Published online Dec 21, 2017. doi: 10.3748/wjg.v23.i47.8355
Peer-review started: October 5, 2017
First decision: October 25, 2017
Revised: October 30, 2017
Accepted: November 14, 2017
Article in press: November 14, 2017
Published online: December 21, 2017
To determine the efficacy of rifaximin for hepatic encephalopathy (HE) with the linkage of gut microbiome in decompensated cirrhotic patients.
Twenty patients (12 men and 8 women; median age, 66.8 years; range, 46-81 years) with decompensated cirrhosis (Child-pugh score > 7) underwent cognitive neuropsychological testing, endotoxin analysis, and fecal microbiome assessment at baseline and after 4 wk of treatment with rifaximin 400 mg thrice a day. HE was determined by serum ammonia level and number connection test (NCT)-A. Changes in whole blood endotoxin activity (EA) was analyzed by endotoxin activity assay. Fecal microbiome was assessed by 16S ribosome RNA (rRNA) gene sequencing.
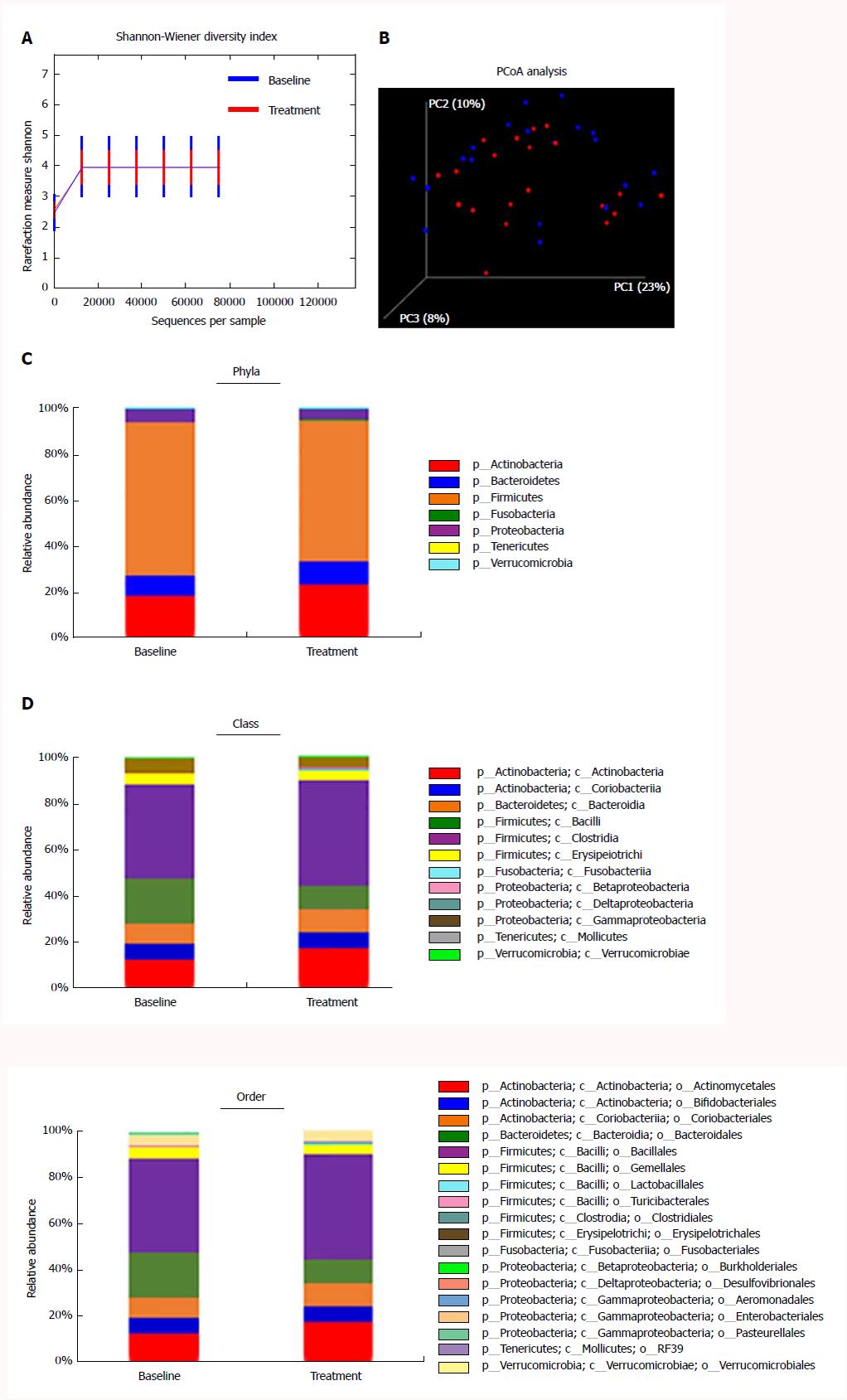
Treatment with rifaximin for 4 wk improved hyperammonemia (from 90.6 ± 23.9 μg/dL to 73.1 ± 33.1 μg/dL; P < 0.05) and time required for NCT (from 68.2 ± 17.4 s to 54.9 ± 20.3 s; P < 0.05) in patients who had higher levels at baseline. Endotoxin activity was reduced (from 0.43 ± 0.03 to 0.32 ± 0.09; P < 0.05) in direct correlation with decrease in serum ammonia levels (r = 0.5886, P < 0.05). No statistically significant differences were observed in the diversity estimator (Shannon diversity index) and major components of the gut microbiome between the baseline and after treatment groups (3.948 ± 0.548 at baseline vs 3.980 ± 0.968 after treatment; P = 0.544), but the relative abundances of genus Veillonella and Streptococcus were lowered.
Rifaximin significantly improved cognition and reduced endotoxin activity without significantly affecting the composition of the gut microbiome in patients with decompensated cirrhosis.
Core tip: Hepatic encephalopathy (HE) is characterized by deficits in cognitive, psychiatric, and motor function and ranges in severity from minimal to overt HE and coma. Rifaximin is used for standard treatment of HE, targeting reduction of ammonia and gut bacterial translocation. This study demonstrates that rifaximin improves hyperammonemia and cognitive impairment with the linkage of decreased endotoxin activity in patients with decompensated cirrhosis. The diversity and major components of gut microbiome analyzed by 16S rRNA gene sequencing are not altered by treatment with rifaximin. This is the first report of systemic and local effects of rifaximin in Japanese patients.
- Citation: Kaji K, Takaya H, Saikawa S, Furukawa M, Sato S, Kawaratani H, Kitade M, Moriya K, Namisaki T, Akahane T, Mitoro A, Yoshiji H. Rifaximin ameliorates hepatic encephalopathy and endotoxemia without affecting the gut microbiome diversity. World J Gastroenterol 2017; 23(47): 8355-8366
- URL: https://www.wjgnet.com/1007-9327/full/v23/i47/8355.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i47.8355
Hepatic encephalopathy (HE) is a spectrum of neuropsychiatric syndromes that form major complications in patients with acute or chronic liver disease[1]. It is characterized by a wide range of changes in the mental state from minimal signs of altered brain function to deep coma. Minimal HE is the earliest stage; it occurs in up to 80% of patients with cirrhosis and manifests as abnormalities in the central nervous system function[2,3]. Recent pathophysiological evidence suggests that alterations in the gut microbiome could be critical to bacterial translocation, hyperammonemia, and systemic inflammation, leading to the development of HE[4-7]. Bacterial overgrowth in the gut microbiome is closely associated with the severity of liver disease, and patients with overt HE reportedly reveal significant changes in the enteric microbiota compared with those with minimal HE[4-7]. Recent randomized clinical trial has reported that fecal microbiota transplantation, a newly developed microbiome-targeted therapy, has the potential to improve HE[8].
Considering the pathogenesis of microbe-based HE, endotoxemia is known to play a potentially cardinal role in the development of systemic inflammation and neuroinflammation. In patients with advanced cirrhosis, endotoxin increases the permeability of the blood-brain barrier and enhances astrocyte swelling via nitric oxide and prostanoid production in the brain microglia[9-11]. Clinical evidence suggests that endotoxemia is correlated with the severity of HE and increased incidence of overt HE[10-12]. Furthermore, accumulating evidences have revealed that microbiota-targeted therapies, such as probiotics, prebiotics, synbiotics, and antibiotics, may cause at least partial improvement of endotoxemia[13-16].
Rifaximin, an oral antibiotic with broad-spectrum activity against aerobic and anaerobic Gram-positive and Gram-negative bacteria, is widely used for the prevention of HE and is proposed to have beneficial effects on overt HE and survival. It could exert antimicrobial activity against ammonia-producing enteric bacteria including (1) Gram-positive aerobic bacteria such as Streptococcus and Bacillus; (2) Gram-negative facultative anaerobic bacteria such as E. coli, Klebsiella, Citrobacter, Enterobacter, and Proteus; (3) Gram-positive obligatory anaerobic bacteria such as Clostridium; and (4) Gram-negative obligatory anaerobic bacteria such as Bacteroides[17]. However, in Japan, it has only been recently available for patients with HE[18]. Several studies of cirrhotic patients in the Western countries have reported the possible mechanisms of action of rifaximin, suggesting that it could decrease endotoxin levels without altering the relative abundance of pathogenic bacteria[19-21]. To the best of our knowledge, the effects of rifaximin on the gut microbiome in patients from the Eastern countries have not been assessed. Moreover, the relationship between the endotoxin activity and microbial alteration at the gene level has not been elucidated.
The present study aimed to evaluate the impact of rifaximin on the endotoxin activity and gut microbiota identified by 16S ribosome RNA (rRNA) gene sequencing in patients with decompensated cirrhosis.
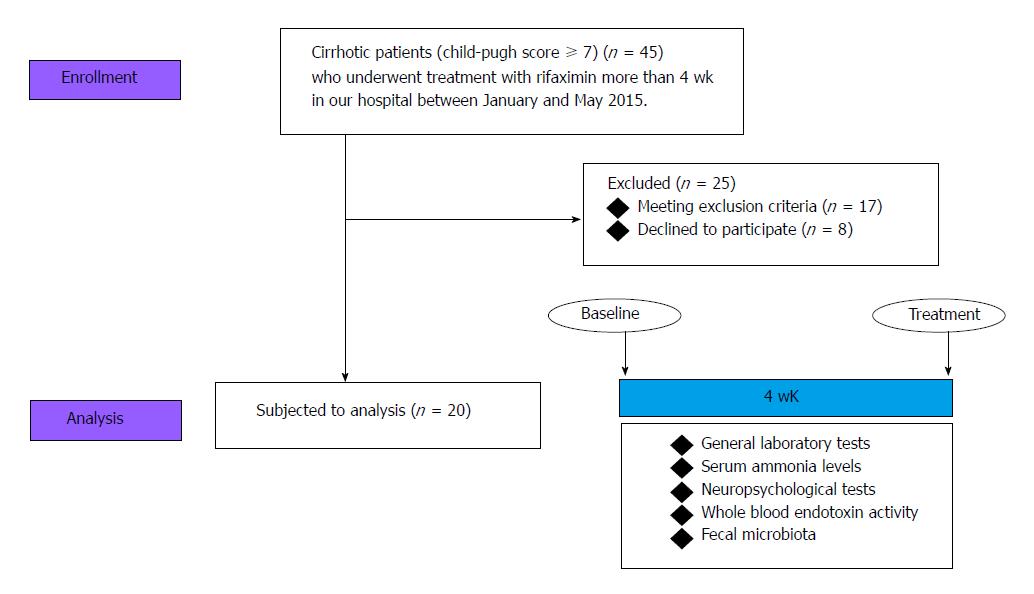
The study was conducted from January to May 2017 at the Third Department of Internal Medicine of Nara Medical University. The subjects were patients with decompensated cirrhosis (Child-Pugh score > 7) due to several causes, aged 18 years or older, who had been diagnosed by clinical, biochemical, and ultrasound findings (n = 45). The exclusion criteria were cardiac and/or respiratory failure or invasive cancer within the past 5 years; renal failure with serum creatinine > 200 μmol/L; clinical or biochemical signs of infection 28 d prior to inclusion; concomitant inflammatory bowel diseases and/or irritable bowel syndrome; previous history of gastrectomy, enterectomy, and/or liver transplantation; and developed portosystemic shunt. Patients who consumed nonabsorbable disaccharides, probiotics, prebiotics, synbiotics, or other antibiotics 28 d prior to inclusion were also excluded. Finally, 20 patients except for the patients to meet the exclusion criteria (n = 17) and decline to participate (n = 8) were finally analyzed.
All subjects were treated with rifaximin 400 mg thrice a day for 4 wk, and the complete investigational program was performed at baseline and after 4 wk of treatment. The program comprised general laboratory tests, measurement of serum ammonia levels, neuropsychological testing, measurement of whole blood endotoxin activity, and analysis of fecal microbiota (Figure 1).
The study protocols conformed to the principles outlined in the 1964 Declaration of Helsinki and its later amendments and were approved by the Ethics Committee of Nara Medical University (approval number 994) and were registered at UMIN000029127. All subjects provided written informed consent prior to their inclusion in the study.
To objectively evaluate cognitive performance, we used the number connection test (NCT)-A distributed by the Japan Society of Hepatology, as previously described[22,23]. The hardware consisted of a touch screen tablet such as iPad (Apple, Cupertino, CA, United States).
Whole blood endotoxin activity was assessed with the commercially available Endotoxin Activity Assay (EAA) kit (Spectral Diagnostics, Toronto, Canada), which uses a luminol chemiluminescence method. In brief, the EAA is based on the principle that endotoxin binds to antiendotoxin antibodies and is delivered to neutrophils by complement receptors. In the presence of β-glucan and luminol, the neutrophils undergo a respiratory burst accompanied by light emission. The light produced is quantified by a chemiluminometer, and its intensity is proportional to the amount of endotoxin present in the sample[24].
Fecal samples were collected before and 4 wk after rifaximin administration and placed in 1.5-mL tubes, snap-frozen on dry ice, and stored at -80 °C. 16S rRNA analysis of fecal samples was performed at Takara Bio (Shiga, Japan). DNA was extracted with the MoBio Powerlyzer Powersoil DNA Isolation Kit (MoBio Laboratories, Carlsbad, CA, United States). The V4 hypervariable region of the bacterial 16S rRNA gene was amplified from the fecal DNA extracts using the modified universal bacterial primer pairs 341F (5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG-3′) and 806R (5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGGACTACHVGGGTWTCTAAT-3′) with Illumina adaptor overhang sequences. Amplicons were generated, cleaned, indexed, and sequenced according to the Illumina MiSeq 16S Metagenomic Sequencing Library Preparation protocol (http://support.illumina.com/downloads/16s_metagenomic_sequencing_library_ preparation.html), with brief modifications.
Sequencing data were combined and sample identification was assigned to multiplexed reads using the MOTHUR software environment[25]. The data were denoised; low-quality sequences, pyrosequencing errors, and chimeras were removed; and then the sequences were clustered into operational taxonomic units (OTUs) at 97% identity using the CD-HITOTU pipeline (available from http://eeizhong-lab.ucsd.edu/cd-hit-otu)[26]. OTUs containing fewer than four reads per individual diet/animal combination were excluded due to the likelihood of a sequencing artifact. The samples were normalized by random resampling sequences used to the lowest number of sequences per sample (each diet/animal combination) using Daisychopper (http://www.festinalente.me/ bioinf/). Taxonomic classification of OTUs was done with the Ribosomal Database Project Classifier[27].
Differences between the paired groups were analyzed by the Mann-Whitney U test. Correlations were calculated with the Spearman rank test. The data are expressed as means ± SD. A two-tailed p-value less than 0.05 was considered to indicate statistical significance. Analyses were performed with EZR (Saitama Medical Center, Jichi Medical University), which is a graphical user interface for R (The R Foundation for Statistical Computing, version 2.13.0). Specifically, EZR is a modified version of R commander (version 1.6-3) that includes statistical functions frequently used in biostatistics[28].
The demographic and baseline clinical characteristics of the patients are presented in Table 1. Twenty patients with decompensated cirrhosis (12 men and 8 women; median age, 66.8 years; range, 46-81 years) were included in the study. The etiology of cirrhosis was identified as alcohol (40%), hepatitis B virus (HBV) (20%), hepatitis C virus (HCV) (20%), nonalcoholic steatohepatitis (NASH) (10%), alcohol and HBV (5%), and alcohol and HCV (5%). The majority of the patients (90%) were classified as Child-Pugh B, and the others (10%) were classified as Child-Pugh C; the median Model of End-stage Liver Disease (MELD) score was 8.6 (range, 2.6-15.0). Administration of rifaximin did not have any adverse effects, including hepatotoxicity and nephrotoxicity (Supplementary Figure 1), on any patient during the research period. Also, no significant changes were observed in MELD score, serum albumin, total bilirubin, prothrombin time, C-reactive protein (CRP), white blood cells (WBC), platelet and branched chain amino acid & tyrosine ratio (BTR) after 4 wk treatment of rifaximin (Table 1). The numbers of patient with the high endotoxin activity/delayed NCT/high ammonia are 11/10/16. All of the patients with high endotoxin activity were included in the high ammonia group. Seven patients with delayed NCT were included in the high ammonia group.
| Baseline | Treatment | P value | |
| Age | 66.8 (46-81) | ||
| Sex (male/female) | 12/8 | ||
| Etiology | |||
| Alcohol | 4 | ||
| HBV | 4 | ||
| HCV | 8 | ||
| NASH | 2 | ||
| Alcohol + HBV | 1 | ||
| Alcohol + HCV | 1 | ||
| Child class (A/B/C) | 0/18/2 | ||
| MELD score | 8.3 (2.6-15.0) | 7.5 (1.2-15.0) | 0.474 |
| AST (U/L) | 50 ± 22 | 53 ± 29 | 0.791 |
| ALT (U/L) | 32 ± 14 | 31 ± 14 | 0.755 |
| Albumin (g/dL) | 3.3 ± 0.6 | 3.3 ± 0.5 | 0.980 |
| Total bilirubin (mg/dL) | 1.8 ± 0.9 | 1.6 ± 0.8 | 0.545 |
| Prothrombin time (INR) | 1.28 ± 0.11 | 1.26 ± 0.11 | 0.630 |
| CRP (mg/dL) | 0.3 ± 0.6 | 0.2 ± 0.2 | 0.533 |
| WBC (103/μL) | 3.4 ± 1.1 | 3.5 ± 1.0 | 0.847 |
| Platelet (104/μL) | 8.1 ± 4.1 | 7.7 ± 3.5 | 0.710 |
| BTR | 3.7 ± 1.5 | 4.2 ± 3.7 | 0.601 |
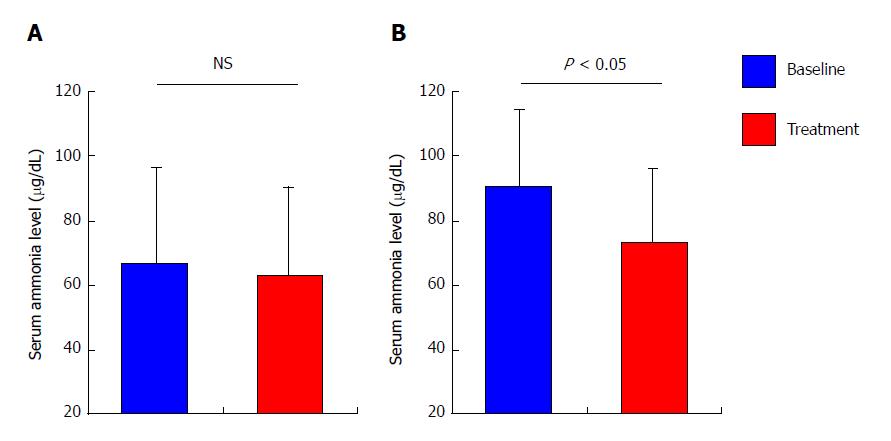
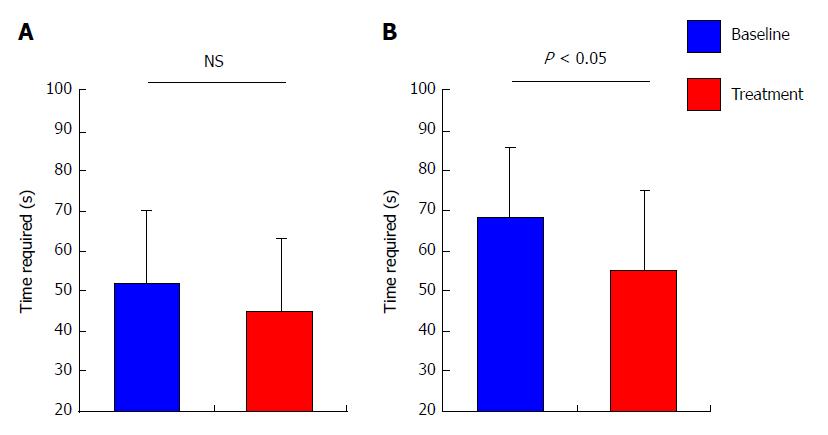
The mean levels of serum ammonia among all patients remain unaltered after 4 wk of treatment with rifaximin as compared with baseline (66.7 ± 29.8 μg/dL at baseline vs 62.7 ± 27.6 μg/dL after treatment, P = 0.440; Figure 2A), although the mean levels among the patients who revealed high levels of serum ammonia (> 70 μg/dL) at baseline were significantly decreased after treatment (90.6 ± 23.9 μg/dL at baseline vs 73.1 ± 33.1 μg/dL after treatment, P < 0.05; Figure 2B). In coincidence with serum ammonia levels, the mean time required for NCT among all patients did not differ from baseline after treatment (51.7 ± 18.7 s at baseline vs 45.0 ± 18.4 s after treatment, P = 0.267; Figure 3A), whereas the mean time required for NCT among patients who revealed prolongation of NCT (> 50 s) at baseline was significantly shortened after treatment (68.2 ± 17.4 s at baseline vs 54.9 ± 20.3 s after treatment, P < 0.05; Figure 3B).
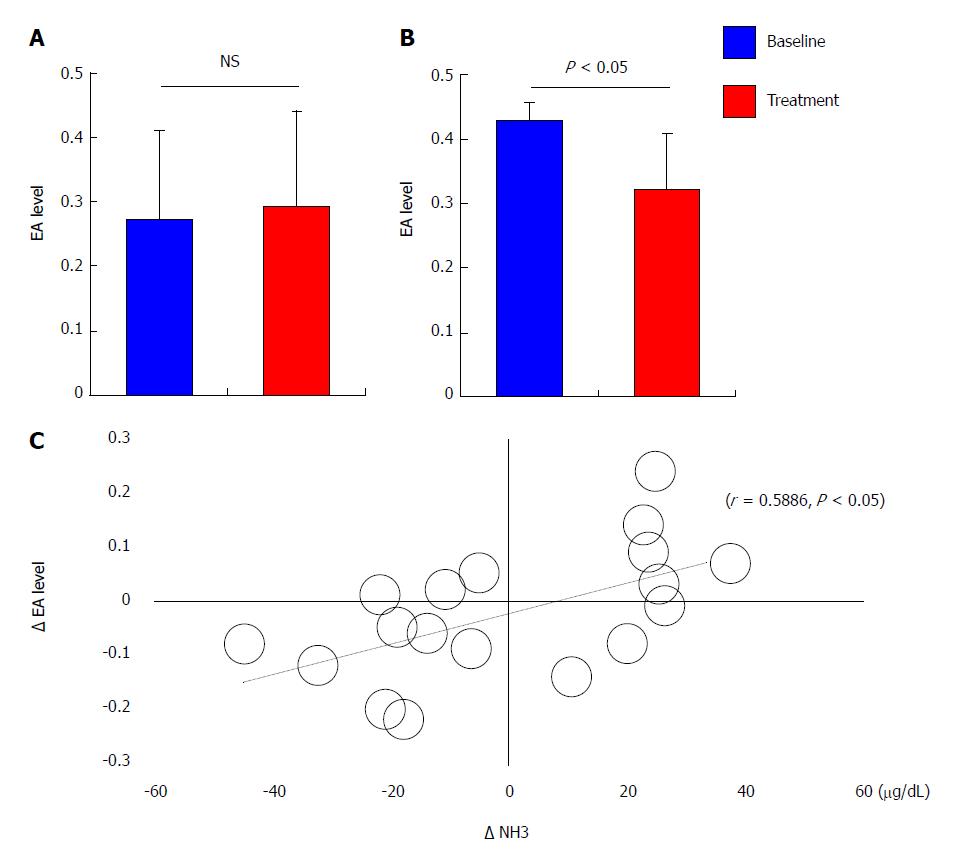
The mean endotoxin activity among all patients remained unaltered after 4 weeks of treatment compared with baseline (0.27 ± 0.14 at baseline vs 0.29 ± 0.15 after treatment, P = 0.641; Figure 4A). The mean endotoxin activity among patients who reported high levels of endotoxin activity (> 0.4) at baseline was significantly decreased after treatment (0.43 ± 0.03 at baseline vs 0.32 ± 0.09 after treatment, P < 0.05; Figure 4B). Univariate correlation analysis demonstrated that the decrease in the endotoxin activity level after 4 weeks of treatment (Δ EA) correlated directly with the decrease in the serum ammonia level (Δ NH3; r = 0.5886, P < 0.05; Figure 4C).
For gut microbiome analysis using 16S rRNA gene sequencing, fecal samples were collected before and after 4 wk of treatment with rifaximin from the 20 patients. In total, 33222538 raw reads were obtained from all 40 fecal samples. After filtering, 17958952 high-quality sequences were produced, with an average of 448473 ± 64690 reads per sample. No statistically significant differences were observed in the diversity estimator (Shannon diversity index) between the baseline and treatment groups (3.948 ± 0.548 at baseline vs 3.980 ± 0.968 after treatment, P = 0.544; Figure 5A). UniFrac principal coordinate analysis (PCoA) also revealed no significant clustering between the microbiota composition before and after rifaximin treatment (Figure 5B). The overall microbiota composition and average relative abundances for each group at the phylum, class, and order levels are shown in Figure 4C-E. There were 7 phyla, 12 classes, and 18 orders in the fecal samples. The dominant phyla of both groups were Firmicutes, Actinobacteria, Bacteroides, and Proteobacteria; the dominant classes of both groups were Clostridia, Actinobacteria, Bacteroidia, Bacilli, and Coriobacteria; and the dominant orders of both groups were Bacillales, Actinomycetales, Bacteroidales, Coriobacteriales, and Bifidobacteriales. At the phylum level, no differences were observed in the average abundance in the patients from baseline to after treatment with rifaximin (Figure 5C). Likewise, we did not observe any changes in the average abundance at the class and order levels (Figure 5D and E).
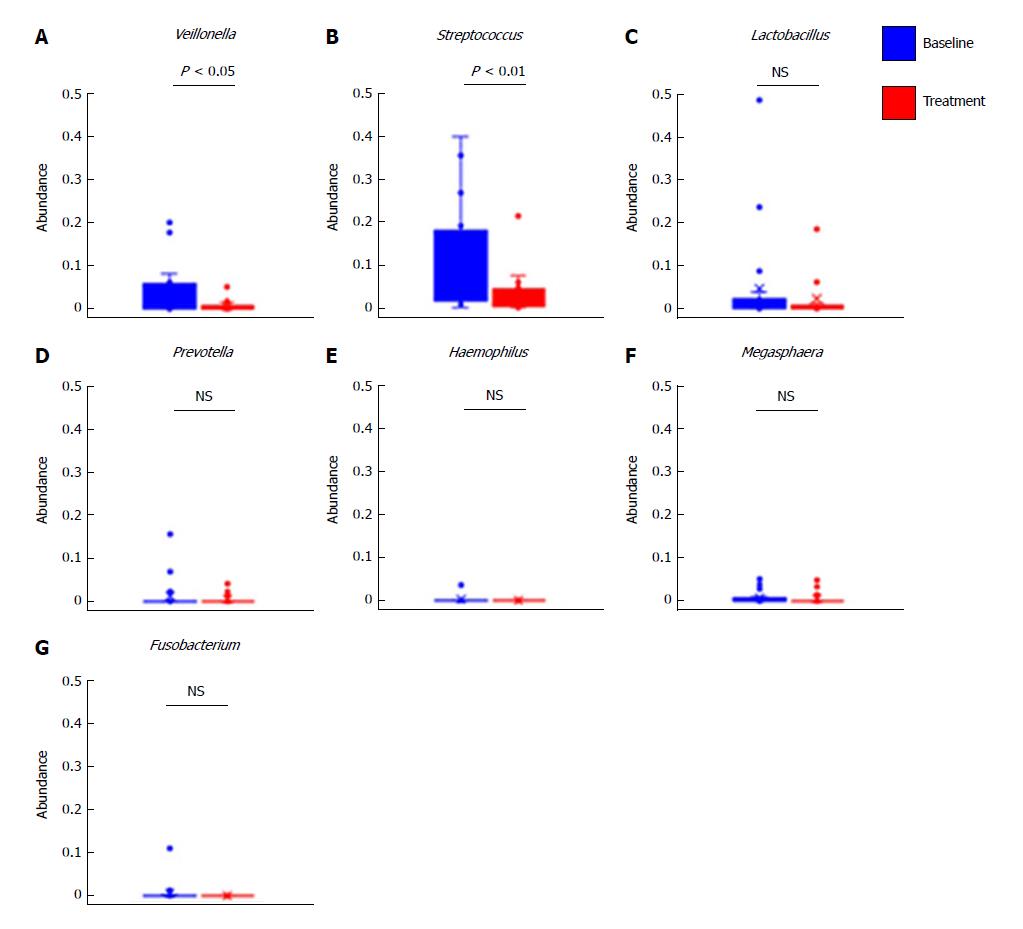
Furthermore, we evaluated the changes in abundance of selected genera after treatment with rifaximin. We selected seven genera revealing elevation in the feces of cirrhotic patients compared with healthy controls, as previously described[29]. The relative abundances of Veillonella and Streptococcus were lower in the treatment group than in the baseline group (Figure 6A and B), whereas no significant differences were observed in the relative abundances of Lactobacillus, Prevotella, Haemophilus, Megaspaera, and Fusobacterium (Figure 6C-G).
The results of this study demonstrate that rifaximin improves cognitive performance with reduced serum ammonia levels and endotoxin activity in patients with decompensated cirrhosis. The 16S rRNA gene analysis found no significant differences in the predominant organisms from before treatment to after treatment, indicating that rifaximin exerts its pharmacological actions independently of modification of the gut microbiota. Evidence reveals the effects of rifaximin on HE with the link between endotoxin and gut microbiota in patients from Western countries[19-21]. Because the microbial taxa involved are slightly different in cirrhotic patients from Eastern and Western countries, we undertook this study to assess the effects of rifaximin on HE in cirrhotic patients from Eastern countries[30,31].
Rifaximin exerts its antibiotic actions via inhibition of bacterial RNA synthesis by binding to the β-subunit of bacterial DNA-dependent RNA polymerase[32]. The activity of rifaximin is targeted to the gastrointestinal tract due to its nonsystemic absorption; thus the use of rifaximin is a viable option as treatment for HE. A meta-analysis including 19 randomized, controlled trials of rifaximin for HE demonstrated that it lowered serum ammonia (mean difference, -7.10 μg/dL; 95%CI: -12.29 to -1.91) and improved NCT (mean difference, -5.29 s; 95%CI: -10.05 to -0.53)[33]. Consistent with this evidence, the present results indicate that 4 wk of administration of rifaximin significantly reduced the serum ammonia levels and shortened the time required for NCT in cirrhotic patients who had reported higher levels at baseline. Recent clinical trials suggest that these beneficial effects of rifaximin on HE are strongly associated with improved endotoxemia[19,21,34]. Basic in vivo studies revealed the mechanistic insight for the ability of rifaximin to lower plasma endotoxin levels. Kang et al[20]. reported that rifaximin improved endotoxemia induced by humanization with stools from patients with minimal HE in germ-free mice[20]. Zhu et al[35] demonstrated that rifaximin attenuates liver fibrosis and portal hypertension by inhibiting the lipopolysaccharides (LPS)/toll-like receptor (TLR) 4 pathway in bile duct-ligation induced fibrotic mice. Unlike other studies, we assessed the plasma endotoxin activity by an EAA. Most quantitative limulus amebocyte lysate (LAL) tests, which are widely used to measure the endotoxin levels, are not endotoxin-specific, as these tests detect both endotoxin from Gram-negative bacteria and (1-3)-β-D-glucan from fungus, which are microbial products translocated from the intestine. Therefore, these tests are unable to detect spillover endotoxemia in liver diseases due to the complexity of the measurement, difficulty in standardization, and low sensitivity. The EAA is a novel and simple method to assess blood levels of endotoxin with higher sensitivity as compared with these tests[24,36]. In fact, our results demonstrated that rifaximin significantly decreased the plasma EA levels, but not endotoxin concentration as detected by LAL tests, in patients with cirrhosis (data not shown). Additionally, our results reveal a direct correlation between Δ NH3 and Δ EA, indicating that a decrease in endotoxin activity is crucial in the effect of rifaximin on hyperammonemia. It has been reported that ammonia and LPS synergistically facilitate cytotoxic edema and precoma in cirrhotic rats[37]. Another report suggested that bacterial LPS inhibit the hepatic ammonia removal via glutamine synthesis[38]; however, a large-scale prospective study would be required to elucidate the exact mechanism of interaction between the generation of ammonia and endotoxemia.
As described in the previous reports, we focused on the modulation of gut microbiota as the major cause of the ability of rifaximin to lower plasma endotoxin activity levels in cirrhotic patients[19-21,33,39]. Remarkably, although rifaximin exerts its effects on a wide spectrum of Gram-positive and Gram-negative organisms, recent evidence suggests that its pharmacological action may involve the alteration of bacterial function and virulence rather than reduction of the bacterial population[39]. In a previous cohort study, Bajaj et al[40] assessed the modulation of the gut microbiome in cirrhotic patients with minimal HE and demonstrated that rifaximin affected neither the overall abundance of bacteria nor the bacterial load. They revealed that rifaximin’s clinical activity might be attributed to effects on metabolic function of the gut microbiota, rather than a change in the relative bacterial abundance[19,40]. Our results also reveal no significant changes in the relative abundances at the phylum, class, and order levels as well as in the overall diversity of fecal microbiota between baseline and follow-up samples. Furthermore, a previous quantitative metagenomic study reported that several genera of bacteria, including Veillonella, Streptococcus, Lactobacillus, Prevotella, Haemophilus, Megaspaera, and Fusobacterium, were more abundant in cirrhotic patients[29]; therefore, we next evaluated the effects of rifaximin on the levels of these organisms. Notably, present metagenomics revealed a marked decrease in Veillonella after treatment with rifaximin. Veillonella is an anaerobic Gram-negative coccus, and previous studies reported that its abundance increased in the colonic mucosa of cirrhotic patients with HE as compared with those without HE, and decreased less in patients treated with rifaximin and nonabsorbable disaccharide than in those receiving nonabsorbable disaccharide monotherapy[34]. These findings indicate that Veillonella may be a candidate fecal marker for the presence of HE. Veillonella primarily requires lactic acid for fermentation, and hence it has a symbiotic relationship with Streptococcus, which produces lactate metabolically[41]. Based on their symbiosis, we also observed a significant decrease in Streptococcus in parallel with Veillonella in the fecal samples from patients treated with rifaximin.
In the present study, endotoxin-generating Gram-negative bacteria were unchanged by treatment with rifaximin. The role of rifaximin in lowering plasma endotoxin levels without modifying the overall composition of the gut microbiome remains unclear. We suggest two possible mechanisms: first, the impact of metabolic modifications in the gut microbiota[19]; second, the possibility that rifaximin may contribute in improvement of the intestinal barrier function. A recent in vitro study using human intestinal epithelial cells reported that Clostridium difficile toxin A-induced cell apoptosis and deprivation of tight junction proteins (TJPs) were suppressed by treatment with rifaximin through the pregnane X receptor-dependent inhibition of theTLR4/MyD88/NF-κB pathway[42]. Our previous report also demonstrated that antibiotics improved the intestinal permeability and enhanced TJP expression in the rat nonalcoholic steatohepatitis model[43]. A further basic analysis is needed to elucidate the association of these mechanisms.
A limitation of this study was the small sample size. Further studies are essential to evaluate the effects of rifaximin in a larger population and in other Asian countries, as well as the effects of its long-term administration. Additionally, assessment of proinflammatory cytokines such as tumor necrosis factor-α and interleukin-6 is currently in progress for deeper analysis of the interaction between endotoxemia and the development of HE.
In conclusion, rifaximin significantly improved cognition and reduced endotoxin activity with minor modification of the gut microbiome in patients with decompensated cirrhosis. This is the first report of systemic and local effects of rifaximin in Japanese patients.
Hepatic encephalopathy (HE) is characterized by deficits in cognitive, psychiatric, and motor function and ranges in severity from minimal to overt HE and coma. Rifaximin is used for standard treatment of HE, targeting reduction of ammonia and gut bacterial translocation. Currently, rifaximin has been suggested to partially affect gut microbiome in the patients with HE.
The effects of rifaximin on the gut microbiome in patients from the Eastern countries have not been assessed. Moreover, the relationship between the endotoxin activity and microbial alteration at the gene level has not been elucidated. Recently, 16S rRNA gene sequencing has been established as a novel method to directly access the genetic content of entire communities of organisms. Some evidence reveals the effects of rifaximin on HE with the linkage of gut microbiota in patients from Western countries. Because the microbial taxa involved are slightly different in cirrhotic patients from Eastern and Western countries, we undertook this study to assess the effects of rifaximin on HE in cirrhotic patients from Eastern countries. Moreover, the relationship between the endotoxin activity and microbial alteration at the gene level.
To determine the efficacy of rifaximin for hepatic encephalopathy (HE), evaluated with serum ammonia level, NCT and endotoxin activity, with the linkage of gut microbiome in decompensated cirrhotic patients.
Twenty patients with decompensated cirrhosis were enrolled for this study. They were treated with rifaximin 400 mg three times a day for 4 wk. The measurement of serum ammonia level and number connection test (NCT)-A were performed to evaluate their status of hepatic encephalopathy before and after treatment of rifaximin. Endotoxemia was assessed by blood endotoxin activity assay (EAA). The 16S ribosome RNA gene sequencing was performed for analysis of fecal microbiome, and the diversity and compositions of gut microbiome were compared between before and after treatment of rifaximin.
This study demonstrates that rifaximin improves hyperammonemia and cognitive impairment with the linkage of decreased endotoxin activity in patients with decompensated cirrhosis. The diversity and major components of gut microbiome analyzed by 16S rRNA gene sequencing are not altered by treatment with rifaximin, although the relative abundances of genus Veillonella and Streptococcus were lowered.
This study demonstrates that rifaximin significantly improves hepatic encephalopathy with minor modification of the gut microbiome in Japanese patients with decompensated cirrhosis. Rifaximin markedly improved cognition and reduced endotoxin activity without significantly affecting the composition of the gut microbiome indicating that the effect of rifaximin is independent of modification of gut microbial diversity. This effect of rifaximin on gut microbiome in Japanese cirrhotic patients is similar to the patients in the West. On the other hands, rifaximin modified minor compositions of gut microbiome such as decreased relative abundances of genus Veillonella and Streptococcus in current subjects. So far, the mechanism of decreased endotoxin activity by rifaximin is still obscure, but we speculate that it is possibly related to the pharmacological action of rifaximin to improve intestinal barrier function. In conclusion, rifaximin is an effective medical agent for the patients with hepatic encephalopathy.
This study demonstrates that rifaximin improves hyperammonemia and cognitive impairment with the linkage of decreased endotoxin activity in patients with decompensated cirrhosis. These effects of rifaximin are independent of alteration of gut microbial diversity, indicating that rifaximin has a potential capacity to decrease ammonia and endotoxin level other than the effect on gut microbiome such as the improvement of intestinal barrier function. Therefore, we will examine the effect of rifaximin on intestinal tight junction protein in the clinical practice to elucidate above hypothesis in near future after the approval of ethical committee. We consider that the best method is to analyze the alteration of intestinal tight junction protein before and after treatment with rifaximin in the biopsy tissues from the patients with decompensated cirrhosis.
The authors gratefully acknowledge the work of ASKA Pharmaceutical Co., Ltd for the support of 16S rRNA gene sequencing analysis.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gencdal G, Hashimoto N, McMillin MA, Stanciu C S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
| 1. | Prakash R, Mullen KD. Mechanisms, diagnosis and management of hepatic encephalopathy. Nat Rev Gastroenterol Hepatol. 2010;7:515-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 202] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 2. | Rikkers L, Jenko P, Rudman D, Freides D. Subclinical hepatic encephalopathy: detection, prevalence, and relationship to nitrogen metabolism. Gastroenterology. 1978;75:462-469. [PubMed] [Cited in This Article: ] |
| 3. | Ortiz M, Jacas C, Córdoba J. Minimal hepatic encephalopathy: diagnosis, clinical significance and recommendations. J Hepatol. 2005;42 Suppl:S45-S53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 195] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 4. | Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, Wang Y, Zhu B, Li L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54:562-572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 674] [Cited by in F6Publishing: 701] [Article Influence: 53.9] [Reference Citation Analysis (1)] |
| 5. | Bajaj JS, Ridlon JM, Hylemon PB, Thacker LR, Heuman DM, Smith S, Sikaroodi M, Gillevet PM. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol. 2012;302:G168-G175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 347] [Cited by in F6Publishing: 373] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 6. | Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, Noble NA, Unser AB, Daita K, Fisher AR. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60:940-947. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 659] [Cited by in F6Publishing: 715] [Article Influence: 71.5] [Reference Citation Analysis (0)] |
| 7. | Bajaj JS. The role of microbiota in hepatic encephalopathy. Gut Microbes. 2014;5:397-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 8. | Bajaj JS, Kassam Z, Fagan A, Gavis EA, Liu E, Cox IJ, Kheradman R, Heuman D, Wang J, Gurry T. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology. 2017;66:1727-1738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 351] [Cited by in F6Publishing: 376] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 9. | Verma S, Nakaoke R, Dohgu S, Banks WA. Release of cytokines by brain endothelial cells: A polarized response to lipopolysaccharide. Brain Behav Immun. 2006;20:449-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 185] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 10. | Luo M, Guo JY, Cao WK. Inflammation: A novel target of current therapies for hepatic encephalopathy in liver cirrhosis. World J Gastroenterol. 2015;21:11815-11824. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 38] [Cited by in F6Publishing: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Jayakumar AR, Rama Rao KV, Norenberg MD. Neuroinflammation in hepatic encephalopathy: mechanistic aspects. J Clin Exp Hepatol. 2015;5:S21-S28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 12. | Ahluwalia V, Betrapally NS, Hylemon PB, White MB, Gillevet PM, Unser AB, Fagan A, Daita K, Heuman DM, Zhou H. Impaired Gut-Liver-Brain Axis in Patients with Cirrhosis. Sci Rep. 2016;6:26800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 144] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 13. | Chiva M, Soriano G, Rochat I, Peralta C, Rochat F, Llovet T, Mirelis B, Schiffrin EJ, Guarner C, Balanzó J. Effect of Lactobacillus johnsonii La1 and antioxidants on intestinal flora and bacterial translocation in rats with experimental cirrhosis. J Hepatol. 2002;37:456-462. [PubMed] [Cited in This Article: ] |
| 14. | Liu Q, Duan ZP, Ha DK, Bengmark S, Kurtovic J, Riordan SM. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004;39:1441-1449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 444] [Cited by in F6Publishing: 426] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 15. | Bajaj JS, Saeian K, Christensen KM, Hafeezullah M, Varma RR, Franco J, Pleuss JA, Krakower G, Hoffmann RG, Binion DG. Probiotic yogurt for the treatment of minimal hepatic encephalopathy. Am J Gastroenterol. 2008;103:1707-1715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 16. | Rishi P, Bharrhan S, Singh G, Kaur IP. Effect of Lactobacillus plantarum and L-arginine against endotoxin-induced liver injury in a rat model. Life Sci. 2011;89:847-853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Scarpignato C, Pelosini I. Rifaximin, a poorly absorbed antibiotic: pharmacology and clinical potential. Chemotherapy. 2005;51 Suppl 1:36-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 162] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 18. | Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, Sigal S, Sheikh MY, Beavers K, Frederick T. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-1081. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 868] [Cited by in F6Publishing: 784] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 19. | Bajaj JS, Heuman DM, Sanyal AJ, Hylemon PB, Sterling RK, Stravitz RT, Fuchs M, Ridlon JM, Daita K, Monteith P. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS One. 2013;8:e60042. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 254] [Cited by in F6Publishing: 302] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 20. | Kang DJ, Kakiyama G, Betrapally NS, Herzog J, Nittono H, Hylemon PB, Zhou H, Carroll I, Yang J, Gillevet PM. Rifaximin Exerts Beneficial Effects Independent of its Ability to Alter Microbiota Composition. Clin Transl Gastroenterol. 2016;7:e187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 21. | Kimer N, Pedersen JS, Tavenier J, Christensen JE, Busk TM, Hobolth L, Krag A, Al-Soud WA, Mortensen MS, Sørensen SJ. Rifaximin has minor effects on bacterial composition, inflammation and bacterial translocation in cirrhosis; A randomized trial. J Gastroenterol Hepatol. 2017; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Bajaj JS, Wade JB, Sanyal AJ. Spectrum of neurocognitive impairment in cirrhosis: Implications for the assessment of hepatic encephalopathy. Hepatology. 2009;50:2014-2021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 234] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 23. | Kawaguchi T, Konishi M, Kato A, Kato M, Kooka Y, Sawara K, Endo R, Torimura T, Suzuki K, Takikawa Y. Updating the neuropsychological test system in Japan for the elderly and in a modern touch screen tablet society by resetting the cut-off values. Hepatol Res. 2017;47:1335-1339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Yaroustovsky M, Plyushch M, Popov D, Samsonova N, Abramyan M, Popok Z, Krotenko N. Prognostic value of endotoxin activity assay in patients with severe sepsis after cardiac surgery. J Inflamm (Lond). 2013;10:8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537-7541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14372] [Cited by in F6Publishing: 12842] [Article Influence: 856.1] [Reference Citation Analysis (0)] |
| 26. | Li W, Fu L, Niu B, Wu S, Wooley J. Ultrafast clustering algorithms for metagenomic sequence analysis. Brief Bioinform. 2012;13:656-668. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 315] [Cited by in F6Publishing: 292] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 27. | Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261-5267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12842] [Cited by in F6Publishing: 12169] [Article Influence: 715.8] [Reference Citation Analysis (0)] |
| 28. | Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9275] [Cited by in F6Publishing: 10634] [Article Influence: 966.7] [Reference Citation Analysis (0)] |
| 29. | Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, Guo J, Le Chatelier E, Yao J, Wu L. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513:59-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1230] [Cited by in F6Publishing: 1330] [Article Influence: 133.0] [Reference Citation Analysis (2)] |
| 30. | Fukui H. Gut-liver axis in liver cirrhosis: How to manage leaky gut and endotoxemia. World J Hepatol. 2015;7:425-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 31. | Nakamoto N, Schnabl B. Does the Intestinal Microbiota Explain Differences in the Epidemiology of Liver Disease between East and West? Inflamm Intest Dis. 2016;1:3-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Jiang ZD, DuPont HL. Rifaximin: in vitro and in vivo antibacterial activity--a review. Chemotherapy. 2005;51 Suppl 1:67-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 33. | Kimer N, Krag A, Møller S, Bendtsen F, Gluud LL. Systematic review with meta-analysis: the effects of rifaximin in hepatic encephalopathy. Aliment Pharmacol Ther. 2014;40:123-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 34. | Bajaj JS, Hylemon PB, Ridlon JM, Heuman DM, Daita K, White MB, Monteith P, Noble NA, Sikaroodi M, Gillevet PM. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol. 2012;303:G675-G685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 354] [Cited by in F6Publishing: 375] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 35. | Zhu Q, Zou L, Jagavelu K, Simonetto DA, Huebert RC, Jiang ZD, DuPont HL, Shah VH. Intestinal decontamination inhibits TLR4 dependent fibronectin-mediated cross-talk between stellate cells and endothelial cells in liver fibrosis in mice. J Hepatol. 2012;56:893-899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 36. | Marshall JC, Walker PM, Foster DM, Harris D, Ribeiro M, Paice J, Romaschin AD, Derzko AN. Measurement of endotoxin activity in critically ill patients using whole blood neutrophil dependent chemiluminescence. Crit Care. 2002;6:342-348. [PubMed] [Cited in This Article: ] |
| 37. | Wright G, Davies NA, Shawcross DL, Hodges SJ, Zwingmann C, Brooks HF, Mani AR, Harry D, Stadlbauer V, Zou Z. Endotoxemia produces coma and brain swelling in bile duct ligated rats. Hepatology. 2007;45:1517-1526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 156] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 38. | Görg B, Wettstein M, Metzger S, Schliess F, Häussinger D. Lipopolysaccharide-induced tyrosine nitration and inactivation of hepatic glutamine synthetase in the rat. Hepatology. 2005;41:1065-1073. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 39. | Jiang ZD, Ke S, Dupont HL. Rifaximin-induced alteration of virulence of diarrhoea-producing Escherichia coli and Shigella sonnei. Int J Antimicrob Agents. 2010;35:278-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 40. | Bajaj JS. Review article: potential mechanisms of action of rifaximin in the management of hepatic encephalopathy and other complications of cirrhosis. Aliment Pharmacol Ther. 2016;43 Suppl 1:11-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 41. | Mikx FH, Van der Hoeven JS. Symbiosis of Streptococcus mutans and Veillonella alcalescens in mixed continuous cultures. Arch Oral Biol. 1975;20:407-410. [PubMed] [Cited in This Article: ] |
| 42. | Esposito G, Nobile N, Gigli S, Seguella L, Pesce M, d’Alessandro A, Bruzzese E, Capoccia E, Steardo L, Cuomo R. Rifaximin Improves Clostridium difficile Toxin A-Induced Toxicity in Caco-2 Cells by the PXR-Dependent TLR4/MyD88/NF-κB Pathway. Front Pharmacol. 2016;7:120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 43. | Douhara A, Moriya K, Yoshiji H, Noguchi R, Namisaki T, Kitade M, Kaji K, Aihara Y, Nishimura N, Takeda K. Reduction of endotoxin attenuates liver fibrosis through suppression of hepatic stellate cell activation and remission of intestinal permeability in a rat non-alcoholic steatohepatitis model. Mol Med Rep. 2015;11:1693-1700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |