBS with 40% paraformaldehyde and 10 μg/mL Hoechst 33258. After washing in PBS for several times, nuclear morphology was observed under a fluorescence microscope (Zeiss, Germany).
To further verify the apoptotic phenotype, cell cultures were also analyzed with an Annexin V-FITC/propidium iodide (PI) kit (Roche, Manheim, Germany), according to the manufacturer’s instructions. Annexin-V immuno-cytofluorescence was detected by flow cytometry. After various treatments, cells were collected and centrifuged. The cell pellet was washed in PBS and centrifuged again. The pellet was resuspended in Annexin-V and PI according to the manufacturer’s protocol. Cells were analyzed on a Beckman flow cytometer (Beckman Coulter, Brea, CA, United States).
Cells were plated in 100-mm2 tissue culture dishes at 60% confluence and incubated overnight. After treatment, cell lysates were obtained using cold radioimmunoprecipitation assay buffer [RIPA buffer contain: 20 mmol/L Tris-HCl (pH 8.0), 100 mmol/L NaCl, 10% glycerol, 1% NP-40, and 0.5% sodium deoxycholate]. Proteins (20 mg) were separated on precasted Bis-Tris NuPAGE gels (Bio-Rad, Hercules, CA, United States), electroblotted to polyvinylidene difluoride membranes (Millipore) and then blocked for 1 h at room temperature in TBS-T [50 mmol/L Tris-HCl (pH 7.5), 150 mmol/L NaCl, and 0.1% Tween 20] containing 5% nonfat milk. Membranes were then incubated overnight at 4 °C or for 1 h at room temperature with the indicated primary antibodies: Notch1 (1:1000), SNAIL1 (1:500), SNAIL2 (1:1,000), E-cadherin (1:1000), IL-24 (1:1000), XIAP (1:500), VEGF (1:1000) and GAPDH (1:5000). Anti-mouse or anti-rabbit secondary antibody conjugated to horseradish peroxidase (HRP) was used to visualize the stained bands with an ECL (enhanced chemiluminescence, Santa Cruz, CA, United States) kit.
HepG2 cells were seeded in 6-well plates and cultured until confluence. A wound was then created by manually scraping the cell monolayer with a 200-microliter pipette tip. The cultures were washed twice with serum free medium to remove floating cells. The cells were then incubated in DMEM supplemented with 1% FBS. Cell migration into the wound was observed at 12 h in eight randomly selected microscopic fields for each condition and time point. Images were acquired with a Nikon DS-5M Camera System mounted on a phase-contrast Leitz microscope.
Scrambled (siCON) and specific siRNAs targeting Notch1 were obtained from Santa Cruz Biotechnology. Transfection of HepG2 cells was performed using the Amaxa system (Amaxa, Cologne, Germany) following their specifications. Firstly, 106 cells in 100 μL of medium was mixed with 3 μg siRNA and then transferred to an Amaxa-certified cuvette. For transfection, we used the program V-01. Transfection efficiency was between 75% and 85% (data not shown), as checked by flow cytometry, using a fluorescein-labeled non-targeted siRNA control (Cell Signaling). After 4 h cells were treated with IL-24 as indicated. Cells were examined for gene downregulation and other properties 48 h after transfection.
The caspase-3/7 activity was detected with the Apo-One Homogeneous caspase-3/7 assay kit (Promega Corporation, Madison, WI). Briefly, cells were seeded into 96-well plates at a density of 2 x 104 cells/well, incubated overnight and subsequently treated with siCON, siNotch1 with or without IL-24. For the caspase assay, the substrate was added after treatment for 48 h (as indicated in the text) and plates were read 2 h later using a Tecan Plate Reader (Tecan, Group. Ltd., Switzerland). All treatments were done in triplicate. Background absorbance was determined by the incubating media with substrate alone or subtracting the values from wells containing cells.
Each experiment was repeated at least three times. All the experimental data are presented as mean ± SD. The differences among means were statistically analyzed by a t-test. All statistical analyses were performed using SPSS13.0 software (Chicago, IL, United States). P < 0.05 was considered statistically significant.
The antiproliferative effects of GSI-I in combination with IL-24 were first examined in two hepatocellular carcinoma cell lines. Using as a single agent or in combination, the dose-response curves of the effects exerted by the compounds on cell viability are showed in Figure 1A. GSI-I at doses of 0-1 μmol/L for 24 h was almost ineffective in the cell lines. GSI-I at a dose of 2.5 μmol/L caused a remarkable reduction in cell viability of about 38% in HepG2 cells. The addition of IL-24 (at a concentration of 50 ng/mL) clearly potentiated the effects of GSI-I on HepG2 cells. IL-24 in combination with GSI-I (1 or 2.5 μmol/L) reduced cell viability of about 30% and 15%, respectively. However, treatment of cells with IL-24 alone did not induce any cytotoxic effect in the HCC cell lines. The addition of IL-24 to GSI-I treated SMMC7721 cells induced modest effects and the reduction of cell viability was only about 25%.
We next examined the effects of the two compounds IL-24 and GSI-I on cell morphology by means of light microscopy. As shown in Figure 2A (top panel), after treatment with the GSI-I/IL-24 combination for 24 h cells appeared rounded, fragmented and floated in the culture medium, while the two drugs, employed separately, did not induce any significant remarkable effect (Figure 2A). In the same experimental conditions, the nuclei stained with Hoechst 33258 and the fluorescence microscopy analysis of nuclei stained with Hoechst 33258 evidenced a significant increase in the number of cells with condensed and fragmented nuclei, which is a typical apoptotic feature (Figure 2A).
We analyzed the externalisation of phosphatidylserine on cell plasma membranes by Annexin V/PI staining to quantify early apoptotic effects. Following GSI-I/IL-24 combined treatment for 6 h, 47.2% of HepG2 cells were Annexin V positive/PI negative, while no significant effect was detected in cells treated with the compounds employed separately (Figure 2B).
Since Notch1 expression was associated with HCC metastasis as previously reported, we further investigated the link between Notch1 expression and the epithelial-mesenchymal transition (EMT) in HCC cells. HepG2 cell line was used for a comparison of the target protein expression profile of Notch1- and EMT-related genes. Decreased expression of Notch1 and its associated proteins SNAIL1 and SNAIL2 was detected in metastatic HCC cells. Increased E-cadherin protein expression was noted in the presence of IL-24 and GSI-I (Figure 3A). Loss of E-cadherin expression is a remarkable feature of EMT. As we all know, the level of E-cadherin is inversely associated with the tumor stage. Programmed cell death can be regulated by certain factors with either pro- or anti-apoptotic action in human cells. The upregulation of the former and/or the downregulation of the latter can be considered as important events to induce cell apoptosis[24]. To this purpose, we examined the expression levels of MMP-2 and IAP family members in HepG2 cells treated with GSI-I and/or IL-24. Furthermore, we proved that increased GSI-I and IL-24 in HepG2 cancer cells were associated with downregulation of MMP-2, XIAP and VEGF (Figure 3B).
Wound healing assay is widely used to analyze cell proliferations and cell migration. As previously reported, for HepG2 cells, the cell invasion has a great corresponding relationship with cell migration and wound healing assay. The invasion properties of cancer cells were detected by the wound closure in these assays. In our study HepG2 cells were treated with IL-24 and/or GSI-I, and the wound closure was analyzed by microscopy. In the absence of treatment, HepG2 cells could migrate into the scratched space in 24 h (Figure 4A). With the treatment of IL-24 or GSI-I, the wound was still open after 24 h, indicating that IL-24 or GSI-I has the ability of inhibiting the HepG2 cell migration or invasion (Figure 4B and C). To further confirm the inhibition effectiveness, we loaded the cells with IL-24 and GSI-I. The distance of the wound closure significantly correlated with the concentrations of IL-24 and GSI-I (Figure 4).
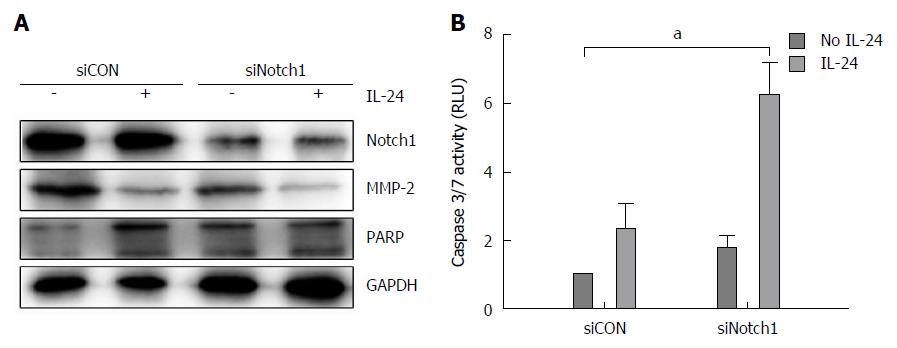
It has been showed that GSIs can act through different biochemical pathways, and we next investigated whether the effects induced by GSI-I/IL-24 combination were related to the specific inhibition of Notch signaling caused by GSI-I. We downregulated the Notch gene and evaluated the biological effects of IL-24 addition. Notch-1, as demonstrated by the authors, is upstream of Notch-4. After confirming the downregulation of Notch-1 level (Figure 5A, upper panel), we analyzed the biological effect of IL-24 addition on HepG2 cell viability. MMP-2 and poly (ADP)-ribose polymerase (PARP) proteins are the key proteins in detecting cell apoptosis. Figure 5A demonstrated that treatment of Notch-1 silenced HepG2 cells with 50 ng/mL IL-24 alone for 48 h induced cytotoxic effects quite similar to those observed in non-silenced cells treated with GSI-I/IL-24 combination.
To investigate the mechanism of apoptosis, we analyzed HepG2 cells treated with siNotch1 or siCON plus IL-24 or not for 48 h by caspase-3/7 activity analysis. Caspase-3/7 activity was increased in the addition of siNotch1 plus IL-24 treatment, indicating that siNotch1 plus IL-24 could sensitize HCC cells to the cytotoxic agents (Figure 5B)
We previously reported that LIGHT protein inhibited the proliferation and induced the apoptosis of HepG2 cells significantly[25]. However, little is known about the mechanism of HCC cell apoptosis. A large number of studies have showed that Notch signaling plays an important role in many kinds of malignant tumors[26-28]. Expression and localization of Notch receptors and their ligands have been observed in the normal human liver tissue in the previous report, while the deregulated Notch signaling has been found in malignant liver tumors[29-31].
This study was to investigate the biological effect of GSI-I, which is one of the most frequently employed GSIs. The association of IL-24 and GSIs arose by the observation that many tumor cells develop IL-24 sensitivity which can be overcome in the presence of different compounds. Although displaying anticancer potential, GSIs often show toxic side effects in some cells. Our data presented in this paper demonstrate a strong synergistic interaction between GSI-I and IL-24 which effectively reduced HCC cell viability for the first time. Noteworthy, GSI-I/IL-24 combined treatment was particularly effective in SMMC7721 and HepG2 cells (Figure 1).
Morphological observations demonstrated that HepG2 cell death was correlated with the induction of cell apoptosis; cells were positive for Αnnexin V/PI staining and the nuclei presented chromatin condensation (Figure 2A and B). After combined treatment, the activation of apoptotic pathway was also confirmed by Western blot.
Many studies indicated that GSI-I could be responsible for sensitization of cells to IL-24-mediated apoptosis and some of these are related to the activity of GSI-I, which is the inhibitor of γ-secretase, the main enzyme responsible for the cleavage and activation of Notch receptor and the following target proteins[32]. Data reported in this paper demonstrated that GSI-I/IL-24 combined treatment was able to modulate the expression level of related proteins in HepG2 cells (Figure 3).
Which GSI-I may sensitize HepG2 cells to apoptosis induced by IL-24 seems to be related to the modulation of pro- and anti-apoptotic factors through? In many experimental studies, the decreased survival factors and the concomitantly increased pro-apoptotic ones trigger a pathway that leads to cell death. Furthermore, a cross-talk has been showed between Notch signaling and the expression of some of these related signal pathway factors.
Sahlgren et al[33] reported that Notch signaling deploys a mechanism that acts in synergy to control the expression of SNAIL1 and SNAIL2, two critical regulators of EMT. Data of our study indicate that in HepG2 cells, besides the effects on SNAIL1 and SNAIL2, treatment with GSI-I/IL-24 combination also induced a clear upregulation of E-cadherin expression, which can be considered a key factor in cell growth and survival, together with that of MMP-2 factor. Moreover, some members of the tumor blood vessels related factors, such as XIAP and VEGF, are also downregulated following combined treatment with the two compounds (Figure 3).
The core role of Notch in HepG2 death pathway induced by GSI-I/IL-24 combination was also confirmed by Notch silencing experiments. In siNotch treated cells, we observed a decrease in the levels of both MMP-2 and PARP similar to that observed after GSI-I/IL-24 treatment (Figure 5A). Moreover, in siNotch silenced cells treated with IL-24 alone we also detected the Caspase 3/7 activity which is similar to that obtained after GSI-I/IL-24 combined treatment (Figure 5B). Overall, data reported seem to indicate that the synergistic effects induced by siNotch1/IL-24 combination are a specific consequence of GSI-I action on γ-secretase activity.
In conclusion, we found that the down-regulation of Notch1 by GSI-I or siRNA combined with IL-24 can sensitize apoptosis and increase expression of E-cadherin and decrease the invasion and migration capabilities of HepG2 cells. Taken together, these results demonstrated that GSI-I/IL-24 combination might represent a novel and potentially effective tool for HCC treatment for the first time.
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide and half of these cases were estimated to occur in China. The major reason for the poor prognosis is that HCC often relapses due to intrahepatic and distant metastases. Thus, the discovery and research of new molecular targets of blocking metastasis are the primary goal for HCC therapy.
Notch signaling pathway plays an important role in HCC metastasis. Notch1, one of the Notch pathway receptors, is significantly higher in HCC than in adjacent non-tumor liver tissue. Abnormal Notch1 expression has been shown to be strongly associated with HCC metastasis, which may be mediated through the Notch1/Snail1/E-cadherin pathway. Interleukin (IL)-24 mediates the inhibition of adhesion and invasion of HepG2 and BEL-7402 cells by increasing the expression of E-cadherin and p-ERK.
To date, there is no study regarding synergistic effect of Notch1 down-regulation and IL-24 on apoptosis, invasion and migration of HCC cells. In this study, the authors found that the down-regulation of Notch1 by γ-secretase inhibitor-I (GSI-I) or siRNA combined with IL-24 can sensitize apoptosis and decrease the invasion and migration capabilities of HepG2 cells. Furthermore, the authors researched the mechanism and found that besides the inhibiting effects on SNAIL1 and SNAIL2, treatment with GSI-I/IL-24 combination also induced a clear up-regulation of E-cadherin and down-regulation of MMP-2.
In understanding the role and mechanism of Notch1/Snail1/E-cadherin pathways in inhibiting invasion and migration of HCC cell lines, this study indicate, for the first time, that GSI-I/IL-24 combination might represent a novel and potentially effective tool for HCC treatment.
All Notch receptors are activated by γ-secretase, and the inhibitors of this enzyme (GSIs) have attracted increasing interest. GSIs were firstly employed in the treatment of Alzheimer’s disease to prevent amyloid precursor protein cleavage and the consequent release of amyloid β-peptide. More recently, it has been observed that GSIs also possess the ability to induce growth arrest and/or apoptosis in some tumor cell lines while other tumor cells were resistant to the molecules.
In this paper the role of Notch 1 deregulation in HCC has been evaluated. It was observed a decrease in HCC cell invasion and migration by Notch1 down-regulation. Notch 1 inhibition by GSI-I increased HCC cell apoptosis induced by IL-24. The down-regulation of Notch1 by specific siRNA or GSI-I increased E-cadherin expression and inhibited HCC cell invasion and migration. It is concluded that a combination therapy of GSI-I/IL-24 may be a novel and potentially effective treatment for HCC.
P- Reviewer: Tsegmed U S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Yu MC, Yuan JM, Govindarajan S, Ross RK. Epidemiology of hepatocellular carcinoma. Can J Gastroenterol. 2000;14:703-709. [PubMed] [Cited in This Article: ] |
| 2. | Singh SD, Ajani UA, Johnson CJ, Roland KB, Eide M, Jemal A, Negoita S, Bayakly RA, Ekwueme DU. Association of cutaneous melanoma incidence with area-based socioeconomic indicators-United States, 2004-2006. J Am Acad Dermatol. 2011;65:S58-S68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10-24. [PubMed] [Cited in This Article: ] |
| 4. | Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770-776. [PubMed] [Cited in This Article: ] |
| 5. | Gao J, Song Z, Chen Y, Xia L, Wang J, Fan R, Du R, Zhang F, Hong L, Song J. Deregulated expression of Notch receptors in human hepatocellular carcinoma. Dig Liver Dis. 2008;40:114-121. [PubMed] [Cited in This Article: ] |
| 6. | Giroldi LA, Bringuier PP, de Weijert M, Jansen C, van Bokhoven A, Schalken JA. Role of E boxes in the repression of E-cadherin expression. Biochem Biophys Res Commun. 1997;241:453-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 105] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1077] [Cited by in F6Publishing: 1057] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 8. | Moody SE, Perez D, Pan TC, Sarkisian CJ, Portocarrero CP, Sterner CJ, Notorfrancesco KL, Cardiff RD, Chodosh LA. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005;8:197-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 541] [Cited by in F6Publishing: 539] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 9. | Bremnes RM, Veve R, Gabrielson E, Hirsch FR, Baron A, Bemis L, Gemmill RM, Drabkin HA, Franklin WA. High-throughput tissue microarray analysis used to evaluate biology and prognostic significance of the E-cadherin pathway in non-small-cell lung cancer. J Clin Oncol. 2002;20:2417-2428. [PubMed] [Cited in This Article: ] |
| 10. | Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493-501. [PubMed] [Cited in This Article: ] |
| 11. | Liu D, Huang C, Kameyama K, Hayashi E, Yamauchi A, Kobayashi S, Yokomise H. E-cadherin expression associated with differentiation and prognosis in patients with non-small cell lung cancer. Ann Thorac Surg. 2001;71:949-954; discussion 954-955. [PubMed] [Cited in This Article: ] |
| 12. | Wei Y, Van Nhieu JT, Prigent S, Srivatanakul P, Tiollais P, Buendia MA. Altered expression of E-cadherin in hepatocellular carcinoma: correlations with genetic alterations, beta-catenin expression, and clinical features. Hepatology. 2002;36:692-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 130] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Bignell GR, Warren W, Seal S, Takahashi M, Rapley E, Barfoot R, Green H, Brown C, Biggs PJ, Lakhani SR. Identification of the familial cylindromatosis tumour-suppressor gene. Nat Genet. 2000;25:160-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 533] [Cited by in F6Publishing: 521] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 14. | Osada T, Sakamoto M, Ino Y, Iwamatsu A, Matsuno Y, Muto T, Hirohashi S. E-cadherin is involved in the intrahepatic metastasis of hepatocellular carcinoma. Hepatology. 1996;24:1460-1467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Tomlinson JS, Alpaugh ML, Barsky SH. An intact overexpressed E-cadherin/alpha,beta-catenin axis characterizes the lymphovascular emboli of inflammatory breast carcinoma. Cancer Res. 2001;61:5231-5241. [PubMed] [Cited in This Article: ] |
| 16. | Wang XQ, Zhang W, Lui EL, Zhu Y, Lu P, Yu X, Sun J, Yang S, Poon RT, Fan ST. Notch1-Snail1-E-cadherin pathway in metastatic hepatocellular carcinoma. Int J Cancer. 2012;131:E163-E172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Jiang H, Lin JJ, Su ZZ, Goldstein NI, Fisher PB. Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene. 1995;11:2477-2486. [PubMed] [Cited in This Article: ] |
| 18. | Allen M, Pratscher B, Roka F, Krepler C, Wacheck V, Schöfer C, Pehamberger H, Müller M, Lucas T. Loss of novel mda-7 splice variant (mda-7s) expression is associated with metastatic melanoma. J Invest Dermatol. 2004;123:583-588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Yacoub A, Mitchell C, Hong Y, Gopalkrishnan RV, Su ZZ, Gupta P, Sauane M, Lebedeva IV, Curiel DT, Mahasreshti PJ. MDA-7 regulates cell growth and radiosensitivity in vitro of primary (non-established) human glioma cells. Cancer Biol Ther. 2004;3:739-751. [PubMed] [Cited in This Article: ] |
| 20. | Huo W, Li ZM, Zhu XM, Bao YM, An LJ. MDA-7/IL-24 suppresses tumor adhesion and invasive potential in hepatocellular carcinoma cell lines. Oncol Rep. 2013;30:986-992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Bergmans BA, De Strooper B. gamma-secretases: from cell biology to therapeutic strategies. Lancet Neurol. 2010;9:215-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 22. | Panza F, Frisardi V, Imbimbo BP, Capurso C, Logroscino G, Sancarlo D, Seripa D, Vendemiale G, Pilotto A, Solfrizzi V. REVIEW: γ-Secretase inhibitors for the treatment of Alzheimer’s disease: The current state. CNS Neurosci Ther. 2010;16:272-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Lin J, Zhang XM, Yang JC, Ye YB, Luo SQ. γ-secretase inhibitor-I enhances radiosensitivity of glioblastoma cell lines by depleting CD133+ tumor cells. Arch Med Res. 2010;41:519-529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3154] [Cited by in F6Publishing: 3325] [Article Influence: 207.8] [Reference Citation Analysis (0)] |
| 25. | Han B, Wu LQ, Ma X, Wang ZH, Li JP, Bi CY, Yong S. Synergistic effect of IFN-γ gene on LIGHT-induced apoptosis in HepG2 cells via down regulation of Bcl-2. Artif Cells Blood Substit Immobil Biotechnol. 2011;39:228-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Sjölund J, Manetopoulos C, Stockhausen MT, Axelson H. The Notch pathway in cancer: differentiation gone awry. Eur J Cancer. 2005;41:2620-2629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Espinoza I, Pochampally R, Xing F, Watabe K, Miele L. Notch signaling: targeting cancer stem cells and epithelial-to-mesenchymal transition. Onco Targets Ther. 2013;6:1249-1259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 28. | Takebe N, Nguyen D, Yang SX. Targeting notch signaling pathway in cancer: clinical development advances and challenges. Pharmacol Ther. 2014;141:140-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 300] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 29. | Nijjar SS, Crosby HA, Wallace L, Hubscher SG, Strain AJ. Notch receptor expression in adult human liver: a possible role in bile duct formation and hepatic neovascularization. Hepatology. 2001;34:1184-1192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 89] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 30. | Zhou L, Wang DS, Li QJ, Sun W, Zhang Y, Dou KF. Downregulation of the Notch signaling pathway inhibits hepatocellular carcinoma cell invasion by inactivation of matrix metalloproteinase-2 and -9 and vascular endothelial growth factor. Oncol Rep. 2012;28:874-882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 31. | Viatour P, Ehmer U, Saddic LA, Dorrell C, Andersen JB, Lin C, Zmoos AF, Mazur PK, Schaffer BE, Ostermeier A. Notch signaling inhibits hepatocellular carcinoma following inactivation of the RB pathway. J Exp Med. 2011;208:1963-1976. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 160] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 32. | Benedito R, Rocha SF, Woeste M, Zamykal M, Radtke F, Casanovas O, Duarte A, Pytowski B, Adams RH. Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF-VEGFR2 signalling. Nature. 2012;484:110-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 273] [Cited by in F6Publishing: 280] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 33. | Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc Natl Acad Sci USA. 2008;105:6392-6397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 568] [Cited by in F6Publishing: 613] [Article Influence: 38.3] [Reference Citation Analysis (0)] |