Published online Dec 15, 2014. doi: 10.4239/wjd.v5.i6.868
Revised: September 23, 2014
Accepted: October 14, 2014
Published online: December 15, 2014
Type-2 diabetes mellitus (T2DM) plays a central role in the development of cardiovascular disease (CVD). However, its relationship to epicardial adipose tissue (EAT) and pericardial adipose tissue (PAT) in particular is important in the pathophysiology of coronary artery disease. Owing to its close proximity to the heart and coronary vasculature, EAT exerts a direct metabolic impact by secreting proinflammatory adipokines and free fatty acids, which promote CVD locally. In this review, we have discussed the relationship between T2DM and cardiac fat deposits, particularly EAT and PAT, which together exert a big impact on the cardiovascular health.
Core tip: Diabetes, a cardiovascular disease equivalent, has considerable effects on the cardiovascular system. Its impact works systemically, but may have more association with epicardial and pericardial adipose tissue locally at the level of the heart. These cardiac tissues have great interplay with diabetic patients and have potential to influence cardiovascular disease.
- Citation: Noyes AM, Dua K, Devadoss R, Chhabra L. Cardiac adipose tissue and its relationship to diabetes mellitus and cardiovascular disease. World J Diabetes 2014; 5(6): 868-876
- URL: https://www.wjgnet.com/1948-9358/full/v5/i6/868.htm
- DOI: https://dx.doi.org/10.4239/wjd.v5.i6.868
More than 25 million United States adults have type-2 diabetes mellitus (T2DM) and this figure will likely reach 50 million by 2050[1,2]. The relationship between metabolic diseases such as T2DM and regional fat deposits, particularly epicardial adipose tissue (EAT) and pericardial adipose tissue (PAT), play an important role in the development of cardiovascular diseases (CVD). Both EAT and PAT are a subset of visceral adipose tissue (VAT) associated with T2DM. They are metabolically active visceral fat deposits found around the heart[3], that are strongly associated with CVD including coronary artery disease (CAD) and the development of cardiac arrhythmias, predominantly due to the secretion of pro-inflammatory mediators and cytokines[4]. In this paper, we review the emerging evidence of impact of T2DM on VAT and the specific role of EAT and PAT both as a cardiac risk marker and as a potentially active player in the development of cardiovascular pathology.
We searched MEDLINE and PubMed for original articles published between 1984 and 2014, focusing on epicardial adipose tissue and type 2 diabetes mellitus. The search terms we used, alone or in combination, were “epicardial fat”, “epicardial adipose tissue”, “pericardial fat”, “pericardial adipose tissue”, “insulin resistance”, “type 2 diabetes mellitus”, “metabolic syndrome”, “cardiovascular disease”, “coronary artery disease”, “congestive heart failure”, and “atrial fibrillation”, which yielded 121 articles. All articles identified were English-language, full-text papers and abstracts. We finally selected 87 articles, which were relevant to our current discussion.
Cardiac disease is the leading cause of death in T2DM, and many have sought to determine the mechanism of development of cardiac dysfunction[5]. Interestingly, diabetic patients with no evidence of CAD or hypertension have also been found with cardiac abnormalities, even when they are asymptomatic. Studies have shown that the metabolic derangements in T2DM primarily contribute to the cardiac problems[6], which, in part, are due to increase in visceral fat deposits and being frequently accompanied by disorders of glucose metabolism[7]. Obesity, specifically abdominal VAT, is an independent risk factor for CVD[8], and is prominent in patients with T2DM[7]. Moreover, studies have shown the correlation between excessive adipose tissue deposition and development of diabetes[9]. Central and VAT is associated with endocrine disorders due to the release of substances such as free fatty acids (FFA), leptin, adiponectin, pro-inflammatory agents, and decreased anti-inflammatory factors. As a result, it often results in unfavorable glucose metabolism and T2DM[10,11]. It has also been well demonstrated that pre-diabetic and diabetic patients are associated with significantly higher PAT burden compared to normoglycemic patients[12]. In a cross sectional study, the impact of obesity and T2DM on adipocytokines (adiponectin, leptin and resistin), inflammatory markers [tumor necrosis factor-α (TNF-α), Interleukin (IL)-6 and high sensitive C-reactive protein (HsCRP)] were evaluated[13]. Obesity was found to significantly lower adiponectin levels, while increasing leptin and IL-6 levels along with HsCRP. There is also a strong association between the increased expression of resistin, another adipocyte-secreted factor, and insulin resistance[14], with the burden of EAT volume being greater in individuals with metabolic syndrome, increased insulin resistance and diabetes mellitus[15,16], and is significantly higher in patients with T2DM than in non-diabetic subjects[4]. The serum profile of coronary artery bypass grafting patients showed significantly higher levels of HsCRP and lower levels of adiponectin compared to body mass index (BMI)-matched controls, supporting the role of VAT in causation of systemic inflammation[17]. Adiponectin has been shown to have a protective role with anti-inflammatory properties suppressing TNF-α and IL-6[13,18]. Hypoadiponectin levels in obesity along with elevated TNF-α, HsCRP and IL-6 were shown to correlate with insulin resistance seen in this population[13]. Interestingly leptin and resistin levels were not shown to consistently correlate with insulin resistance.
EAT and omental fat were shown to have broadly comparable pathogenic mRNA profile[17]. EAT and PAT are both forms of VAT, which store lipids and have demonstrated increased expression of the above mentioned hormones, chemokines and cytokines, with the addition of monocyte chemotactic protein-1 and IL-1β[19]. These adipokines also impair insulin-signaling pathways leading to insulin resistance and reduced nitric oxide (NO) synthesis, causing unopposed vasoconstriction[20]. Thus, the endocrine function of EAT and PAT play a significant role in patients with metabolic syndrome. In fact, the examination of EAT and PAT found that PAT is associated with VAT and metabolic syndrome features such as T2DM, than that of EAT[21]. On the other hand, EAT thickness showed independent positive correlation with metabolic parameters including postprandial glucose (P = 0.049), HbA1c level (P < 0.001), and homeostasis model assess of insulin resistance (P = 0.047)[22]. EAT accumulation was seen to strongly correlate with serum fibroblast growth factor 21, which is known to improve insulin sensitivity despite an increment in its serum levels in T2DM patients. Thus, excessive EAT in T2DM patients may exert bivalent, unfavorable and adaptive effects on progression of cardiovascular diseases[23].
In obese patients with T2DM, adipocytes from epicardial fat infiltrate the myocardium, which refers to a strong association of intra-myocardial fat content to the echocardiographic epicardial fat thickness. Similarly, EAT has been found to be significantly related to intra-abdominal visceral fat, suggested by echocardiographic studies[24,25], and PAT may increase up to 400 g in T2DM patients (with 100 g in healthy lean people)[26]. Yang et al[12] demonstrated the burden of PAT in diabetic and pre-diabetic subjects, revealing that PAT volume was much higher in pre-diabetics and diabetics as compared to normoglycemic subjects.
However, it is important to distinguish EAT and PAT from obesity-specific lipotoxic cardiomyopathy, in which excessive fat proliferates inside cardiac muscle causing left ventricular remodeling and eventually cardiomyopathy. This develops after subcutaneous adipose tissues and VAT are unable to accommodate the excess fat in the obese patients leading to intracellular accumulation of lipids and FFA, eventually forming myocardial steatosis[27].
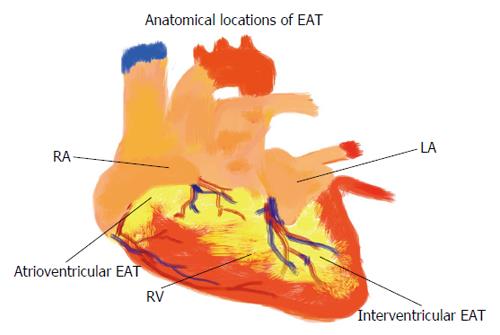
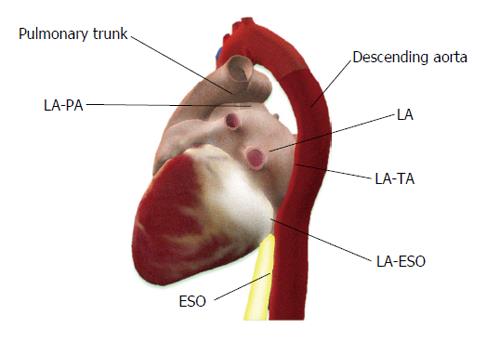
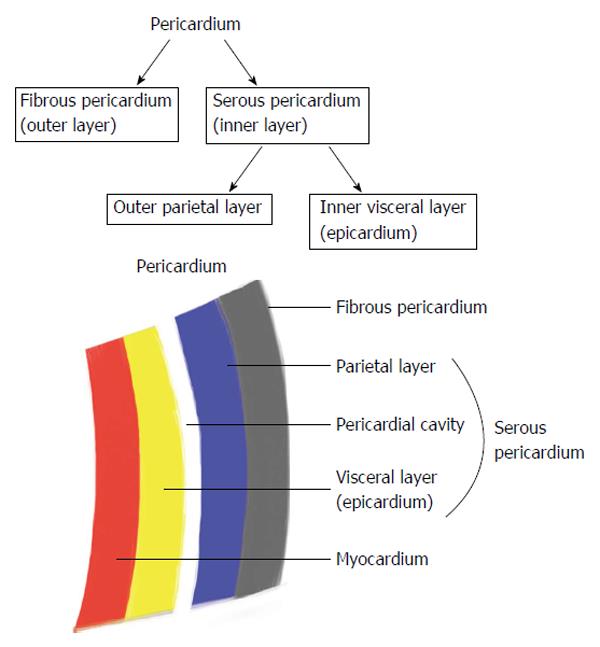
Epicardial and pericardial adipose tissue are close, however anatomically clearly different. EAT is not symmetrically distributed around the heart (Figure 1). EAT volume and thickness varies depending on the location (Figure 2). PAT (Figure 3) has a different embryonic origin than that of EAT as it originates from the embryonic primitive thoracic mesenchyme[24], and clinically are different. In the existing literature, the terminologies have often been erroneously overlapped without clear differentiation between these two entities. Some suggest the use of a terminology, which encompasses three types of fat around the heart: epicardial, pericardial and paracardial fats. In this terminology, paracardial fat often refers to the fat located on the external surface of the parietal pericardium, while the term pericardial fat is used to represent EAT plus paracardial fat. It is important to be familiar with these terms to avoid confusion. In our opinion, it is rather more important to differentiate the “true pericardial fat” from “paracardial fat” as these two have different endocrine and metabolic properties. The true pericardial fat (epi-pericardial fat) should encompass the epicardial and pericardial fat (i.e., fat located above the myocardium and up to the parietal pericardium; epicardial fat being located between the outer wall of the myocardium and the visceral layer of pericardium and pericardial fat being located between the visceral and the parietal pericardium), while paracardial fat should clearly be considered as the fat located outside the parietal pericardium.
EAT is a metabolically active visceral fat deposit found around the heart, between the pericardium and myocardium[3]. EAT can be found in highest concentration in the atrioventricular and interventricular grooves and alongside the coronary arteries, and lesser so around the atria, over the free wall of the right ventricle and over the apex of the left ventricle. PAT may be defined as EAT plus paracardial fat, whereas paracardial fat is located on the external surface of the parietal pericardium within the mediastinum[28]. EAT varies from PAT and other local fat depots in the size of its adipocytes, where as epicardial adipocytes are smaller in size and high in number (high number of pre-adipocytes). The best imaging tool for quantification of both EAT and PAT remains uncertain. Their thicknesses and volumes can be evaluated by echocardiography, computed tomography (CT) or magnetic resonance imaging (MRI)[24,29]. Due to distinct attenuation values of fat on chest or cardiac CT and MRI, EAT and PAT are both readily identified with ability to calculate the tissue volume and thickness. Furthermore, MRI accurately correlates with EAT and PAT seen on echocardiography imaging[30].
Biochemically, EAT and PAT are different. Investigation into EAT and PAT suggests that these two tissues have different metabolic and physiologic properties[31]. Under physiological situations, EAT is cardioprotective which can be explained by its anti-atherogenic/anti-inflammatory properties, high FFA release and uptake and low glucose requirements, serving as a major source of energy to the heart and thermoregulatory properties[32]. It is also known to provide mechanical support to the coronary arteries as well as anti-toxic effects by protecting heart from high levels of FFA. In diabetics, lack of insulin impairs cardiac glucose transport and oxidation, resulting in FFA becoming the preferred means of energy supply[33]. To make available this increased requirement of the heart for FFA, the diabetic heart upregulates its luminal lipoprotein lipase (LPL) activity, which can result in abnormal FFA supply and utilization by the heart tissue, potentially initiating cardiac dysfunction[33]. Importantly, EAT has low levels of LPL and acetyl-CoA as compared to subcutaneous fat[34], though the cardio-protective role of PAT is not clear[31]. Despite these protective qualities, EAT in excess can become cardio-toxic resulting in local inflammatory changes and cardiac dysfunction[32,35]. In non-diabetic patients with excessive EAT, the presence of fatty acid binding protein-4 in epicardial adipocytes, and its increased expression, promotes the development of metabolic syndrome[32] and T2DM.
PAT and EAT have firmly been recognized as a contributor to the development of CAD[36-41], and several cross sectional studies (Table 1) have shown similar results. PAT is emerging as a novel risk factor for CVD development[42] and progression[43], as CAD has been shown to correlate with PAT more consistently than other general measures of adiposity like body mass index or waist circumference[42]. PAT volume has been a predictor of increased death and disability for CVD[44], and independently linked with coronary artery calcification (CAC)[45]. EAT has also been shown to correlate with CAC[43] and has a statistically significant correlation between EAT and CAC in both diabetic and non-diabetic patients (P = 0.01, r = 0.60; P = 0.02, r = 0.38, respectively)[46]. The Multi-Ethnic Study of Atherosclerosis study showed a stronger correlation between PAT and the incidence of future coronary heart events in a group of patients without history of CAD, than that of other cardiac risk factors such as BMI or waist circumference[42].
| Ref. | Year | Diagnostic modality | Results |
| Taguchi et al[86] | 2001 | Computerized tomogram | Pericardial fat was the strongest independent variable for severity of CAD, determined by coronary angiogram |
| Jeong et al[41] | 2007 | Echocardiogram | Epicardial fat thickness significantly correlated with the severity of CAD in patients with known CAD |
| Ahn et al[38] | 2008 | Echocardiogram | Epicardial adipose tissue was an independent predictor of CAD |
| Greif et al[36] | 2009 | Computerized tomogram | Patient with any coronary plaque showed a significantly higher pericardial adipose tissue volume compared to patients without coronary plaques |
| Shemirani et al[40] | 2012 | Echocardiogram | Confirms the presence of association between epicardial fat thickness and severity of CAD |
EAT has been studied more extensively than PAT. EAT differs from PAT, not only in its location, but also by its blood supply. EAT derives its blood supply from coronary circulation, whereas PAT is supplied by non-coronary sources[32]. There is a functional and anatomic relationship between EAT and muscular components of the heart as these components share the same coronary blood supply, due to the lack of fascia separating the adipose tissue and myocardial layers[3]. Because of the highly metabolic paracrine and endocrine functions of EAT, it has been proposed to play a role in the pathogenesis of CVD by contributing to increased carotid intima media thickness (CIMT) in those with metabolic syndrome[47], CAD[37-41], increased left ventricle (LV) mass[48] and diastolic dysfunction[49,50]. The release of pro-inflammatory and pro-atherogenic factors into the circulation advancing CVD is more significantly linked to VAT accumulation, metabolic syndrome and other situations related to oxidative stress[32]. Pathophysiological effects of abnormal EAT may be explained by the expression of an enzyme-sPLA2-IIA which is generally found in human atherosclerotic lesions[32]. In patients with CAD, catalase levels in EAT are lower than in subcutaneous fat resulting in higher oxidative stress, which further contributes to atherosclerosis.
It is the close anatomical relationship between EAT and the coronary arteries, combined with its biologically active properties that participates in the pathogenesis of diabetic coronary atherosclerosis[4,51]. Iacobellis et al[52] demonstrated that the expression of anti-inflammatory and antiatherogenic properties of adiponectin was approximately 40% lower in the EAT of patients with CAD than in that of normal controls.
Apart from above, EAT was also shown to play an important role in the prediction of no-reflow phenomenon in ST elevation myocardial infarction treated with primary percutaneous intervention (PCI)[53]. The no-reflow was defined as < 70% ST-segment resolution following primary PCI. EAT has also been shown to be one of the independent factors associated with restenosis post-stenting warranting target vessel revascularization[54]. Smooth muscle proliferation, secondary to the local inflammatory mediators, have been postulated as mechanism of restenosis in this population[54].
EAT volume also has a significant role in promoting CVD and was shown to be positively and independently related to coronary atherosclerotic burden[55], and was significantly increased in patients with acute coronary syndrome[14]. Multivariate logistic regression analysis indicated that EAT thickness was an independent indicator for significant coronary artery stenosis after adjusting for traditional risk factors (OR = 1.403, P = 0.026)[22] assessed by cardiovascular magnetic resonance imaging in asymptomatic T2DM patients. Echocardiographic measurement of EAT thickness ≥ 7 mm was shown to identify individuals with higher probability of coronary atherosclerosis[56]. Furthermore, EAT thickness ≥ 5 mm in general population may identify individuals with higher likelihood of detectable carotid atherosclerosis, but did not have any significant association with CIMT[57]. However, EAT thickness in patients with metabolic syndrome showed a linear positive correlation with CIMT[47]. Similar association was also found in human immunodeficiency virus receiving highly active antiretroviral therapy[58]. These studies establish that the correlation between EAT and CIMT is stronger in high-risk individuals prone to atherosclerosis than in the general population. It also demonstrates the existence of independent paracrine effects in addition to the endocrine effect, to account for the consistent association of EAT and coronary atherosclerosis[59].
EAT and associated inflammatory cytokines, particularly hypoadiponectin levels and reduced NO synthesis, may have direct effect on myocardium causing dysfunction independent of ischemic pathophysiology[60]. PAT was shown to be significantly associated with LV diastolic dysfunction in people with CAD and normal ejection fraction independent of other risk factors including diabetes and hypertension[61]. Variation in regional fat distribution has been reported in patients on peritoneal dialysis[62]. Increased EAT thickness determined by echocardiogram in such patients was shown to be the most powerful determinant of LV diastolic dysfunction among other variables[63]. In addition to the paracrine metabolic effect as discussed earlier, mechanical effect of increased PAT has also been shown to contribute to the pathophysiology of diastolic dysfunction[63]. Additionally, patients with LV diastolic dysfunction had significantly increased EAT volumes[64].
On contrary, in patients with congestive heart failure (CHF) and severely reduced left ventricular ejection fraction (LVEF), EAT has been found to be significantly reduced[65]. LV function in such patients correlated best with EAT/Left Ventricular Remodeling Index ratio[65], raising a possible protective role of EAT to remodeling myocardium. Khawaja et al[66] demonstrated similar results with a stepwise decrease in EAT volume from controls to patients with moderate CHF (LVEF 35%-55%) and severe heart failure (LVEF < 35%). Though the paracrine metabolic effects and possible role as source of FFA to myocardium in demand has been postulated as mechanism for this correlation[65], the exact pathophysiology remains elusive. Further study is needed to access the possible confounding role of lipid lowering therapies to this finding in such patients.
Obesity is a well-established risk factor for atrial fibrillation (AF), as altered atrial electrical function is considered an important mechanism for the relation of obesity and increased AF risk. Atrial tissue in diabetic subjects demonstrates persistent oxidative stress compared with nondiabetics; which can potentially play a role in the development of interatrial conduction delay[67]. Evidence on the impact of EAT thickness, particularly in the area of posterior left atrium, is associated with persistent AF[68,69]. PAT is also associated with a higher incidence of AF, both paroxysmal (OR = 1.11, 95%CI: 1.01-1.23, P = 0.04) and persistent (OR = 1.18, 95%CI: 1.05-1.33, P = 0.004), independent of other risk factors[69]. PAT’s unique anatomic proximity to the myocardium and atrial conduction system may modify atrial electrophysiology and promote subsequent risk for arrhythmogenesis[70]. Based on PAT’s influence on altered P-wave indices (PWI), potential mechanisms by which increases in PAT may lead to changes in atrial conduction include prolonged atrial depolarization, diminished voltage, and heterogeneous atrial activation related to fibrosis, hypertrophy, and fatty myocardial infiltration[70].
Two independent studies reported significant association of pericardial fat volume with AF both paroxysmal and persistent even after adjustment for traditional risk factors[69,71]. The possible mechanisms speculated were secondary to increase in left atrial size associated with pericardial fat[72,73] and local inflammatory effects induced by pericardial adipose tissue as discussed earlier via paracrine and endocrine route. This speculation was based on the evidence that systemic inflammation marked by CRP was associated with presence of AF and also predicted the patients at risk for future development of AF[74].
PWI and PAT were found to be associated independent of ectopic visceral and intra-thoracic fat depots[70], supporting the role of PAT in atrial conduction. Voltage-dependent PWI (P-Wave amplitude, P wave area and P wave terminal force) may be enhanced by hypertrophy of left atrium seen with pericardial fat. At the same time it may also be decreased due to fibrosis and effects on summation vector secondary to insulation effect[70]. The insulation effect does not affect the voltage-independent PWI (P wave duration and PR interval), however hypertrophy and fibrosis may still affect the conduction time[70]. P-wave terminal force is more closely associated with pericardial fat than other voltage-dependent PWI[70]. This is due to the fact that blocked posterior inter-atrial bundles seen with PAT causes anterior to posterior activation of left atrium resulting in a terminal negative deflection on the electrocardiogram in lead V1. PAT has been questioned to contribute to the P wave dispersion seen in obese individuals[71].
With further advancements in imaging, thickness of the posterior peri-atrial fat pad between left atrium and the esophagus was found to correlate with the AF burden[68]. Their proximity to the pulmonary vein ostia would explain the correlation, as triggers for AF initiation are located in the pulmonary vein ostia[75]. EAT total and inter-atrial septal thickness was shown to be related to left atrial volume independently even after adjustment for other confounding factors[76]. PAT has also been associated with increased risk of AF recurrence after ablation[77]. PAT volume has also been identified as a novel risk factor for post-operative AF after coronary artery bypass grafting[78].
As excessive cardiac adipose tissue have correlations with poor cardiovascular outcomes, research into possible reversal of the tissue has been studied. Weight loss through bariatric surgery and calorie restriction has shown a corresponding decrease in EAT volume and thickness. EAT thickness decreased in obese subjects who underwent an aggressive 6-mo long weight loss program (mean 20 kg) by adhering to a very low-calorie diet (900 kcal/d)[79]. Similarly, weight loss after bariatric surgery (average weight loss of 40 kg) was associated with a decrease in EAT thickness[80]. Conversely, the compared effects of pioglitazone and metformin treatment in T2DM patients demonstrated an increase in PAT volume in pioglitazone-treated patients after 24 wk[81]. Nonetheless, the correlation between increased cardiac adipose tissue has been associated with several features of metabolic syndrome, including fasting insulin[82]. Further studies are needed to show the effects of controlling these measures with changes in size of the cardiac adipose tissues.
Cardiac adipose tissue is metabolically active and associated with various metabolic derangements in the body leading to insulin resistance, atherosclerosis, metabolic syndrome and CVD. It has become clear that the adipose tissue around the heart is a critical indicator of CVD burden. Lifestyle and medical improvements may reduce this impact, as the evidence through the use of ultrasound has documented that weight loss is associated with a decrease in pericardial fat stores in both non-diabetic[79,83,84] and diabetic[85] subjects. In diabetics, metabolic derangements are significantly linked with cardiac adiposity, thus it should be considered screening for EAT or PAT as CVD risk factors in diabetic patients. Many aspects between EAT and PAT overlap. Clinicians and researchers must have a clear understanding of their physiological and pathological differences to expand on screening, managing and reducing the impact that EAT and PAT have on CVD.
P- Reviewer: Gillessen A, Saeki K, Tziomalos K S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
| 1. | Centers for Disease Control and Prevention. National Estimates and General Information on Diabetes and Prediabetes in the United States. USA (Atlanta): National Diabetes Fact Sheet 2011; . [Cited in This Article: ] |
| 2. | Chhabra L, Liti B, Kuraganti G, Kaul S, Trivedi N. Challenges in the management of type 2 diabetes mellitus and cardiovascular risk factors in obese subjects: what is the evidence and what are the myths? Int J Endocrinol. 2013;2013:856793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2:536-543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 619] [Cited by in F6Publishing: 663] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 4. | Wang CP, Hsu HL, Hung WC, Yu TH, Chen YH, Chiu CA, Lu LF, Chung FM, Shin SJ, Lee YJ. Increased epicardial adipose tissue (EAT) volume in type 2 diabetes mellitus and association with metabolic syndrome and severity of coronary atherosclerosis. Clin Endocrinol (Oxf). 2009;70:876-882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 155] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 5. | Goldfine AB, Fonseca V. Management of diabetes mellitus in patients with cardiovascular disease in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Circulation. 2010;121:2447-2449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | van der Meer RW, Rijzewijk LJ, de Jong HW, Lamb HJ, Lubberink M, Romijn JA, Bax JJ, de Roos A, Kamp O, Paulus WJ. Pioglitazone improves cardiac function and alters myocardial substrate metabolism without affecting cardiac triglyceride accumulation and high-energy phosphate metabolism in patients with well-controlled type 2 diabetes mellitus. Circulation. 2009;119:2069-2077. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 173] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 7. | Matsuzawa Y, Shimomura I, Nakamura T, Keno Y, Tokunaga K. Pathophysiology and pathogenesis of visceral fat obesity. Diabetes Res Clin Pract. 1994;24 Suppl:S111-S116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 8. | Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898-918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1901] [Cited by in F6Publishing: 1894] [Article Influence: 99.7] [Reference Citation Analysis (0)] |
| 9. | Iacobellis G, Malavazos AE. Pericardial adipose tissue, atherosclerosis, and cardiovascular disease risk factors: the Jackson Heart Study: comment on Liu et Al. Diabetes Care. 2010;33:e127; author reply e128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Bays HE. “Sick fat,” metabolic disease, and atherosclerosis. Am J Med. 2009;122:S26-S37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 11. | Bays HE, González-Campoy JM, Henry RR, Bergman DA, Kitabchi AE, Schorr AB, Rodbard HW. Is adiposopathy (sick fat) an endocrine disease? Int J Clin Pract. 2008;62:1474-1483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Yang FS, Yun CH, Wu TH, Hsieh YC, Bezerra HG, Liu CC, Wu YJ, Kuo JY, Hung CL, Hou CJ. High pericardial and peri-aortic adipose tissue burden in pre-diabetic and diabetic subjects. BMC Cardiovasc Disord. 2013;13:98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Rajkovic N, Zamaklar M, Lalic K, Jotic A, Lukic L, Milicic T, Singh S, Stosic L, Lalic NM. Relationship between obesity, adipocytokines and inflammatory markers in type 2 diabetes: relevance for cardiovascular risk prevention. Int J Environ Res Public Health. 2014;11:4049-4065. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Harada K, Amano T, Uetani T, Tokuda Y, Kitagawa K, Shimbo Y, Kunimura A, Kumagai S, Yoshida T, Kato B. Cardiac 64-multislice computed tomography reveals increased epicardial fat volume in patients with acute coronary syndrome. Am J Cardiol. 2011;108:1119-1123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Kerr JD, Holden RM, Morton AR, Nolan RL, Hopman WM, Pruss CM, Garland JS. Associations of epicardial fat with coronary calcification, insulin resistance, inflammation, and fibroblast growth factor-23 in stage 3-5 chronic kidney disease. BMC Nephrol. 2013;14:26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Seidell JC, Han TS, Feskens EJ, Lean ME. Narrow hips and broad waist circumferences independently contribute to increased risk of non-insulin-dependent diabetes mellitus. J Intern Med. 1997;242:401-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 150] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Baker AR, Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, Kumar S, McTernan PG. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 460] [Cited by in F6Publishing: 485] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 18. | Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930-1935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1862] [Cited by in F6Publishing: 1912] [Article Influence: 83.1] [Reference Citation Analysis (0)] |
| 19. | Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O’Brien S, Keiper EA, Johnson AG. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460-2466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1279] [Cited by in F6Publishing: 1333] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 20. | Yudkin JS, Eringa E, Stehouwer CD. “Vasocrine” signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet. 2005;365:1817-1820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 395] [Cited by in F6Publishing: 405] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 21. | Sicari R, Sironi AM, Petz R, Frassi F, Chubuchny V, De Marchi D, Positano V, Lombardi M, Picano E, Gastaldelli A. Pericardial rather than epicardial fat is a cardiometabolic risk marker: an MRI vs echo study. J Am Soc Echocardiogr. 2011;24:1156-1162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Kim HM, Kim KJ, Lee HJ, Yu HT, Moon JH, Kang ES, Cha BS, Lee HC, Lee BW, Kim YJ. Epicardial adipose tissue thickness is an indicator for coronary artery stenosis in asymptomatic type 2 diabetic patients: its assessment by cardiac magnetic resonance. Cardiovasc Diabetol. 2012;11:83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Chen WW, Li L, Yang GY, Li K, Qi XY, Zhu W, Tang Y, Liu H, Boden G. Circulating FGF-21 levels in normal subjects and in newly diagnose patients with Type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2008;116:65-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 24. | Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr. 2009;22:1311-1319; quiz 1417-1418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 396] [Cited by in F6Publishing: 415] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 25. | Malavazos AE, Di Leo G, Secchi F, Lupo EN, Dogliotti G, Coman C, Morricone L, Corsi MM, Sardanelli F, Iacobellis G. Relation of echocardiographic epicardial fat thickness and myocardial fat. Am J Cardiol. 2010;105:1831-1835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Iozzo P. Myocardial, perivascular, and epicardial fat. Diabetes Care. 2011;34 Suppl 2:S371-S379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 187] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 27. | Zhang Y, Ren J. Role of cardiac steatosis and lipotoxicity in obesity cardiomyopathy. Hypertension. 2011;57:148-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. 2007;153:907-917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 636] [Cited by in F6Publishing: 674] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 29. | Gorter PM, van Lindert AS, de Vos AM, Meijs MF, van der Graaf Y, Doevendans PA, Prokop M, Visseren FL. Quantification of epicardial and peri-coronary fat using cardiac computed tomography; reproducibility and relation with obesity and metabolic syndrome in patients suspected of coronary artery disease. Atherosclerosis. 2008;197:896-903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 196] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 30. | Dey D, Nakazato R, Li D, Berman DS. Epicardial and thoracic fat - Noninvasive measurement and clinical implications. Cardiovasc Diagn Ther. 2012;2:85-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 32] [Reference Citation Analysis (0)] |
| 31. | Iacobellis G. Epicardial and pericardial fat: close, but very different. Obesity (Silver Spring). 2009;17:625; author reply 626-627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 32. | Iacobellis G, Bianco AC. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol Metab. 2011;22:450-457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 326] [Cited by in F6Publishing: 347] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 33. | Pulinilkunnil T, Rodrigues B. Cardiac lipoprotein lipase: metabolic basis for diabetic heart disease. Cardiovasc Res. 2006;69:329-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Rabkin SW. Epicardial fat: properties, function and relationship to obesity. Obes Rev. 2007;8:253-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 279] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 35. | Ouwens DM, Sell H, Greulich S, Eckel J. The role of epicardial and perivascular adipose tissue in the pathophysiology of cardiovascular disease. J Cell Mol Med. 2010;14:2223-2234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 160] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 36. | Greif M, Becker A, von Ziegler F, Lebherz C, Lehrke M, Broedl UC, Tittus J, Parhofer K, Becker C, Reiser M. Pericardial adipose tissue determined by dual source CT is a risk factor for coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:781-786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 209] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 37. | Eroglu S, Sade LE, Yildirir A, Bal U, Ozbicer S, Ozgul AS, Bozbas H, Aydinalp A, Muderrisoglu H. Epicardial adipose tissue thickness by echocardiography is a marker for the presence and severity of coronary artery disease. Nutr Metab Cardiovasc Dis. 2009;19:211-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 38. | Ahn SG, Lim HS, Joe DY, Kang SJ, Choi BJ, Choi SY, Yoon MH, Hwang GS, Tahk SJ, Shin JH. Relationship of epicardial adipose tissue by echocardiography to coronary artery disease. Heart. 2008;94:e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 227] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 39. | Iwasaki K, Matsumoto T, Aono H, Furukawa H, Samukawa M. Relationship between epicardial fat measured by 64-multidetector computed tomography and coronary artery disease. Clin Cardiol. 2011;34:166-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 40. | Shemirani H, Khoshavi M. Correlation of echocardiographic epicardial fat thickness with severity of coronary artery disease-an observational study. Anadolu Kardiyol Derg. 2012;12:200-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Jeong JW, Jeong MH, Yun KH, Oh SK, Park EM, Kim YK, Rhee SJ, Lee EM, Lee J, Yoo NJ. Echocardiographic epicardial fat thickness and coronary artery disease. Circ J. 2007;71:536-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 271] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 42. | Ding J, Hsu FC, Harris TB, Liu Y, Kritchevsky SB, Szklo M, Ouyang P, Espeland MA, Lohman KK, Criqui MH. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2009;90:499-504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 335] [Cited by in F6Publishing: 341] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 43. | Yerramasu A, Dey D, Venuraju S, Anand DV, Atwal S, Corder R, Berman DS, Lahiri A. Increased volume of epicardial fat is an independent risk factor for accelerated progression of sub-clinical coronary atherosclerosis. Atherosclerosis. 2012;220:223-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 181] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 44. | Cheng VY, Dey D, Tamarappoo B, Nakazato R, Gransar H, Miranda-Peats R, Ramesh A, Wong ND, Shaw LJ, Slomka PJ. Pericardial fat burden on ECG-gated noncontrast CT in asymptomatic patients who subsequently experience adverse cardiovascular events. JACC Cardiovasc Imaging. 2010;3:352-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 45. | Greif M, Leber AW, Saam T, Uebleis C, von Ziegler F, Rümmler J, D’Anastasi M, Arias-Herrera V, Becker C, Steinbeck G. Determination of Pericardial Adipose Tissue Increases the Prognostic Accuracy of Coronary Artery Calcification for Future Cardiovascular Events. Cardiology. 2012;121:220-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 46. | Tonbul HZ, Turkmen K, Kayıkcıoglu H, Ozbek O, Kayrak M, Biyik Z. Epicardial adipose tissue and coronary artery calcification in diabetic and nondiabetic end-stage renal disease patients. Ren Fail. 2011;33:770-775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Sengul C, Cevik C, Ozveren O, Oduncu V, Sunbul A, Akgun T, Can MM, Semiz E, Dindar I. Echocardiographic epicardial fat thickness is associated with carotid intima-media thickness in patients with metabolic syndrome. Echocardiography. 2011;28:853-858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 48. | Liu J, Fox CS, Hickson DA, May WL, Ding J, Carr JJ, Taylor HA. Pericardial fat and echocardiographic measures of cardiac abnormalities: the Jackson Heart Study. Diabetes Care. 2011;34:341-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 49. | Kazlauskaite R, Doukky R, Evans A, Margeta B, Ruchi A, Fogelfeld L, Kelly RF. Predictors of diastolic dysfunction among minority patients with newly diagnosed type 2 diabetes. Diabetes Res Clin Pract. 2010;88:189-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Cavalcante JL, Tamarappoo BK, Hachamovitch R, Kwon DH, Alraies MC, Halliburton S, Schoenhagen P, Dey D, Berman DS, Marwick TH. Association of epicardial fat, hypertension, subclinical coronary artery disease, and metabolic syndrome with left ventricular diastolic dysfunction. Am J Cardiol. 2012;110:1793-1798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 51. | Kremen J, Dolinkova M, Krajickova J, Blaha J, Anderlova K, Lacinova Z, Haluzikova D, Bosanska L, Vokurka M, Svacina S. Increased subcutaneous and epicardial adipose tissue production of proinflammatory cytokines in cardiac surgery patients: possible role in postoperative insulin resistance. J Clin Endocrinol Metab. 2006;91:4620-4627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 52. | Iacobellis G, Pistilli D, Gucciardo M, Leonetti F, Miraldi F, Brancaccio G, Gallo P, di Gioia CR. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine. 2005;29:251-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 147] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 53. | Zencirci E, Zencirci AE, Değirmencioğlu A, Karakuş G, Uğurlucan M, Ozden K, Erdem A, Güllü AU, Ekmekçi A, Velibey Y. The relationship between epicardial adipose tissue and ST-segment resolution in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Heart Vessels. 2014;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Park JS, Choi BJ, Choi SY, Yoon MH, Hwang GS, Tahk SJ, Shin JH. Echocardiographically measured epicardial fat predicts restenosis after coronary stenting. Scand Cardiovasc J. 2013;47:297-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 55. | Bettencourt N, Toschke AM, Leite D, Rocha J, Carvalho M, Sampaio F, Xará S, Leite-Moreira A, Nagel E, Gama V. Epicardial adipose tissue is an independent predictor of coronary atherosclerotic burden. Int J Cardiol. 2012;158:26-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 56. | Nabati M, Saffar N, Yazdani J, Parsaee MS. Relationship between epicardial fat measured by echocardiography and coronary atherosclerosis: a single-blind historical cohort study. Echocardiography. 2013;30:505-511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 57. | Nelson MR, Mookadam F, Thota V, Emani U, Al Harthi M, Lester SJ, Cha S, Stepanek J, Hurst RT. Epicardial fat: an additional measurement for subclinical atherosclerosis and cardiovascular risk stratification? J Am Soc Echocardiogr. 2011;24:339-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 58. | Iacobellis G, Sharma AM, Pellicelli AM, Grisorio B, Barbarini G, Barbaro G. Epicardial adipose tissue is related to carotid intima-media thickness and visceral adiposity in HIV-infected patients with highly active antiretroviral therapy-associated metabolic syndrome. Curr HIV Res. 2007;5:275-279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 59. | Soliman EZ, Ding J, Hsu FC, Carr JJ, Polak JF, Goff DC. Association between carotid intima-media thickness and pericardial fat in the Multi-Ethnic Study of Atherosclerosis (MESA). J Stroke Cerebrovasc Dis. 2010;19:58-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 60. | Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, Laing I, Yates AP, Pemberton PW, Malik RA. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation. 2009;119:1661-1670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 425] [Cited by in F6Publishing: 444] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 61. | Konishi M, Sugiyama S, Sugamura K, Nozaki T, Matsubara J, Akiyama E, Utsunomiya D, Matsuzawa Y, Yamashita Y, Kimura K. Accumulation of pericardial fat correlates with left ventricular diastolic dysfunction in patients with normal ejection fraction. J Cardiol. 2012;59:344-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 62. | Choi SJ, Kim NR, Hong SA, Lee WB, Park MY, Kim JK, Hwang SD, Lee HK. Changes in body fat mass in patients after starting peritoneal dialysis. Perit Dial Int. 2011;31:67-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 63. | Lin HH, Lee JK, Yang CY, Lien YC, Huang JW, Wu CK. Accumulation of epicardial fat rather than visceral fat is an independent risk factor for left ventricular diastolic dysfunction in patients undergoing peritoneal dialysis. Cardiovasc Diabetol. 2013;12:127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 64. | Vural M, Talu A, Sahin D, Elalmis OU, Durmaz HA, Uyanık S, Dolek BA. Evaluation of the relationship between epicardial fat volume and left ventricular diastolic dysfunction. Jpn J Radiol. 2014;32:331-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 65. | Doesch C, Haghi D, Flüchter S, Suselbeck T, Schoenberg SO, Michaely H, Borggrefe M, Papavassiliu T. Epicardial adipose tissue in patients with heart failure. J Cardiovasc Magn Reson. 2010;12:40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 66. | Khawaja T, Greer C, Chokshi A, Chavarria N, Thadani S, Jones M, Schaefle K, Bhatia K, Collado JE, Shimbo D. Epicardial fat volume in patients with left ventricular systolic dysfunction. Am J Cardiol. 2011;108:397-401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 67. | Chhabra L, Devadoss R, Chaubey VK, Spodick DH. Interatrial block in the modern era. Curr Cardiol Rev. 2014;10:181-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 68. | Batal O, Schoenhagen P, Shao M, Ayyad AE, Van Wagoner DR, Halliburton SS, Tchou PJ, Chung MK. Left atrial epicardial adiposity and atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:230-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 174] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 69. | Al Chekakie MO, Welles CC, Metoyer R, Ibrahim A, Shapira AR, Cytron J, Santucci P, Wilber DJ, Akar JG. Pericardial fat is independently associated with human atrial fibrillation. J Am Coll Cardiol. 2010;56:784-788. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 284] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 70. | Friedman DJ, Wang N, Meigs JB, Hoffmann U, Massaro JM, Fox CS, Magnani JW. Pericardial fat is associated with atrial conduction: the Framingham Heart Study. J Am Heart Assoc. 2014;3:e000477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 71. | Thanassoulis G, Massaro JM, O’Donnell CJ, Hoffmann U, Levy D, Ellinor PT, Wang TJ, Schnabel RB, Vasan RS, Fox CS. Pericardial fat is associated with prevalent atrial fibrillation: the Framingham Heart Study. Circ Arrhythm Electrophysiol. 2010;3:345-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 321] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 72. | Iacobellis G, Leonetti F, Singh N, M Sharma A. Relationship of epicardial adipose tissue with atrial dimensions and diastolic function in morbidly obese subjects. Int J Cardiol. 2007;115:272-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 162] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 73. | Greif M, von Ziegler F, Wakili R, Tittus J, Becker C, Helbig S, Laubender RP, Schwarz W, D’Anastasi M, Schenzle J. Increased pericardial adipose tissue is correlated with atrial fibrillation and left atrial dilatation. Clin Res Cardiol. 2013;102:555-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 74. | Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006-3010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 972] [Cited by in F6Publishing: 1032] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 75. | Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659-666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5432] [Cited by in F6Publishing: 5155] [Article Influence: 198.3] [Reference Citation Analysis (0)] |
| 76. | Shin SY, Yong HS, Lim HE, Na JO, Choi CU, Choi JI, Kim SH, Kim JW, Kim EJ, Park SW. Total and interatrial epicardial adipose tissues are independently associated with left atrial remodeling in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2011;22:647-655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 77. | Wong CX, Abed HS, Molaee P, Nelson AJ, Brooks AG, Sharma G, Leong DP, Lau DH, Middeldorp ME, Roberts-Thomson KC. Pericardial fat is associated with atrial fibrillation severity and ablation outcome. J Am Coll Cardiol. 2011;57:1745-1751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 309] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 78. | Drossos G, Koutsogiannidis CP, Ananiadou O, Kapsas G, Ampatzidou F, Madesis A, Bismpa K, Palladas P, Karagounis L. Pericardial fat is strongly associated with atrial fibrillation after coronary artery bypass graft surgery†. Eur J Cardiothorac Surg. 2014;46:1014-1020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 79. | Iacobellis G, Singh N, Wharton S, Sharma AM. Substantial changes in epicardial fat thickness after weight loss in severely obese subjects. Obesity (Silver Spring). 2008;16:1693-1697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 175] [Article Influence: 10.9] [Reference Citation Analysis (1)] |
| 80. | Willens HJ, Byers P, Chirinos JA, Labrador E, Hare JM, de Marchena E. Effects of weight loss after bariatric surgery on epicardial fat measured using echocardiography. Am J Cardiol. 2007;99:1242-1245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 81. | Jonker JT, Lamb HJ, van der Meer RW, Rijzewijk LJ, Menting LJ, Diamant M, Bax JJ, de Roos A, Romijn JA, Smit JW. Pioglitazone compared with metformin increases pericardial fat volume in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2010;95:456-460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 82. | Iacobellis G, Ribaudo MC, Assael F, Vecci E, Tiberti C, Zappaterreno A, Di Mario U, Leonetti F. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003;88:5163-5168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 572] [Cited by in F6Publishing: 572] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 83. | Kim MK, Tanaka K, Kim MJ, Matuso T, Endo T, Tomita T, Maeda S, Ajisaka R. Comparison of epicardial, abdominal and regional fat compartments in response to weight loss. Nutr Metab Cardiovasc Dis. 2009;19:760-766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 84. | Bosy-Westphal A, Kossel E, Goele K, Blöcker T, Lagerpusch M, Later W, Heller M, Glüer CC, Müller MJ. Association of pericardial fat with liver fat and insulin sensitivity after diet-induced weight loss in overweight women. Obesity (Silver Spring). 2010;18:2111-2117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 85. | Snel M, Jonker JT, Hammer S, Kerpershoek G, Lamb HJ, Meinders AE, Pijl H, de Roos A, Romijn JA, Smit JW. Long-term beneficial effect of a 16-week very low calorie diet on pericardial fat in obese type 2 diabetes mellitus patients. Obesity (Silver Spring). 2012;20:1572-1576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 86. | Taguchi R, Takasu J, Itani Y, Yamamoto R, Yokoyama K, Watanabe S, Masuda Y. Pericardial fat accumulation in men as a risk factor for coronary artery disease. Atherosclerosis. 2001;157:203-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 199] [Article Influence: 8.7] [Reference Citation Analysis (0)] |