INTRODUCTION
Hyperkalemia, usually defined by a potassium concentration greater than 5.5 or 6.0 mEq/L (mmol/L)[1], is a common clinical condition and is potentially life-threatening due to the risk of ileus paralysis and fatal arrhythmias[2]. Among the various causes of hyperkalemia, hypoaldosteronism should be considered in any patient with persistent hyperkalemia for which there is no clear cause, such as renal failure, the use of potassium supplements or a potassium-sparing diuretic. The causes of hypoaldosteronism include both acquired (secondary mineralocorticoid deficiency) and, less often, inherited disorders (primary mineralocorticoid deficiency), which can affect adrenal aldosterone synthesis or renal (and maybe adrenal) renin release. The most common secondary mineralocorticoid deficiency cause is hyporeninemic hypoaldosteronism (HH), including cases related to diabetes mellitus (DM), pharmacologic inhibition of angiotensin II, the use of potassium-sparing diuretics, and non-steroidal anti-inflammatory drugs (NSAIDs) or calcineurin inhibitors[3]. Primary hypoaldosteronism can be the result of acquired or congenital errors in renal juxtaglomerular function, angiotensin generation or activity, or aldosterone synthesis. Secondary hypoaldosteronism (pseudohypoaldosteronism), in contrast, occurs as a consequence of mutations in genes that may adversely affect aldosterone-mediated electrolyte homeostasis[4].
For decades, it has been known that there is a relationship between the metabolism of glucose and potassium; the lack of insulin predisposes one to hyperkalemia, exogenous insulin lowers serum potassium, and potassium deficiency interferes with insulin release, leading to glucose intolerance[5]. In addition, hyperkalemia occurs more frequently in patients with DM than in the general population[6]. Various mechanisms are involved in the development of hyperkalemia in patients with DM, for example hyperosmolality, insulin deficiency or resistance, HH, potassium-sparing drugs, and raised glucagon concentrations[2]. HH is related to a secondary mineralocorticoid deficiency, leads to hyperkalemia accompanied by urinary salt wasting[7] and is commonly seen in association with diabetic nephropathy (DN). HH normally occurs when there is some underlying renal pathology causing volume expansion[8].
A few years ago, a series of publications addressed the close relationship between HH and DM in case reports and studies on physiology and applied pathophysiology. However, most of these studies were published before the spread of the use of medications that interfere with the renin-angiotensin-aldosterone system (RAAS), such as inhibitors of the angiotensinogen-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs). Currently, few studies have reported on the implications of the diagnosis of HH in patients with DM, the concerns of a diagnosis of HH for the management of antihypertensive medications (ACEIs and ARBs) and the natural history of nephropathy in patients with DM. The objective of this review is to highlight the pathophysiology and diagnosis of HH in patients with DM, as well as the consequences of this condition for the treatment of arterial hypertension (AH) and proteinuria in these patients.
PATHOPHYSIOLOGY
For the purpose of maintaining homeostasis, the urinary excretion of potassium is typically equal to the quantity ingested minus the quantity excreted in the feces[6]. In normal individuals, most of the potassium filtered at the glomerulus is reabsorbed in the proximal tubule and in the ascending limb of Henle’s loop, and most of the potassium excreted in the urine is that secreted by the distal convoluted tubule and the cortical collecting tubule (CCT)[7]. Consequently, potassium secretion in the cortical collecting duct is the major determinant of urinary potassium excretion[6]. However, the amount of potassium finally excreted in the urine is typically less than the amount secreted by earlier segments because there is a considerable quantity of potassium reabsorbed at the outer medullary collecting duct[3]. Potassium enters the tubular cell in exchange for sodium by the action of the Na-K ATPase located at the basolateral membrane, an active transport mechanism that moves three sodium ions out of the cell while simultaneously carrying two potassium ions into the cell. This process is able to maintain a high potassium and a low sodium concentration in the cell. Aldosterone stimulates Na-K ATPase activity directly and increases luminal membrane permeability to sodium. It also increases the permeability of the luminal membrane to potassium[6]. Aldosterone thus plays a major role in regulating the renal excretion of potassium. The action of aldosterone is to increase the number of open sodium channels in the luminal membrane of the principal cells in the CCT, leading to increased sodium reabsorption. The subsequent elimination of sodium from the tubular fluid makes the lumen electronegative, thereby creating an electrical gradient that stimulates the secretion of potassium into the lumen through potassium channels in the luminal membrane[7].
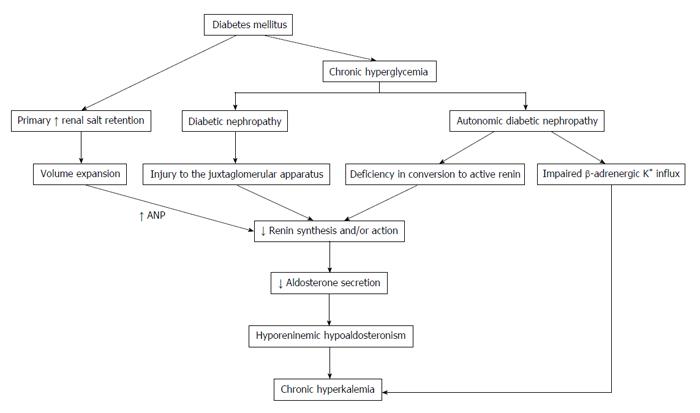
HH is a syndrome that is related to a reduction in the production of aldosterone from the adrenal gland and in the release of renin from the juxtaglomerular cells. A reduced renin release results in a decreased systemic and intra-adrenal angiotensin II (AngII) production, which contributes to the decline in aldosterone secretion[9]. AngII is a cofactor, along with potassium, in aldosterone synthesis by the adrenal gland. The production of renin occurs in the kidney by the juxtaglomerular cells and is stimulated primarily by the reduction in intravascular volume. Therefore, the RAAS plays a key role in blood pressure regulation[7]. HH is most common among patients with mild to moderate renal insufficiency due to DN or chronic interstitial nephritis[10]. As seen in Figure 1, the reduction in renin release in patients with DM may be secondary to[10]: (1) injury to the juxtaglomerular apparatus (such as an afferent arteriolar hyalinization); (2) defects in the stimulation factors or suppressed plasma renin activity (PRA) because of the deficiency in conversion of prorenin (big renin) to active renin; (3) autonomic dysfunction (as part of the autonomic diabetic neuropathy); or (4) an primary increase in renal salt retention with volume expansion, which suppresses renin synthesis (paradoxically, if compared to individuals without DM). The volume expansion leads to the suppression of renin release because of an increase in release of atrial natriuretic peptide, thus contributing to HH[11]. It is important to emphasize that any condition in which the renin-angiotensin-aldosterone axis is interrupted can potentially produce type IV renal tubular acidosis (RTA); therefore, type IV RTA and HH are often considered synonymous. Renal tubular damage may cause inadequate renin production and release, adrenal dysfunction may lead to inadequate aldosterone production, and the principal cells of the CCT may not respond normally to aldosterone. In addition, atrophy of the juxtaglomerular apparatus may be present, and this may be more prevalent among diabetic patients. This atrophy is related to autonomic neuropathy[12], chronic hyperkalemia and volume expansion[11]. In addition, any combination of these factors may cause HH or type IV RTA. Indeed, all of these factors (or any combination of them) may be present in some patients[13].
Figure 1 Pathophysiology of hyporeninemic hypoaldosteronism related to diabetes mellitus.
ANP: Atrial natriuretic peptide; K+: Potassium.
Because there is a reduced secretion of potassium, which can lead to chronic hyperkalemia, the resulting hyperkalemia impairs NH4+ production in the collecting duct. This leads to impaired generation of acid for excretion and metabolic acidosis[14], usually hyperchloremic metabolic acidosis, due to the deficiency in potassium and hydrogen secretion[15]. The degree of acidosis varies and may be related to the underlying chronic kidney disease (CKD). In type I (i.e., distal) RTA, the defect is in proton secretion with a resulting high urine pH (> 5.3), whereas in type IV RTA, the primary defect is in ammoniagenesis. This defect, albeit significant, still permits elaboration of acidic (pH < 5.3) urine. Hyperkalemia inhibits renal ammoniagenesis in several ways that involve the direct effects of one on the other: Modulation of ion transport by aldosterone, lowering of ammonia formation, and defective medullary ammonium handling. Furthermore, it may produce acidosis by shifting protons from inside the cells to the extracellular space as homeostatic mechanisms attempt to buffer potassium by intracellular uptake[2].
EPIDEMIOLOGY
HH predominantly occurs in patients 50 to 70 years of age with DN and/or chronic tubulointerstitial disease who have mild to moderate kidney failure[16]. It is more frequent among women. Therefore, patients with these characteristics should be monitored[17]. Other common clinical conditions associated with HH include various forms of interstitial disease, such as amyloid, monoclonal gammopathies and the interstitial nephritis associated with NSAIDs in particular[2,18-20]. In addition, there is a close relationship between diabetes and hyperkalemia. Insulin deficiency, kidney disease, HH, and use of medications such as ACEIs and ARBs that increase the risk of hyperkalemia are mechanisms involved in this relationship[21]. Therefore, hyperkalemia can be observed in type 1 DM patients due to their insulin deficiency and ketone-prone condition, as well as in type 2 DM patients, in whom an association has been found between serum potassium concentration and incidence of hyperkalemia and insulin resistance (estimated by the homeostasis model assessment)[21]. In addition, serum potassium concentration is likely to be more increased in patients with poorly controlled type 2 DM with insulin resistance than in those without DM[22]. Clinicians must also be aware that hyperkalemia in patients with type 1 DM may be due to concurrent adrenal insufficiency in the case of autoimmune polyglandular syndrome[23]. Specifically about HH, in many cases, particularly DN, hypoaldosteronism and low renin levels are present. In previous studies, about half of the subjects with HH were found to be diabetics[10]. Moreover, although HH usually occurs with persistent high potassium levels, some cases of HH are not accompanied by hyperkalemia despite suppression of renin and aldosterone levels. A previous study assessed the renin-aldosterone axis in 13 normokalemic patients with DM and creatinine clearances of < 40 mL/min and showed that approximately 92.3% of them had HH[24]. However, no recent studies have assessed the prevalence of HH in individuals with DM, particularly among those with normal renal function and/or without hyperkalemia. Similarly, the incidence of hyperkalemia in patients with HH could not be found. However, such studies are difficult to conduct because HH is often underdiagnosed and habitually only manifests when the patient is challenged by excess dietary potassium or by exposure to medications; furthermore, HH improves upon the removal of the exacerbating agents. In addition, it is worth noting that these older studies were conducted when the use of ACEIs and ARBs was not widespread. Recently, only a few studies have been conducted with moot methodology to determine the current incidence and prevalence of HH among populations that include patients using these medications. One recent study[25] evaluated the prevalence and role of type IV RTA in the development of significant hyperkalemia in patients admitted to a hospital for over 1 year and found significant hyperkalemia (> 6.0 mEq/L) in 3.8% of hospital admissions. Type IV RTA was diagnosed in 42% of these patients, of whom 71% had pre-existing renal insufficiency due to DN or tubulointerstitial nephritis. In the same study, patients with type IV RTA more frequently had a history of DM (50% vs 24%, P = 0.07) and were more likely to have pre-existing kidney failure (71% vs 38%, P < 0.05). No significant difference in the use of ACEIs, ARBs, NSAIDs, or another potassium-sparing diuretics was found between the groups with or without type IV RTA[25]. In another pharmacovigilance study, life-threatening hyperkalemia was found to be related to polypharmacy (use of more than 5 drugs), age (greater than 74 years), gender (female) and glomerular filtration rate (GFR) < 60 mL/min[26].
CLINICAL FEATURES AND DIAGNOSIS
In patients with persistent hyperkalemia with no obvious cause (such as renal failure, the use of potassium supplements or a potassium-sparing diuretic), the diagnosis of hypoaldosteronism should be considered[9]. These patients typically have elevated potassium serum levels disproportional to their renal function and potassium intake. Actually, HH accounts for most cases of unexplained hyperkalemia in patients in whom GFR and potassium intake would not be expected to result in hyperkalemia[2]. Regarding its clinical manifestation, most patients with HH are asymptomatic and have mild to moderate hyperkalemia. However, patients with HH may have significant hyperkalemia with no manifestations for long periods of time and may occasionally be identified in routine laboratory tests[6]. Conversely, an acute event (renal dysfunction or salt restriction) or medication (such as ACEIs, ARBs, potassium-sparing diuretics or heparin) may sometimes precipitate hyperkalemia in a patient whose disease has not been recognized because his or her plasma potassium has not exceeded the normal range. In those cases, acute hyperkalemia can disturb excitable tissues and provide different manifestations depending on the potassium serum level. Nausea, muscle weakness, paresthesias and fasciculations may occur and could progress to paralysis in severe cases. The progressive effects on the heart can be seen in the electrocardiogram (ECG), namely the peaking of T waves, ST-segment depression, widening of the PR interval, widening of the QRS interval, loss of the P wave, and development of a sine-wave pattern[3], and may even culminate in ventricular fibrillation. Generally, with acute onset of hyperkalemia, ECG changes appear at a serum potassium level of 6-7 mEq/L (6-7 mmol/L). However, with chronic hyperkalemia, the ECG may remain normal up to a concentration of 8-9 mEq/L (8-9 mmol/L)[3]. Despite these findings, a retrospective study revealed a poor correlation between serum potassium concentrations and cardiac manifestations[27]. Furthermore, hypoaldosteronism has been related with mild hyperchloremic metabolic acidosis. Metabolic acidosis is primarily the result of impaired renal ammoniagenesis caused by hyperkalemia (type IV RTA), reduced aldosterone levels, and reduced distal delivery of sodium. The acidosis is hyperchloremic because the renal insufficiency is mild and the retention of uremic anions is slight. Patients’ urinary pH is characteristically acidic because impaired ammoniagenesis reduces the buffering capacity of urine; occasionally, patients cannot acidify urine because of an associated distal tubular defect in hydrogen ion secretion[6]. Chronic hyperkalemia, per se, is usually asymptomatic, but chronic acidosis contributes to long-term morbid conditions, including bone demineralization[28]. Hyponatremia is uncommon in HH because there is no hypovolemia-induced stimulation to release antidiuretic hormone (ADH) and the plasma cortisol level, a tonic inhibitor of ADH release, is normal. Otherwise, when hyponatremia is present, other disorders such as primary adrenal insufficiency should be suspected[9].
Regarding the diagnosis of HH, it is important to remember that middle-aged or elderly patients with chronic hyperkalemia, diabetes, and/or with renal insufficiency are at risk. However, patients with DM at any age and metabolic conditions can be at risk. A study performed some years ago described a 31-year-old man with insulin-dependent DM and previous normal renal function who presented with symptomatic hyperkalemia and reversible impairment of renal function when treated with enalapril[29]. Before performing laboratory and dynamic tests to confirm HH, patients should be questioned about increased dietary potassium intake (including fruit juices and herbal preparations, such as noni), cell lysis (rhabdomyolysis), and the use of medications that interfere with potassium levels, such as ACEIs, ARBs, NSAIDs, beta-blockers, calcineurin inhibitor (cyclosporine, for example) and heparin, as well as human immunodeficiency virus (HIV) infection[6]. Patients with HIV are at risk for adrenal insufficiency, which may present as hyperkalemia. Nevertheless, the adrenal defect is sometimes selective for mineralocorticoid production. Furthermore, trimethoprim, a drug commonly used in chemoprophylaxis regimens for patients with AIDS, may impair tubular potassium excretion and may cause hyperkalemia[3]. In women, the use of some oral contraceptives should be evaluated because the progestin drospirenone retains mineralocorticoid blocking effects similar to those seen with spironolactone[30]. Other rarer diseases that can also cause nephropathy and HH may need to be excluded, for example, multiple myeloma, amyloidosis, systemic lupus erythematosus, and genetic disorders (pseudohypoaldosteronism)[7]. Some researchers also recommend excluding alcohol consumption, hemolysis, rhabdomyolysis, and/or metabolic acidosis[2]. In addition, as revealed before, clinicians must also be aware that hyperkalemia in patients with type 1 DM may be due to concurrent adrenal insufficiency in the case of autoimmune polyglandular syndrome.
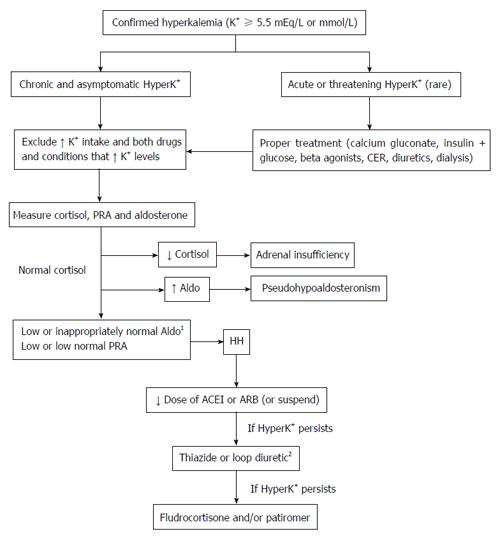
As previously mentioned, HH should be suspected in cases of unexplained chronic hyperkalemia in patients in whom GFR and potassium intake would likely not result in hyperkalemia. After exclusion of those possible causes, to confirm and to perform a differential diagnosis of hypoaldosteronism, it is recommended that the PRA, serum aldosterone, and serum cortisol should be measured (Figure 2). Some authors[9] recommend that these tests should be performed after the administration of a loop diuretic (furosemide) or after three hours in the upright position, which increases renin and aldosterone release in normal individuals. This ensures activation of the RAAS and the reliability of the findings, especially in the HH context. Hyperkalemia in patients with chronic kidney failure that is well short of end-stage (stage V) is typically characterized by plasma aldosterone levels that are inappropriately low for the degree of hyperkalemia[13]. Therefore, the authors of this article believe that, after excluding other causes, the existence of significant hyperkalemia (potassium levels > 5.5-6.0 mEq/L) accompanied by low or inappropriately normal aldosterone levels is indicative of hypoaldosteronism. In addition, HH is characterized by a low (or low normal) PRA and commonly normal serum cortisol[9]. Conversely, in the absence of significant hyperkalemia, it is important to ensure activation of the RAAS by the furosemide or upright test. These tests have commonly been used to differentiate between aldosterone-producing adenoma and idiopathic hyperaldosteronism in cases of primary aldosteronism[31], but they have recently been used increasingly less often for this purpose[32]. In brief, after a minimum of 1 wk on a 90 mmol/d sodium diet and in a potassium-replete state, the furosemide test can be performed after administration of a dose of 2 mg/kg of body weight orally at 06:00. The dose is then repeated 6 h later at 12:00 (if systolic blood pressure is ≥ 120 mmHg without orthostatic hypotension). At 1800 (6 h after the second dose of furosemide), a blood sample to measure PRA and aldosterone, sodium, and potassium concentrations is obtained after 5 min in the sitting position[31]. Other authors have used a combined test (upright position with administration of 60 mg furosemide)[33] for clinical research purposes in particular. In cases of primary adrenal insufficiency, both serum aldosterone and cortisol concentrations are typically low and PRA high. Ultimately, unlike primary hyperaldosteronism, there are no cutoffs for the diagnosis of HH. Consequently, reference values of laboratory tests are commonly used. Considering the lack of recent data concerning the diagnosis of HH, the impact of ACEI and ARB use is unknown. These medications are known to alter the aldosterone:PRA ratio by increasing the PRA[34], potentially reducing aldosterone levels. Therefore, there may be doubt regarding whether these medications interfere with the diagnosis of HH. In the absence of specific studies, the authors of this article believe that the suspension of ACEIs and ARBs is not essential to establish a diagnosis of HH. If a patient using these drugs develops hyperkalemia without impaired renal function and has low or inappropriately normal aldosterone and PRA levels, the most likely diagnosis is HH. However, if the suspension of drugs is necessary (either because of continuing concerns about the diagnosis or because of hyperkalemia), aldosterone and PRA should be repeated. Potassium-sparing diuretics (such as spironolactone and eplerenone), however, must be suspended before hormonal laboratory evaluation. Finally, further studies are clearly needed to clarify these issues.
Figure 2 Diagnosis and management of hyporeninemic hypoaldosteronism related to diabetes mellitus.
1In any cases, it might be necessary to activate the renin-angiotensin-aldosterone system (using a loop diuretic or the upright position); 2After using diuretics, the use of ACEIs or ARBs should be restarted (if suspended). ACEIs: Angiotensinogen-converting enzyme inhibitors; Aldo: Aldosterone; ARBs: Angiotensin receptor blockers; CER: Calcium exchange resins; HyperK+: Hyperkalemia; K+: Potassium; PRA: Plasma renin activity; HH: Hyporeninemic hypoaldosteronism.
RELATION TO OTHER DIABETIC MICROVASCULAR COMPLICATIONS
As previously stated, HH is most often found in patients with DN. It is therefore more likely to diagnose HH in patients with other diabetic microvascular complications. Considering the limited number of recent studies, few (if any) advances in the understanding of this issue have been obtained. Obviously, a key point in the explanation of these findings is hyporeninemia. Its association with DN is at least in part related to the damage to the juxtaglomerular apparatus and problems in the conversion of prorenin to active renin in diabetic patients[16]. The most comprehensive hypothesis accounting for the hyporeninemia is the associated renal disease-associated destruction of renin-producing cells (JG cells) or impaired function of the juxtaglomerular apparatus. However, the pathological findings of the juxtaglomerular apparatus in HH are limited in number and are likely nonspecific (they are found in essential hypertension, for instance)[10]. In addition, previous studies have suggested that impaired conversion of prorenin to active renin may cause a clinically significant reduction of PRA[35]. Nevertheless, the molecular mechanisms that explain how renal dysfunctions affect this conversion remain uncertain. A study designed to clarify the cause of selective HH divided 118 normokalemic patients based on presence or absence of diabetic neuropathy and/or nephropathy, and the authors observed that the development of abnormalities was associated with diabetic neuropathy and/or nephropathy[33]. According to a different study[36], autonomic neuropathy may be more relevant in HH than nephropathy: In patients with type 2 DM and autonomic neuropathy, levels of PRA were lower and inactive renin levels were higher, regardless of proteinuria and GFR. In addition, a Japanese study on the postural test and neuropathic diabetes showed that autonomic dysfunction was a major factor in impairing the processing of prorenin to active renin in diabetic patients and that severe autonomic dysfunction may impair the biosynthesis of prorenin in patients with HH[37]. In fact, autonomic neuropathy is known to result in impaired beta2-mediated influx of potassium into cells[38]. In addition, the stimulation of beta-adrenergic receptors induces renin release[39]; autonomic neuropathy with consequent sympathetic insufficiency might therefore lead to an impairment of renin production in patients with DM[10]. Theoretically, the suppression of PRA that results from sympathetic dysfunction should lead to a reduction in blood pressure; however, most reported patients with HH have AH[10]. Thus, these data reveal a significant association between other diabetic microvascular complications and HH, but no single pathophysiological aspect is able to accurately explain the findings observed related to DM-HH. It is most likely that there is a combination of mechanisms that together explain HH within the spectrum of microvascular diabetic complications.
IMPLICATIONS FOR THE MANAGEMENT OF AH AND PROTEINURIA
Patients with DM commonly use ACEIs and ARBs when they have concurrent hypertension and/or DN or for cardiovascular protection[40]. Despite the decrease in PRA in DN[34], the RAAS is fundamental in DN pathogenesis; there is an increased production of AngII secondary to stimulation by hyperglycemia and advanced glycation end products. AngII promotes an increase in intraglomerular pressure and adrenal aldosterone production. In addition, prorenin and aldosterone contribute to renal fibrosis[34]. Therefore, ACEIs and ARBs are essential for nephroprotection. However, one of the main adverse effects of these medications is hyperkalemia. The patients at the highest risk for hyperkalemia include those with either diabetes or those with impaired renal function in whom a defect in the excretion of renal potassium may already exist[41], including patients with HH. The development of hyperkalemia as a direct or indirect consequence of decreased aldosterone concentrations is typically observed when aldosterone concentrations have already decreased prior to the administration of drugs[41]. DN is the most common cause of HH, ranging from 43% to 63% of cases[9,42]. In addition, the risk of hyperkalemia increases with the progression of DM as a result of insulin deficiency, by limiting the body’s ability to shift potassium into cells[41]. ACEIs and ARBs impair the urinary excretion of potassium by inhibiting the stimulatory effect of AngII on aldosterone secretion in the adrenal gland. ACEIs act by blocking the formation of AngII, whereas ARBs prevent AngII from binding to its adrenal receptor, both systemically and perhaps within the adrenal zona glomerulosa[43]. However, while the effects of these medications in patients with HH can be inferred, they have not been evaluated through specific clinical studies.
The role of RAAS inhibition in postponing the progression of DN by using ACEIs or ARBs has been well established in multiple controlled trials; these medications are thus the drugs of choice in the treatment of hypertension and/or proteinuria in patients with DM[41]. In adult patients with diabetes and kidney disease, ACEIs and ARBs have been the most effective strategies in preventing progression to end-stage kidney disease[44]. Unfortunately, the true prevalence of HH in patients with DM, especially those with DN, is not known. However, given the vast prevalence of DM worldwide, it is thought that there are many patients with HH (even with normal levels of potassium) using ACEIs or ARBs. The large clinical trials that evaluated the efficacy of ACEIs, ARBs, and direct renin inhibitors on the natural history of DN did not perform post hoc analyses regarding the status of the RAAS (baseline levels of aldosterone and PRA, for example). HH has been suggested to be nephroprotective on its own, as it leads to lower levels of aldosterone and PRA[45]. Thus, one might suggest that the use of medications that interfere with the RAAS would have a modest to no effect on the progression of DN and would only increase the risk of hyperkalemia. However, these issues have not been specifically evaluated and are therefore only suppositions. In the absence of contrary evidence, the authors still believe that ACEIs and ARBs can be used in patients with HH and may even have clinical benefits, since their potassium levels do not present an obstacle and are closely monitored. In fact, the systematic monitoring of potassium levels in patients using RAAS blockers prevents cases of severe hyperkalemia. A study found that patients who received potassium monitoring were 50% less likely to experience a hyperkalemia-associated adverse event [adjusted relative risk of 0.50 (95%CI: 0.37-0.66)] compared to patients without monitoring. In patients with CKD, the adjusted relative risk was even lower at 0.29 (95%CI: 0.18-0.46)[46].
Given the underlying pathophysiology, there was hope that dual RAAS blockade could reduce the progression of DN even further[34]. The potential benefit of this dual blockade has been tested in three large randomized clinical trials, and unfortunately, their results demonstrated a lack of benefit with regard to renal or cardiovascular outcomes in diabetic patients[47-49]. In contrast, a recent meta-analysis found that the progression to end-stage renal disease was significantly less likely after combined treatment with an ARB and an ACEI[44]. However, one of the main safety concerns with more intensive RAAS blockade is hyperkalemia. Thus, careful monitoring of potassium in CKD patients in whom a mineralocorticoid receptor blocker is to be used in combination with an ACEI or ARB is of utmost importance, especially if the patient also has diabetes. While precaution may suggest that this combination should be avoided, it is widely used, especially in cases of heart failure. Prior studies have shown that the risk factors for hyperkalemia with the use of RAAS blockers include older age, lower GFR, higher baseline potassium levels, and the use of more than one medication that interferes with potassium excretion[50]. In fact, dual RAAS blockade has been shown to increase the risk of hyperkalemia and acute kidney injury. New agents for the treatment of hyperkalemia may increase the feasibility of dual blockade of RAAS; however, further research is still needed[34]. Nevertheless, withholding or withdrawing drugs that block the RAAS on the basis of impaired kidney function or on diagnosis of HH alone may potentially deprive many patients of the cardiovascular benefit they would receive; instead, there are numerous steps that can be taken to minimize the risk of hyperkalemia[3,41]. The initial approach should be to estimate the GFR and the potassium levels to assess the specific risk of hyperkalemia as well as to review the patient’s medications profile. Drugs that can impair renal potassium excretion[41,51] should be discontinued. It is important to inquire specifically about the use of over-the-counter NSAIDs and herbal remedies, as herbs can be a hidden source of intake potassium. A low-potassium diet should then be prescribed with specific counseling against the use of potassium-containing salt substitutes. Next, therapy with a low dose of ACEIs or ARBs should be initiated. It is essential to monitor patients’ potassium levels within 1 wk after initiating therapy or after increasing dosage[52]. If potassium levels remain normal, the dose of the drug can be titrated upwards. If potassium is higher than 5.5 mEq/L (5.5 mmol/L) despite the steps described above, ACEIs and ARBs may need to be avoided[41,51,52]. When albuminuria and proteinuria occur in DN, RAAS inhibitors should be used aggressively. However, the antiproteinuric effects of ACEIs and ARBs can be observed soon after starting treatment and may decrease the effectiveness throughout the treatment[40,53]. In addition, when an ARB is combined with an ACEI or direct renin inhibitor (such as aliskiren), there has been no evidence of clinical efficacy and adverse reactions have in fact been increased[40]. Despite the fact that ACEIs and ARBs are key drugs for the treatment of DM nephropathy, new treatment strategies are needed to achieve improved effectiveness. Finally, studies evaluating the efficacy and safety of medications in the treatment of hypertension and albuminuria in patients with HH are highly needed.
MANAGEMENT OF HYPERKALEMIA IN PATIENTS WITH HH
The incidence of hyperkalemia is higher in diabetic patients than in the general population[52], and the most common causal factor of chronic hyperkalemia in patients with diabetes is the reduced tubular secretion of potassium due to HH. The development of overt hyperkalemia is most common in patients with other risk factors that further impair the efficiency of potassium excretion, such as renal insufficiency, volume depletion, or the use of medications that interfere through the deterioration of intravascular volume contraction[2]. Indeed, worsening of renal function and hyperkalemia may occur in patients who are using the novel sodium glucose cotransporter 2 inhibitors, particularly those predisposed to hyperkalemia due to impaired renal function, medications, or other medical conditions[54].
There are no recognized recommendations or guidelines regarding when to initiate hyperkalemia treatment, but it is usually necessary to treat hyperkalemia because of the potential clinical manifestations in excitable tissue and the risk of progression to respiratory failure and fatal arrhythmias[3]. A summary of the management of hyperkalemia in patients with DM is presented in Figure 2. In cases of rapid elevation, very high potassium levels, and in life-threatening conditions, emergency treatment should be promptly initiated. Hyperkalemia must be acutely treated to counter its cardiac effects, using calcium gluconate or chloride to decrease the membrane excitability of the cardiac cells and reverse the locking depolarization caused by hyperkalemia. Drugs that provide potassium redistribution between the intra and extracellular fluid should be used. Insulin shifts the potassium into the cell, and the recommended dose is 10 units of regular insulin intravenously together with 50 mL of 50% glucose to prevent hypoglycemia in patients with glycemia below 200-250 mg/dL. Beta agonists, such as salbutamol, are also used to redistribute the potassium and to act synergistically with insulin. The recommended dose is 10-20 mg of nebulized salbutamol in 4 mL of saline solution for 10 min. For potassium removal, calcium exchange resins, diuretics and/or dialysis can be used. The use of bicarbonate infusion should be restricted to patients with associated metabolic acidosis and when it is non-gap metabolic acidosis[27,30]. In addition, the most widely used calcium exchange resin is sodium polystyrene sulfonate. As its effect is slow and there is a potential risk of intestinal injury, it is recommended that the use of this medication be restricted to the acute management of hyperkalemia only and when dialysis is not available or indicated[32]; it must not be used chronically.
In patients with HH in particular, the use of medications that affect the RAAS should be reassessed. In cases of hyperkalemia (serum potassium concentration up to 5.5 mEq/L or mmol/L) in patients with HH using ACEIs, dose reduction may be initially attempted[3]. In some cases, potassium concentration will improve, allowing the patient to remain on the renin-angiotensin blocker, although at a lower dose. ARBs and direct renin inhibitors should be used with the same caution. It is important to remember to recommend a low potassium diet and to avoid the use of NSAIDs, including selective cyclooxygenase-2 inhibitors[41]. Some years ago, a case of selective HH triggered by the use of NSAID was reported[55]. Dual RAAS blockade must be avoided (combinations of ACEIs and ARBs or direct renin inhibitors).
However, the use of aldosterone antagonists (spironolactone or eplerenone) is relatively common. If indicated, the dose of spironolactone should not exceed 25 mg daily when used with an ACEI or ARB, and this combination should be avoided when the GFR is < 30 mL/min. Patients with HH and decompensated diabetes (with significant hyperglycemia, particularly if there is concomitant loss of weight) have an additional increased risk of hyperkalemia; insulin deficiency contributes to both a low serum aldosterone concentration and to an increased concentration of extracellular potassium. In addition to the risk associated with insulin deficiency, hyperglycemia creates a differential osmolality, resulting in a hypertonic extracellular fluid. Consequently, water and ions, as potassium, are attracted to the extracellular fluid. In these patients, the use of insulin for the treatment of hyperglycemia may be recommended[3]. If these actions do not have the expected result and the risk benefit profile is in favor of maintaining the RAAS blockade, the use of fludrocortisone or diuretics can be attempted. Fludrocortisone is a potent mineralocorticoid that promotes increased reabsorption of sodium and loss of potassium from renal distal tubules[9]. While being administered, it is necessary to monitor the concentration of serum sodium and potassium because of the potential risk of hypokalemia and hypokalemic alkalosis[20,31,52]. The typical dose of fludrocortisone required to normalize the serum potassium is usually higher than the dose in primary adrenal insufficiency (0.05 to 0.2 mg daily). Nevertheless, the authors recommend starting with 0.1 to 0.2 mg once a day and increasing the dosage based on potassium levels and signs of hypervolemia. However, fludrocortisone is not widely used; patients with HH often have hypertension and/or edema (heart failure, for instance) and thus should either use fludrocortisone with caution or not use it at all because of its effect on plasma volume expansion. In those cases, hyperkalemia can be treated with low potassium ingestion and drugs that eliminate potassium, such as diuretics[33,35], preferably thiazide or loop ones, because of their efficiency in reducing hyperkalemia[41]. In patients with a GFR < 30 mL/min, a loop diuretic is ideal, as thiazide diuretics are less effective[52]. Alternatively, other authors have initially chosen to suspend the use of ARBs and start diuretics to first control potassium levels and then restart RAAS blockade successfully[56]. However, the use of loop diuretics may lead to the deterioration of renal function because of intravascular volume depletion[20]. If challenges in managing hyperkalemia persist, it is recommended to discontinue medications that act on the RAAS[41]; however, new agents are being evaluated in the treatment of hyperkalemia. Sodium zirconium cyclosilicate, a selective cation exchanger, was tested in a multicenter study and led to a significant reduction in potassium levels at 48 h (approximately 0.5 to 0.7 mEq/L)[57]. However, the most promising drug to date is patiromer, a non-absorbed potassium binder, which has been tested in patients with hyperkalemia and with use of RAAS blockers. In a multicenter study, patiromer was able to significantly reduce the level of potassium by approximately 1 mEq/L (mmol/L) in hyperkalemic patients using ACEIs or ARBs[58].
CONCLUSION
In clinical practice, it is common for patients with DM to present with hyperkalemia, especially if they are monitored for electrolytes. The literature states that the main cause of hyperkalemia in those patients, particularly those who already have diabetic microvascular complications (such as autonomic neuropathy), is HH. However, the recent literature on this topic is quite limited. Despite its pathophysiological importance, the diagnosis of HH is actually difficult to make because of the high frequency of concomitant comorbidities as well as the use of different medications and clinical variability. Clinically, HH is most often found in patients with DN, and the patients typically have asymptomatic, mild to moderate hyperkalemia. The prevalence of HH in the general diabetic population remains unclear, but it is believed to be underdiagnosed by physicians, including diabetologists. ACEIs and ARBs may precipitate hyperkalemia in a patient whose disease has not been recognized and increase the risk of severe hyperkalemia in patients with previously mild hyperkalemia. Although ACEIs and ARBs are considered to be essential for nephroprotection and are key drugs in the treatment of hypertension and DN, new treatment strategies are needed to achieve better effectiveness and control of potassium imbalances. Therefore, preventive actions should be routinely taken when treating such patients, including the proper evaluation of patients with initial borderline hyperkalemia to detect HH and the monitoring of patient potassium levels after initiating or modifying medications that block the RAAS. Undoubtedly, further studies are required to clarify critical issues regarding the syndrome of HH.
ACKNOWLEDGMENTS
The authors would like to thank Prof. Josivan Lima for his support and contribution in shaping this paper.