Published online Aug 15, 2002. doi: 10.3748/wjg.v8.i4.707
Revised: June 1, 2002
Accepted: June 3, 2002
Published online: August 15, 2002
AIM: Stress-activated signaling pathways responsible for hepatic ischemia reperfusion injury and their modulation by protective interventions are widely unknown. Preconditioning of rat livers with Atrial Natriuretic Peptide (ANP) attenuates ischemia reperfusion injury (Gerbes et al[21]Hepatology 1998, 28:1309-1317). Since ANP has recently been shown to be a regulator of the p38 MAPK pathway in endothelial cells (Kiemer et al[25]Circ Res 2002, 90:874-881), aim of this study was to investigate activities of MAPK during ischemia and reperfusion and effects of ANP on MAPK.
METHODS: Rat livers were perfused with KH-buffer in the presence or absence of ANP for 20 min, kept in cold UW solution for 24 h, and reperfused for up to 120 min. Activities of p38 MAPK and JNK was determined by in vitro phosphorylation assays using MBP and c-jun as substrates. After SDS/PAGE electrophoresis, gels were quantified by phosphorimaging.
RESULTS: Activity of p38 MAPK in control organs decreased in the course of ischemia and reperfusion by 85%, whereas ANP increased p38 activity by up to 30-fold. JNK activation of control livers increased in the course of ischemia and reperfusion by up to three-fold. This increase in JNK activity was slightly elevated in ANP preconditioned organs.
CONCLUSION: This work represents a systematic investigation of MAPK activation during liver ischemia and reperfusion. Employing ANP, for the first time a pharmacological approach to modulate these central signal transduction molecules is presented.
- Citation: Kiemer AK, Kulhanek-Heinze S, Gerwig T, Gerbes AL, Vollmar AM. Stimulation of p38 MAPK by hormal preconditioning with atrial natriuretic peptide. World J Gastroenterol 2002; 8(4): 707-711
- URL: https://www.wjgnet.com/1007-9327/full/v8/i4/707.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i4.707
Ischemia reperfusion (I/R) injury is the main cause of severe complications following liver transplantation[1,2]. Therefore, measures to reduce I/R injury are of highest clinical interest. However, there is still incomplete understanding of the pathomechanisms leading to I/R injury and of signalling pathways responsible for protective interventions.
Mitogen activated protein kinases (MAPK) are dually phosphorylated serine/threonine protein kinases (for review see[3,4]. They regulate an extensive range of cellular processes including gene transcription, cytoskeletal organization, metabolic homeostasis, cell growth, and apoptosis[3,4]. They represent signalling molecules which can be activated by various cellular stresses and cytokines and which therefore play a crucial role in the stress response[5]. Their role in the stress response, however, either detrimental or protective, seems to strongly depend on the system looked at[6].
In mammalian cells, three closely related parallel cascades of MAPK are known, the p38 MAPK, the c-jun N-terminal kinase (JNK), as well as the extracellular signal-regulated protein kinases p42/p44 (ERK)[4]. ERKs are critical regulators of gene transcription in cell proliferation and differentiation, while JNK and p38 pathways seem to be involved in cellular responses to environmental stresses and inflammatory cytokines[3].
Several groups have observed activation of MAPK in the course of warm ischemia and reperfusion[7-11] whereas there is only limited information on activation of MAPK after cold ischemic storage[12-14]. However, these reports show controversial data on the activation pattern of MAPK.
Since recent data show that protective preconditioning strategies, such as whole animal heat shock[15,16] or short time hypoxic[17] or ischemic preconditioning[18,19] significantly activate stress-activated MAPKs, these kinases are suggested as central signal transduction pathways mediating protection from I/R injury. Therefore, we felt that a systematic investigation of MAPK activation after cold ischemic storage should lead to a more complete understanding of their role in I/R injury. Moreover, the potential pharmacological modulation of MAPK by the hepatoprotective Atrial Natriuretic Peptide (ANP) should be investigated. This cardiovascular hormone has previously been shown to attenuate I/R injury after both warm[20] and cold[21] ischemia of the rat liver. Protection conveyed by ANP involves the attenuated activation of pro-inflammatory transcription factors and the reduced expression of TNF-α[22] as well as induction of heat shock proteins[23]. The initial signalling events responsible for the protective potential of hormonal preconditioning by ANP, however, are still unknown. ANP has recently been reported by us and others as a regulator of the p38 MAPK cascade[24,25]. By investigating an effect of ANP on MAPK activation, an information on signalling events potentially involved in liver protection should be obtained.
Rat ANP 99-126 was purchased from Calbiochem/Novabiochem, Bad Soden, Germany. [γ32P]-ATP (3000 Ci/mmol) was from Amersham Pharmacia (Braunschweig, Germany); Complete® was from Roche (Heidelberg, Germany). Recombinant c-jun 1-79, polyclonal rabbit anti-p38, and anti-JNK antibodies were purchased from Calbiochem-Novabiochem (Bad Soden, Germany). Protein A-agarose, myelin basic protein, and all other materials were from Sigma, Deisenhofen, Germany.
Liver perfusion Male Sprague-Dawley rats weighing 250-300 g were purchased from SAVO (Kisslegg, Germany) and housed in a climatized room with a 12-hour light-dark cycle. The animals had free access to chow (Standard-Diet, Altromin 1314 Lage, Germany) and water up to the time of the experiments. After anaesthetizing the animals with pentobarbital (50 mg/kg body weight, intraperitoneally), the portal vein was cannulated and the livers were perfused in situ with hemoglobin-free and albumin-free, bicarbonate-buffered Krebs-Henseleit (KH) solution (pH7.4, 37 °C) gassed with 95% O2 and 5% CO2. The perfusion medium was pumped through the livers with a membrane pump at a constant flow rate of 3.0-3.5 mL × min-1× g liver-1 in a non-recirculating fashion. After 10 min controlling the stability of the system, ANP (200 nM) was added to the perfusate for 20 min, livers were then perfused with 30 mL of cold (4 °C) University of Wisconsin (UW) solution for 1 min. Then the organs were kept in 150 mL UW solution at 4 °C for 24 h. Following the period of ischemia, livers of each group were reperfused with KH buffer for 2 h. At the indicated times, i.e. before ischemia, at the end of ischemia and after 45 and 120 min of reperfusion livers were snap-frozen and stored at -85 °C until further analysis. Five independent experiments were performed.
The "Principles of laboratory animal car" (NIH publication No 86-23, revised 1985) as well as the German Law on the Protection of Animals were followed. The study was registered with the local animal welfare committee.
Immunoprecipitation and in vitro phosphorylation assay Tissue lysates were prepared from frozen liver sections. Briefly, tissue samples (100 μg) were homogenized in ice-cold lysis buffer (containing 2 mM EDTA, 137 mM NaCl, 10% glycerol, 2 mM tetrasodium pyrophosphate, 20 mM Tris, 1% Triton® X-100, 20 mM sodium glycerophosphate, 10 mM sodium fluoride, 2 mM sodium vanadate, 1 mM PMSF, 1 × Complete® with a dounce homogenizer, and centrifuged at 11180 ×g for 10 min at 4 °C. Aliquots of the supernatant were taken for determination of protein concentrations and the tissue extract was frozen at -85 °C. The protein concentrations were estimated after the method of Pierce. Equal amounts of protein were incubated with the respective antibody (1.5 μL of anti-p38, 3 μL of anti-JNK; polyclonal rabbit antibodies, Calbiochem-Novabiochem, Bad Soden, Germany) shaking for 2 h. Afterwards immunoprecipitation was performed with protein A agarose (5 μL) shaking overnight at 4 °C. After centrifugation (11180 ×g, 4 min, 4 °C) the precipitates were washed three times with lysis buffer and once with kinase buffer (containing 20 mM Hepes pH7.5, 20 mM MgCl2, 25 mM sodium glycerophosphate, 100 μM sodium vanadate, 2 mM DTT). Immunoprecipitates were resuspended in 20 μL of kinase buffer, 3 μL of substrate solution (1 mg/300 μL MBP for p38, Sigma, Deisenhofen, Germany and 1 mg/mL of recombinant c-jun 1-79, Calbiochem-Novabiochem, Bad Soden, Germany) and a 10 μL volume of ATP mix was added, containing kinase buffer with 10 mCi/mL [γ32P]-ATP, (3000 Ci/mmol, Amersham, Braunschweig, Germany), 5 mM ATP and 2 M MgCl2. The reaction mixture was incubated at 30 °C for 20 min shaking. Phosphorylation was stopped by the addition of 6 μL 5 × Laemmli buffer and heating for 3 min at 90 °C. 30 μL of the reaction mixture were resolved in a 12% (MBP) or 15% (c-jun) SDS polyacrylamide gel in a Laemmli system at 200 V. Band intensities were quantified by phosphorimaging (Packard, Meriden, USA). Ratio of digital light units (DLU) of respective values vs controls were determined.
Data are expressed as means ± SEM of three to five independent experiments. A P value of < 0.05 was considered significant (unpaired student's t test, Graph Pad Prism, version 3.02).
At the end of cold ischemic storage for 24 h rat livers displayed a significantly reduced activation of p38 MAPK activity (Figure 1). The p38 activity even further decreased in the course of reperfusion and at the end of the 120 min reperfusion period displayed only 15% of pre-ischemic values (Figure 1).
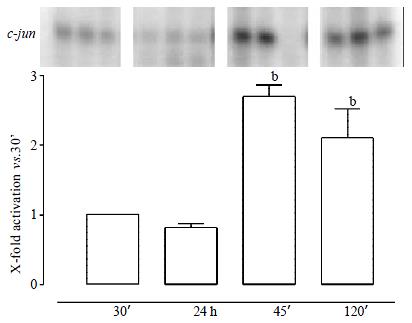
The c-jun N-terminal kinase (JNK) was activated in the reperfusion period (up to 3-fold). No increase of c-jun phosphorylation could be measured at the end of ischemia (Figure 2).
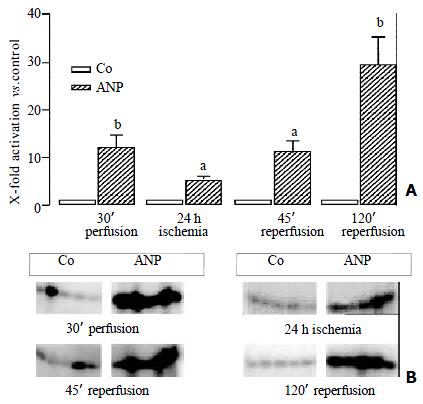
When rat livers were pre-conditioned by adding 200 nM of ANP to the pre-ischemic perfusion buffer the livers displayed a completely different pattern of MAPK activities. At the end of the preconditioning period ANP treated organs displayed a tremendous increase in p38 activity by 12-fold (Figure 3). In the course of the experiment the p38 activity even further increased compared to the respective control organs (Figure 3).
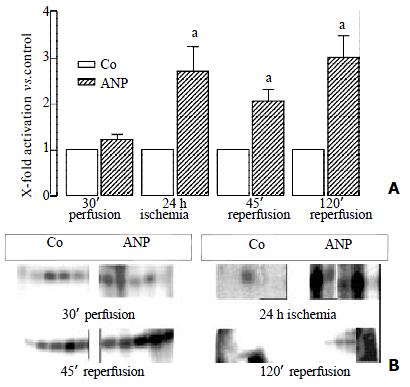
ANP did not affect JNK activity during preconditioning. However, ANP-pretreated organs showed elevated post-ischemic JNK activities (Figure 4).
This work represents a systematic characterization of the stress-activated p38 MAPK and JNK activities during ischemia and reperfusion of the isolated perfused rat liver. The following results were obtained: (I) p38 shows a decreasing activity in the whole course of ischemia and reperfusion whereas (II) JNK is only activated during reperfusion. (III) Even more importantly, our paper reports for the first time that preconditioning with Atrial Natriuretic Peptide exerts a tremendous increase of p38 MAPK activation but shows no effect on JNK.
Our data on the time course of MAPK activation during cold ischemia and reperfusion adds further information to the increasing discussion on the role of p38 MAPK during I/R. Decreased p38 activity at the end of cold ischemia was also seen by Iesalnieks et al[14] using a rat liver transplantation model, but not in the work of the Brenner group which reported no change of p38 activity[12]. After transplantation activation[14] as well as no effect on p38 MAPK[12] has been demonstrated. Since p38 is referred to as a stress-induced MAPK[5] the decline of p38 MAPK activities we observed in ischemia as well as reperfusion might surprise. However, p38 activity might not solely reflect a stress response, but also mediate the induction of protective mechanisms.
For JNK convincing evidence is available that this MAPK is stimulated in the course of reperfusion. The data presented here are supported by several other observations of increased activity of JNK during reperfusion for models of both cold[12,14] and warm[7] ischemic livers.
Taken together, MAPK are affected by I/R. The differences between our data and other work most likely reflect the different experimental set-up and therefore different basal MAPK activities and time dependency of activation.
The most important question arising from this discussion is the role of MAPKs in I/R injury. To address this question known protective strategies have to be examined for their effect on MAPKs during I/R. Up to now, these kinds of studies in the liver are quite rare. The outcome of our work, namely that preconditioning with ANP leads to a striking increase of p38 MAPK activities but is without effect on JNK, is therefore of great importance. Both hyperthermic[26] as well as ischemic[18,19] preconditioning have also been shown to represent protective strategies against I/R injury. Both JNK and p38 MAPK were reported to be activated by hyper thermia[15,18] and a short time ischemic period of 10 min as used for ischemic preconditioning[18,19] was shown to activate JNK[8].
Our data point to p38 MAPK activation as a central signal transduction pathway of hepatoprotection during I/R. Hypoxic preconditioning of hepatocytes has recently been shown to also activate p38 MAPK[17], thus supporting our assumption. For the heart, in fact, activation of p38 is reported to be a key signal in protection by ischemic preconditioning[27]. Moreover, the hepatoprotective action of CO has very recently been reported to be exerted via p38 MAPK activation[28].
ANP must be administered at least 20 min before ischemia in order to protect against I/R injury of the rat liver[21]. Thus, the strong activation of p38 MAPK at the end of the preconditioning period i.e. before is chemia is suggested to be the crucial step in mediating hepatoprotection by ANP.
The meaning of increased MAPK activities by ANP at the end of reperfusion periods is less easy to explain. The augmented MAPK activities might simply reflect the increased number of vital hepatocytes in ANP preconditioned liver as compared to untreated tissue.
In summary, our data represent a systematic investigation of stress-activated MAPK in the course of ischemia and reperfusion. Second and even more importantly the pharmacological activation of MAPK as a mediator of preconditioning was achieved by the administration of the cardiovascular hormone ANP. The observation that protective strategies such as pre-treatment with ANP profoundly interact with the MAPK signalling cascade suggests them as crucial mediators of preconditioning.
We thank Ingrid Li B for excellent technical assistance.
Edited by Zhang JZ
| 1. | Bilzer M, Gerbes AL. Preservation injury of the liver: mechanisms and novel therapeutic strategies. J Hepatol. 2000;32:508-515. [PubMed] [Cited in This Article: ] |
| 2. | Jaeschke H. Preservation injury: mechanisms, prevention and consequences. J Hepatol. 1996;25:774-780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 109] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Cobb MH. MAP kinase pathways. Prog Biophys Mol Biol. 1999;71:479-500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 637] [Cited by in F6Publishing: 625] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 4. | Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143-180. [PubMed] [Cited in This Article: ] |
| 5. | Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807-869. [PubMed] [Cited in This Article: ] |
| 6. | Cross TG, Scheel-Toellner D, Henriquez NV, Deacon E, Salmon M, Lord JM. Serine/threonine protein kinases and apoptosis. Exp Cell Res. 2000;256:34-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 519] [Cited by in F6Publishing: 516] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 7. | Bendinelli P, Piccoletti R, Maroni P, Bernelli-Zazzera A. The MAP kinase cascades are activated during post-ischemic liver reperfusion. FEBS Lett. 1996;398:193-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 56] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Onishi I, Tani T, Hashimoto T, Shimizu K, Yagi M, Yamamoto K, Yoshioka K. Activation of c-Jun N-terminal kinase during ischemia and reperfusion in mouse liver. FEBS Lett. 1997;420:201-204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Zwacka RM, Zhang Y, Zhou W, Halldorson J, Engelhardt JF. Ischemia/reperfusion injury in the liver of BALB/c mice activates AP-1 and nuclear factor kappaB independently of IkappaB degradation. Hepatology. 1998;28:1022-1030. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 151] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Onishi I, Shimizu K, Tani T, Hashimoto T, Miwa K. JNK activation and apoptosis during ischemia-reperfusion. Transplant Proc. 1999;31:1077-1079. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Crenesse D, Gugenheim J, Hornoy J, Tornieri K, Laurens M, Cambien B, Lenegrate G, Cursio R, De Souza G, Auberger P. Protein kinase activation by warm and cold hypoxia- reoxygenation in primary-cultured rat hepatocytes-JNK (1)/SAPK (1) involvement in apoptosis. Hepatology. 2000;32:1029-1036. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Bradham CA, Stachlewitz RF, Gao W, Qian T, Jayadev S, Jenkins G, Hannun Y, Lemasters JJ, Thurman RG, Brenner DA. Reperfusion after liver transplantation in rats differentially activates the mitogen-activated protein kinases. Hepatology. 1997;25:1128-1135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 169] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Bradham CA, Schemmer P, Stachlewitz RF, Thurman RG, Brenner DA. Activation of nuclear factor-kappaB during orthotopic liver transplantation in rats is protective and does not require Kupffer cells•L. iver Transpl Surg. 1999;5:282-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Iesalnieks I, Rentsch M, Lengyel E, Mirwald T, Jauch K, Beham A. JNK and p38MAPK are activated during graft reperfusion and not during cold storage in rat liver transplantation. Transplant Proc. 2001;33:931-932. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Bendinelli P, Piccoletti R, Maroni P, Bernelli-Zazzera A. The liver response to in vivo heat shock involves the activation of MAP kinases and RAF and the tyrosine phosphorylation of Shc proteins. Biochem Biophys Res Commun. 1995;216:54-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Maroni P, Bendinelli P, Zuccorononno C, Schiaffonati L, Piccoletti R. Cellular signalling after in vivo heat shock in the liver. Cell Biol Int. 2000;24:145-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Carini R, De Cesaris MG, Splendore R, Vay D, Domenicotti C, Nitti MP, Paola D, Pronzato MA, Albano E. Signal pathway involved in the development of hypoxic preconditioning in rat hepatocytes. Hepatology. 2001;33:131-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Fung JJ. Ischemic preconditioning: application in clinical liver transplantation. Liver Transpl. 2001;7:300-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Arai M, Thurman RG, Lemasters JJ. Ischemic preconditioning of rat livers against cold storage-reperfusion injury: role of nonparenchymal cells and the phenomenon of heterologous preconditioni ng•L. iver Transpl. 2001;7:292-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Bilzer M, Witthaut R, Paumgartner G, Gerbes AL. Prevention of ischemia/reperfusion injury in the rat liver by atrial natriuretic peptide. Gastroenterology. 1994;106:143-151. [PubMed] [Cited in This Article: ] |
| 21. | Gerbes AL, Vollmar AM, Kiemer AK, Bilzer M. The guanylate cyclase-coupled natriuretic peptide receptor: a new target for prevention of cold ischemia-reperfusion damage of the rat liver. Hepatology. 1998;28:1309-1317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Kiemer AK, Vollmar AM, Bilzer M, Gerwig T, Gerbes AL. Atrial natriuretic peptide reduces expression of TNF-alpha mRNA during reperfusion of the rat liver upon decreased activation of NF-kappaB and AP-1. J Hepatol. 2000;33:236-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Kiemer AK, Gerbes AL, Bilzer M, Vollmar AM. The atrial natriuretic peptide and cGMP: novel activators of the heat shock response in rat livers. Hepatology. 2002;35:88-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Tsukagoshi H, Shimizu Y, Kawata T, Hisada T, Shimizu Y, Iwamae S, Ishizuka T, Iizuka K, Dobashi K, Mori M. Atrial natriuretic peptide inhibits tumor necrosis factor-alpha production by interferon-gamma-activated macrophages via suppression of p38 mitogen-activated protein kinase and nuclear factor-kappa B activation. Regul Pept. 2001;99:21-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Kiemer AK, Weber NC, Fürst R, Bildner N, Kulhanek-Heinze S, Vollmar AM. Inhibition of p38 MAPK activation via induction of MKP-1: atrial natriuretic peptide reduces TNF-alpha-induced actin polymerization and endothelial permeability. Circ Res. 2002;90:874-881. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 147] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 26. | Terajima H, Enders G, Thiaener A, Hammer C, Kondo T, Thiery J, Yamamoto Y, Yamaoka Y, Messmer K. Impact of hyperthermic preconditioning on postischemic hepatic microcirculatory disturbances in an isolated perfusion model of the rat liver. Hepatology. 2000;31:407-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Nakano A, Cohen MV, Downey JM. Ischemic preconditioning: from basic mechanisms to clinical applications. Pharmacol Ther. 2000;86:263-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 112] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 28. | Amersi F, Shen XD, Anselmo D, Melinek J, Iyer S, Southard DJ, Katori M, Volk HD, Busuttil RW, Buelow R. Ex vivo exposure to carbon monoxide prevents hepatic ischemia/reperfusion injury through p38 MAP kinase pathway. Hepatology. 2002;35:815-823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 183] [Article Influence: 8.3] [Reference Citation Analysis (0)] |