Published online Oct 26, 2012. doi: 10.4252/wjsc.v4.i10.101
Revised: August 23, 2012
Accepted: September 18, 2012
Published online: October 26, 2012
AIM: To investigate the effect of human umbilical cord stem cells, both mesenchymal and hematopoietic (CD34+), in the treatment of arthritis.
METHODS: Mesenchymal stem cells (MSCs) and hematopoietic (CD34+) stem cells (HSC) were isolated from human umbilical cord blood obtained from the umbilical cord of healthy pregnant donors undergoing full-term normal vaginal delivery. MSC, HSC, methotrexate (MTX) and sterile saline were injected intra-articularly into the rat hindpaw with complete freunds adjuvant (CFA) induced arthritis after the onset of disease (day 34), when arthritis had become well established (arthritis score ≥ 2). Arthritic indices were evaluated and the levels of interleukin (IL)-1, tumor necrosis factor (TNF)-α and interferon (IFN)-γ and anti-inflammatory cytokine IL-10 in serum were determined using enzyme-linked immunosorbent assay. Animals of all groups were sacrificed 34 d after beginning treatment, except positive control (PC) which was sacrificed at 10, 21 and 34 d for microscopic observation of disease progression. We used hematoxylin, eosin and Masson’s trichrome stains for histopathological examination of cartilage and synovium.
RESULTS: The mean arthritis scores were similar in all groups at 12 and 34 d post immunization, with no statistical significant difference. Upon the injection of stem cells (hematopoietic and mesenchymal), the overall arthritis signs were significantly improved around 21 d after receiving the injection and totally disappeared at day 34 post treatment in MSC group. Mean hindpaw diameter (mm) in the MSC rats was about half that of the PC and MTX groups (P = 0.007 and P = 0.021, respectively) and 0.6 mm less than the HSC group (P = 0.047), as indicated by paw swelling. Associated with these findings, serum levels of TNF-α, IFN-γ and IL-1 decreased significantly in HSC and MSC groups compared to PC and MTX groups (P < 0.05), while the expression of IL-10 was increased. Histopathological examination with H and E stain revealed that the MTX treated group showed significant reduction of leucocytic infiltrate and hypertrophy of the synovial tissue with moderate obliteration of the joint cavity. Stem cells treated groups (both hematopoietic CD34+ and mesenchymal), showed significant reduction in leucocytic infiltrate and hypertrophy of the synovial tissue with mild obliteration of the joint cavity. With Masson’s trichrome, stain sections from the PC group showed evidence of vascular edema of almost all vessels within the synovium in nearly all arthritic rats. Vacuoles were also visible in the outer vessel wall. The vessel became hemorrhagic and finally necrotic. In addition, there was extensive fibrosis completely obliterating the joint cavity. The mean color area percentage of collagen in this group was 0.324 ± 0.096, which was significantly increased when compared to the negative control group. The mean color area percentage of collagen in hematopoietic CD34+ and mesenchymal groups was 0.176 ± 0.0137 and 0.174 ± 0.0197 respectively, which showed a marked decrement compared to the PC group, denoting a mild increase in synovial tissue collagen fibers.
CONCLUSION: MSC enhance the efficacy of CFA-induced arthritis treatment, most likely through the modulation of the expression of cytokines and amelioration of pathological changes in joints.
- Citation: Greish S, Abogresha N, Abdel-Hady Z, Zakaria E, Ghaly M, Hefny M. Human umbilical cord mesenchymal stem cells as treatment of adjuvant rheumatoid arthritis in a rat model. World J Stem Cells 2012; 4(10): 101-109
- URL: https://www.wjgnet.com/1948-0210/full/v4/i10/101.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v4.i10.101
Rheumatoid arthritis (RA) is an autoimmune disease caused by loss of immunological self-tolerance that leads to chronic inflammation in the joints and subsequent cartilage destruction and bone erosion. The crucial process underlying disease initiation is the induction of autoimmunity to collagen-rich joint components; later events evolve a destructive inflammatory process[1]. In RA, proinflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-1 and IL-17, play dominant pathological roles. Aberrant T helper cells (Th) 17 and Th1 responses have been linked to pathogenesis of RA[2-4]. Furthermore, evidence is accumulating that a defect in number or function of regular T cells is important in the immune imbalance that culminates in RA[5,6].
RA imparts a massive burden on health services worldwide. A desirable therapeutic approach would be to prevent the activation of inflammatory and autoimmune components of the disease[6-10]. Efforts to discover new target therapies have achieved considerable success. For instance, TNF-α inhibitors and B-cell-depleting therapies have benefited many RA patients[7]. However, these approaches are expensive and none of the currently widely used biological agents reaches long-term drug-free remission[8,9]. Therefore, it is important to develop a new and more effective therapy for RA.
Mesenchymal stem cells (MSCs) are mesoderm-derived cells that reside in the stroma of solid organs and function as precursors of nonhematopoietic connective tissues[11,12]. They can exert profound immunosuppression by modulating T and B cell proliferation and differentiation, dendritic cell maturation and NK activity. These immunoregulatory properties encourage a possible use of these cells to modulate autoimmune responses and in the treatment of autoimmune diseases[13]. Several recent studies have shown that bone marrow-derived MSCs (BM-MSCs) regulate the immune response, including in vitro inhibition of T cell proliferation, B cell function and dendritic cell maturation[14].
However, the specific molecular and cellular mechanisms involved in the immunoregulatory activity of BM-MSCs remain a subject of controversy[15-18]. In addition, aspirating bone marrow is an invasive procedure. In addition, the number and the differentiating potential of BM-MSCs decrease with age[19,20]. Human umbilical cord blood-derived MSCs (hUCB-MSCs) have a capacity similar to that of BM-MSCs for multi-lineage differentiation[21]. In addition, hUCB-MSCs also possess activities for immune modulation, tumor tropism and nursing effect[22,23].
Recent evidence has demonstrated that hUCB-MSCs can suppress, not only the function of mature dendritic cells, but also increase the portion of regulatory T cells related to immune regulation[24,25]. In fact, hUCB-MSCs have much higher chondrogenic differentiation potential among mesodermal differentiation potentials, which might lead to regeneration of damaged cartilage. These properties might be due in part to specific secreted factors, including some types of cytokines and growth factors. For instance, it has been reported that thrombospondin-1, 2 functions as an anti-inflammatory factor in RA by suppressing production of proinflammatory mediators, such as interferon (IFN)-γ and TNF-α, inducing depletion of synovium residing T cells and reducing infiltration of monocytes/macrophages in articular tissues[26,27]. However, very little is known about UC-MSCs, with one report about UC-MSCs in the treatment of RA[28].
Consequently, to understand and utilize the immune regulation properties of hUCB-MSCs for application in the treatment of a number of human immunological diseases, the molecular mechanism underlying the immune modulatory functions of hUCB-MSCs needs further investigations.
This study was performed in the Stem Cell Unit in the Physiology Department, Faculty of Medicine, Suez Canal University. It was performed according to the national laws on animal experiments and with the permission of the local university ethics commission. We used forty apparently healthy male rats weighing 100-150 g in this study. We purchased the rats from the National Centre of Research (Cairo, Egypt), housed in clean cages under hygienic conditions and allowed to acclimatize for 7 d before starting the experiment. Rats were kept on a standard chow and water ad libitum with a reversed dark-light cycle. All possible measures were made to minimize animal suffering and to reduce the number of used animals.
Animals were randomly divided into five groups each of eight rats: negative control (NC), positive control (PC), methotrexate (MTX), MSC and hematopoietic (CD34+) stem cells (HSC).
RA was induced in all groups (except NC), in briefly anaesthetized rats, using a single intradermal injection of 0.1 mL complete freunds adjuvant (CFA) (Sigma, United Kingdom) into the right hindpaw[29]. Rats were monitored for signs of arthritis onset based on paw swelling and clinical scores[30]. Paw swelling was assessed by measuring the mean thickness of both hindpaws with 0-10-mm calipers. Clinical arthritis was scored on a scale of 0-4, where 0 = no swelling, 1 = redness, 2 = swelling, 3 = digit deformity, and 4 = ankle deformity (ankylosis). All clinical score (CS) measurements were performed independently by two individuals, without awareness of the rats’ clinical score history. Full ankylosis was scored clinically by lack of motion of the extremity in question. An animal could have a CS of 16 if all paws were fully ankylosed.
Umbilical cord blood samples collection: After obtaining informed consent, hUCB samples were obtained from the umbilical cord of healthy pregnant donors undergoing full-term normal vaginal delivery. Females with a history of hepatitis, infectious diseases, diabetes mellitus, severe hypertension, abortions or abnormal obstetric history were excluded. UCB was collected while the placenta was in utero. By strict aseptic techniques, 50 mL of hUCB were withdrawn from the umbilical vein and collected in sterile tubes containing five milliliters of citrate phosphate dextrose adenine-l anticoagulant and stored at 4 °C in a refrigerator and processed within 24 h[31].
Umbilical cord blood samples processing: Cord blood sample was diluted 1:1 with isolation buffer. A 3.0 mL histopaque-1077 was placed in a 15 mL centrifuge tube and brought to room temperature, then 6 mL diluted blood was carefully layered onto the histopaque-1077 and centrifuged at 400 ×g for exactly 30 min at normal room temperature. After centrifugation, we carefully aspirated the upper layer with a Pasteur pipet to within 0.5 cm of the opaque interface containing mononuclear cells and it was discarded. The opaque interface was carefully transferred to a clean centrifuge tube with a Pasteur pipet. About 6-10 mL isolation buffer was added to the tube and was mixed by gentle aspiration, then centrifuged at 400 ×g for 20 min. We aspirated and discarded the supernatant. Then, the cell pellet was suspended with 5.0 mL isolation buffer and mixed by gentle aspiration with a Pasteur pipet and then centrifuged at 400 ×g for 10 min. The previous steps were repeated three times; each time supernatant was discarded and cell pellet was suspended in 0.5 mL isolation buffer. The quantity of the isolated mononuclear cells was assessed by automated cell counter and the viability was determined by a trypan blue exclusion test.
Separation and purification of CD34+ cells: Separation of UCB CD34+ stem cells was carried out according to the method described by an immuno-magnetic separation technique[32]. By mixing and incubation, CD34+ cells were bound to Dynabeads M-450 CD34. The formed rosettes were isolated from the suspension using a DYNAL Magnetic Particle Concentrator (DYNAL MPC). Subsequent incubation with DETACHaBEAD, CD34 gently detached isolated cells from the beads. A DYNAL MPC was then used to separate the purified, positively selected CD34+ cells from the released Dynabeads M-450 CD34. The quantity of the isolated CD34+ cells was assessed by putting the sample in an automated cell counter. The quality of the isolated CD34+ cells was determined by using a trypan blue dye exclusion test where the viable cells were not stained[33].
Culture of MSC from mononuclear cells: MSCs isolation is possible due to its capacity of adhesion to the flasks. Cultures were initiated in culture flasks of 25 cm2 at a density of 1.0 × 106 mononuclear cells/cm2. Cells were nurtured with culture medium á-MEM supplemented with 20% fetal bovine serum, 1% antibiotic/antimycotic and 1% glutamine, then incubated at 37 °C, humidified atmosphere containing 5% CO2. After overnight incubation, non-adherent cells were removed and fresh medium was added to the culture flask. Later on, the culture medium was changed every 4 d and cellular growth assessed daily under an inverted microscope. When the cells reached 50%-60% confluence, they were detached with 0.25% trypsin-EDTA (Invitrogen), the trypsin was inactivated with fresh media, and the cells centrifuged at 250 g for 5 min[34].
Treatment was begun after the onset of disease (day 34), when arthritis had become well established (arthritis score ≥ 2). All rats in four groups received a single intraarticular injection in the right hindpaw where the NC and PC group rats received 5 μL sterile saline, 0.9% PBS, the MSC group received 1 × 106 MSCs, and the HSC group received 1 × 106 hematopoietic stem cell. However, MTX received 0.3 mg/kg twice a week for 5 wk.
Animals in all groups were sacrificed 34 d after beginning treatment, except the PC group. Animals of the PC group were divided equally and sacrificed at 10, 21 and 34 d for microscopic observation of disease progression.
At the end of the experiment, according to each group schedule, hindpaws were taken from skinned sacrificed animals and placed in 10% buffered phosphate formalin for at least 1 wk before being subjected to acid decalcification. The paws were decalcified by immersion in 3% nitric acid solution and maintained at room temperature for an average of 5-7 d. De-calcified paws were embedded in paraffin, longitudinally sectioned through the center of the tibia-tarsal joint, and stained with hematoxylin and eosin and Masson’s trichrome. Quantitative measurements were carried out using the image analyzer (Super eye-Heidi soft) to measure the color area percentage of the green color (collagen fibers) in Masson’s trichrome stained sections. The image analyzer was calibrated for color before utilization. Thirty fields, at least, were captured and analyzed for each group.
TNF-α, IL-1, IL-10 and IFN-γ were estimated 34 d after beginning treatment in the rat serum by sandwich enzyme-linked immunosorbent assays using capture/biotinylated detection antibodies (Sigma, United Kingdom).
Student’s t test was used to analyze significance between groups. A P value of less than 0.05 was considered a significant difference.
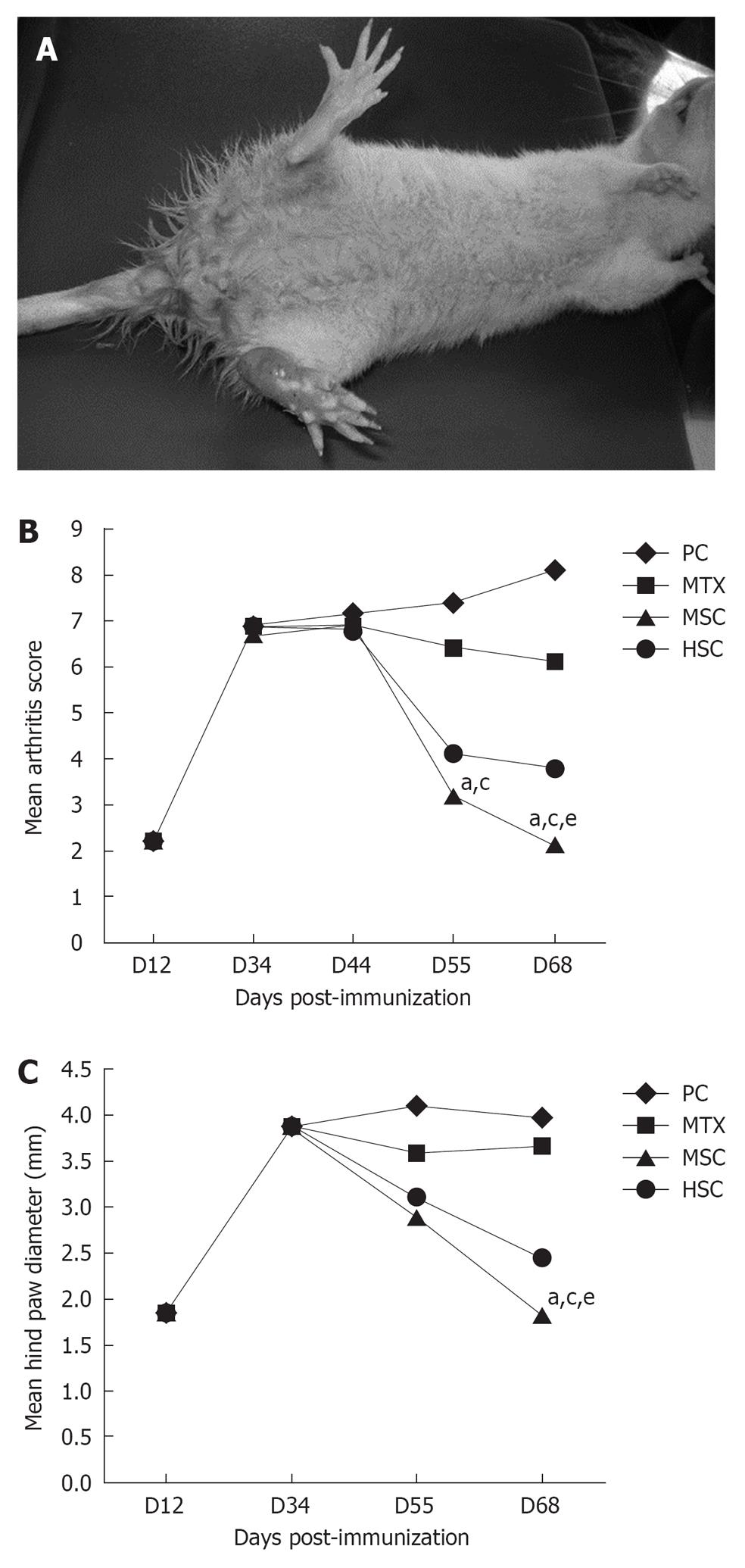
To study the therapeutic effect of MSC and HSC, we first established adjuvant arthritis rat models according to established protocol. The arthritis that develops in rats after a single intradermal treatment with CFA shows a rapid clinical progression. Animals showed clinical signs of disease starting from day 10-12 after immunization with CFA; these initial signs were restricted to redness in one or both hindpaws. These early clinical signs of disease rapidly progressed to swelling, digit deformity and finally to hindpaw ankylosis in 100% of immunized rats by day 34. Figure 1A compares the gross appearance of a normal rat hindpaw and a fully ankylosed hindpaw from a CFA-immunized rat at day 34. Incidence of forepaw involvement in immunized animals was lower and more variable than the hindpaw disease, affecting 20%-90% of rats and generally after day 21. We examined the immune-suppression effect of stem cells on the CFA mouse models by injecting MSC, HSC, MTX or PBS into rats once paw swelling had fully developed (34 d post immunization). Grossly, we found that the swelling of those rats receiving PBS further worsened or showed no significant improvement. The mean arthritis score was similar in all groups at 12 and 34 d post immunization with no statistical significant difference. Mean arthritis score was assessed in all groups at 44, 55 and 68 d post immunization (10, 21 and 34 d post treatment) and results revealed a significant decrease in both MSC and HSC groups compared to MTX and PC groups, started at 55 d post immunization and reached its maximum at day 68. In addition, there was a statistical significant difference between MSC and HSC groups at day 68 (P = 0.036) (Figure 1B).
We observed that paw swelling in rats of the HSC and MSC groups began to disappear around 21 d after receiving stem cell injection and totally disappeared at day 34 post treatment in the MSC group. The mean hindpaw diameter (mm) in the MSC rats was about half that of the PC and MTX groups (P = 0.007 and P = 0.021, respectively) and 0.6 mm less than the HSC group (P = 0.047) (Figure 1C).
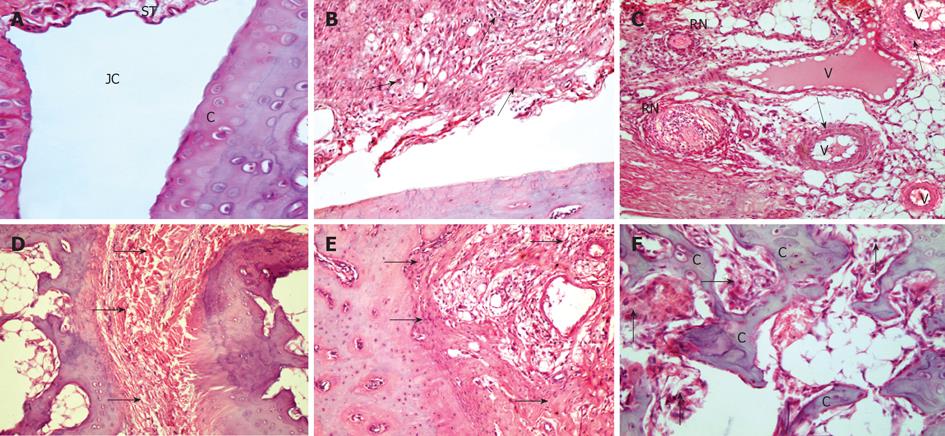
H and E stained sections in hindpaws of the NC group showed intact articular cartilage with normal joint space and synovial tissue (Figure 2A). Sections from the PC group showed a microscopic analysis of disease progression in CFA-immunized rats. The first histological signs of disease appeared at day 10, when hypertrophy of the synovial lining was observed. Massive mononuclear cell infiltration of the synovium was also visible in the same section (Figure 2B).
Nearly, all arthritic rats showed evidence of hypervascularity and congestion. Mononuclear cells infiltration was also visible within vessels wall (Figure 2C). Chronic inflammatory nodules and rheumatoid nodules in the synovial connective tissue of arthritic rats were also found. These nodule-like structures have a defined morphology, consisting of a central zone composed of necrotic tissue and polymorphonuclear cells, surrounded by a layer of radially oriented mononuclear cells (Figure 2C). With disease progression, by day 21 the synovial tissue became hypercellular and well vascularized, so that, at this stage, the synovial lining had been replaced by an inflammatory granulation tissue, or pannus obliterating the joint space (Figure 2D and E). By day 34, infiltrating pannus significantly eroded the articular cartilage and subchondral bone (Figure 2F), both from the joint margins and the periarticular space, resulting in complete fibrous ankylosis.
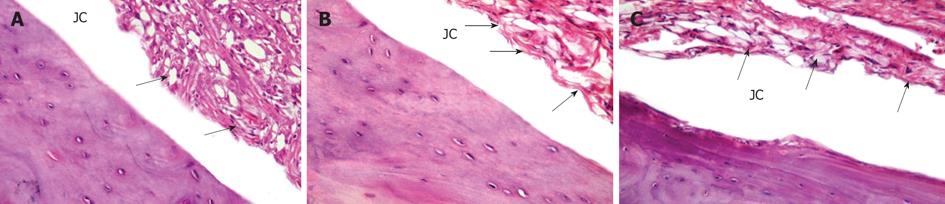
The MTX group showed significant reduction of leucocytic infiltrate and hypertrophy of the synovial tissue with moderate obliteration of the joint cavity (Figure 3A). However, stem cells treatment, both CD34+ (HSC) (Figure 3B) and mesenchymal (MSC) (Figure 3C), showed more or less similar results, with significant reduction of leucocytic infiltrate and hypertrophy of the synovial tissue with mild obliteration of joint cavity.
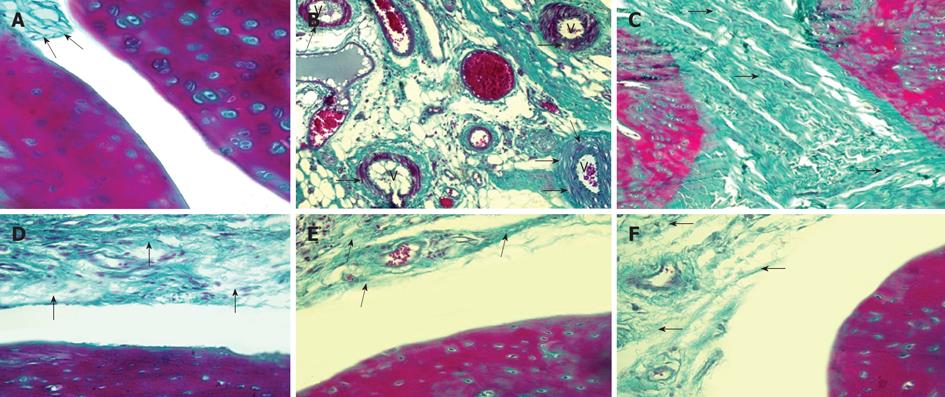
With Masson’s trichrome stain, the NC group showed a normal synovial tissue with delicate collagen fibers (Figure 4A). The mean color area percentage of collagen in this group was 0.121 ± 0.0147 (Table 1). Sections from the PC group showed evidence of vascular edema of almost all vessels within the synovium in nearly all arthritic rats. Vacuoles were also visible in the outer vessel wall (Figure 4B). The vessel became hemorrhagic and finally necrotic. This histological progression resembles necrotizing vasculitis seen in human RA. Sections from the same group also showed extensive fibrosis completely obliterating the joint cavity (Figure 4C). The mean color area percentage of collagen in this group was 0.324 ± 0.096, which was significantly increased when compared to the control group (Table 1).
The MTX group revealed a moderate increase in collagen fibers in the synovial tissue (Figure 4D). The mean color area percentage of collagen in this group was 0.183 ± 0.0148, which was significantly increased compared to the NC group; however, it was markedly decreased compared to the PC group (Table 1). Stem cell treatment, both HSC (Figure 4E) and MSC (Figure 4F), showed similar results with a mild increase in collagen fibers in the synovial tissue. The mean color area percentage of collagen in the HSC and MSC groups was 0.176 ± 0.0137 and 0.174 ± 0.0197 respectively, which was significantly increased when compared to the NC group; however, it was markedly decreased compared to the PC group (Table 1).
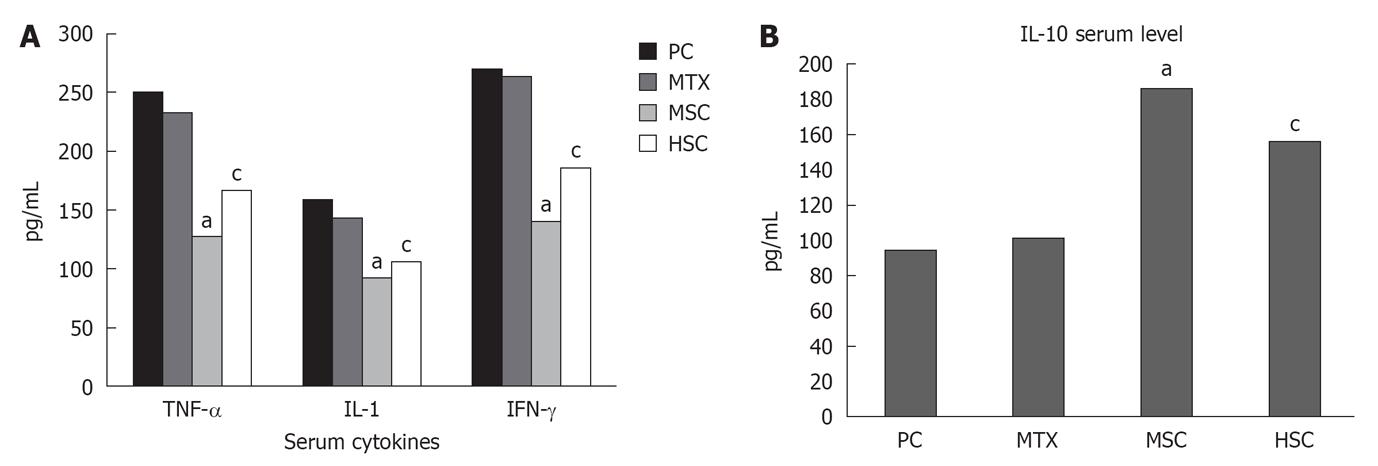
In addition, the results of immunoassay showed that the serum levels of TNF-α, IL-1 and IFN-γ were lower in the HSC and MSC groups compared to the PC and MTX groups (P < 0.05). Both groups also showed elevated anti-inflammatory cytokine IL-10 in comparison to PC and MTX. Despite the level of these cytokines being lower in the MSC group than the HSC group, this difference was not statistically significant (Figure 5A and B).
Analysis of the histopathology of CFA induced arthritis in rat shows the progression of arthritic disease resembling human RA. These similarities support the use of CFA injected rat as an appropriate model for testing potential therapeutics for the treatment of RA[35].
Generally, MSCs have an immune suppressive function in addition to its roles in tissue repair. In addition, they have the capability of self-renewal and differentiation into different lineages of mesenchymal tissues. Moreover, MSCs have been consistently shown to exert a potent immunosuppressive effect superior in magnitude to any other immunosuppressive cell types thus far described[36]. Compared with those BM-MSCs, hUCB-MSCs have higher proliferative potency, lower risk for viral contamination and stronger differentiation capacity[34]. Several researchers have conducted studies to demonstrate the therapeutic potential of BM-MSCs for RA[37,38], but few studies have reported on hUCB-MSCs.
Zhou et al[39] found that human MSC could significantly inhibit the autoimmune progression in MRL/lpr mice. In our study, we further researched the possible clinical utility of hUCB-MSCs in the treatment of RA. Grossly, we noticed that injection of human umbilical cord blood-derived stem cells (both mesenchymal and hematopoietic) into CFA rats led to a significant reduction in the severity of arthritis. Paw swelling had completely disappeared 21-34 d after stem cell administration, but with a significant decrease in the MSC group, more than the PC group, MTX group and even the HSC group.
These results were in accordance with Fei Mao and his colleagues[37], who found that injection of MSCs into collagen induced arthritis mice led to a significant reduction in arthritic signs with complete disappearance of paw swelling 21 d after MSC administration in CIA/MSC mice.
Microscopically, in HE stained sections there was a significant reduction of leucocytic infiltrate and hypertrophy of the synovial tissue with mild obliteration of the joint cavity in both HSC and MSC groups in comparison to the PC and MTX group. These findings were similar to Liu et al[28], who observed that control mice exhibited a marked mononuclear cell infiltration, severe synovitis, pannus formation and bone erosion, while the majority of joints from mice injected with umbilical cord-MSCs had normal morphology with a smooth articulation cartilage surface and an absence of inflammatory cell infiltrate and pannus formation.
With Masson’s trichrome stain there was a mild increase in collagen fibers in the synovial tissue in both HSC and MSC groups and a moderate increase in the MTX group, denoting that the increase in collagen fibers and fibrosis associated with RA had been decreased with stem cell injection. In addition, these changes were also in favor of the MSC group, more than HSC, suggesting that MSC may be a more potent therapeutic in alleviating the arthritic burden, even more than HSC. In conclusion, we observe that microscopically, human umbilical MSCs can prevent tissue damage in RA.
Furthermore, we investigated the effect of stem cells on production of inflammatory mediators that are linked to RA and found that HSC and MSC injections significantly downregulated TNF-α, IL-1 and IFN-γ, as well as upregulated the regulatory and anti-inflammatory cytokine (IL-10). These results are consistent with previous findings by others[37] showing the immunosuppressive effect of MSCs and our results showed that human umbilical stem cells can be a potent candidate for therapeutic treatment for RA.
In conclusion, many new therapeutic possibilities for RA have been proposed recently. Most of these target individual proinflammatory cytokines or cytokine-producing cells as anti-TNF drugs. Here, we believe that human umbilical MSCs could be a better therapeutic option for the treatment of RA.
Rheumatoid arthritis (RA) is an autoimmune disease that leads to chronic inflammation in the joints and subsequent cartilage destruction and bone erosion. RA imparts a massive burden on health services worldwide. A desirable therapeutic approach would be to prevent the destructive effects of RA. New target therapies have achieved considerable success; however, these approaches are expensive and none of the currently widely used biological agents reaches long-term drug-free remission. Human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs) have a potential capacity to regulate the immune response, including in vitro inhibition of T cell proliferation, B cell function and dendritic cell maturation.
hUCB-MSCs are regarded as an alternative source of bone marrow-derived MSCs because collection of cord blood is less invasive than that of bone marrow. In addition, hUCB-MSCs also possess activities for immune modulation, tumor tropism and nursing effect. However, very little is known about hUCB-MSCs in the treatment of RA. Consequently, to understand and use the immune regulation properties of hUCB-MSCs for application in the treatment of a number of human immunological diseases, the molecular mechanism underlying the immune modulatory functions of hUCB-MSCs should be further investigated.
In this study, the authors tried to understand and utilize the immune regulation properties of hUCB-MSCs for the treatment of RA.
The study results suggest that MSCs enhance the efficacy of complete freunds adjuvant-induced arthritis treatment, most likely through the modulation of the expression of inflammatory cytokines and amelioration of joint damage.
RA is a chronic systemic inflammatory disease of unknown cause. The hallmark feature of this condition is persistent symmetric polyarthritis (synovitis) that affects the hands and feet, although any joint lined by a synovial membrane may be involved. Extra-articular involvement of organs such as the skin, heart, lungs and eyes can be significant. hUCB-MSCs, isolated from the umbilical cord of healthy pregnant donors undergoing full-term normal vaginal delivery, are a promising candidate source of MSCs. Apart from their prominent advantages of abundant supply, painless collection and faster self-renewal, hUCB-MSCs have shown the potencies to differentiate into a variety of cells of three germ layers (such as bone, cartilage, adipose, skeletal muscle, cardiomyocyte, endothelium, hepatocyte-like cluster, islet-like cluster, neuron, astrocyte and oligodendrocyte).
This is interesting work that is in line with other findings in induced arthritis models.
P-Reviewer Driscoll D S- Editor Wen LL L- Editor Roemmele A E- Editor Zheng XM
| 1. | Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2468] [Cited by in F6Publishing: 2504] [Article Influence: 119.2] [Reference Citation Analysis (0)] |
| 2. | Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173-6177. [PubMed] [Cited in This Article: ] |
| 3. | Chiang EY, Kolumam GA, Yu X, Francesco M, Ivelja S, Peng I, Gribling P, Shu J, Lee WP, Refino CJ. Targeted depletion of lymphotoxin-alpha-expressing TH1 and TH17 cells inhibits autoimmune disease. Nat Med. 2009;15:766-773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 4. | Gonzalez-Rey E, Chorny A, Varela N, O'Valle F, Delgado M. Therapeutic effect of urocortin on collagen-induced arthritis by down-regulation of inflammatory and Th1 responses and induction of regulatory T cells. Arthritis Rheum. 2007;56:531-543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200:277-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 894] [Cited by in F6Publishing: 917] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 6. | Morgan ME, Sutmuller RP, Witteveen HJ, van Duivenvoorde LM, Zanelli E, Melief CJ, Snijders A, Offringa R, de Vries RR, Toes RE. CD25+ cell depletion hastens the onset of severe disease in collagen-induced arthritis. Arthritis Rheum. 2003;48:1452-1460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 242] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 7. | Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775-787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3445] [Cited by in F6Publishing: 3595] [Article Influence: 224.7] [Reference Citation Analysis (0)] |
| 8. | Morgan ME, Flierman R, van Duivenvoorde LM, Witteveen HJ, van Ewijk W, van Laar JM, de Vries RR, Toes RE. Effective treatment of collagen-induced arthritis by adoptive transfer of CD25+ regulatory T cells. Arthritis Rheum. 2005;52:2212-2221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 302] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 9. | van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004;50:2775-2785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 364] [Cited by in F6Publishing: 372] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 10. | Yudoh K, Matsuno H, Nakazawa F, Yonezawa T, Kimura T. Reduced expression of the regulatory CD4+ T cell subset is related to Th1/Th2 balance and disease severity in rheumatoid arthritis. Arthritis Rheum. 2000;43:617-627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 11. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15372] [Cited by in F6Publishing: 14773] [Article Influence: 590.9] [Reference Citation Analysis (0)] |
| 12. | Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5703] [Cited by in F6Publishing: 5568] [Article Influence: 242.1] [Reference Citation Analysis (0)] |
| 13. | Sun L, Akiyama K, Zhang H, Yamaza T, Hou Y, Zhao S, Xu T, Le A, Shi S. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009;27:1421-1432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 463] [Cited by in F6Publishing: 433] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 14. | Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755-1761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1050] [Cited by in F6Publishing: 1032] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 15. | Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499-3506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1289] [Cited by in F6Publishing: 1288] [Article Influence: 75.8] [Reference Citation Analysis (1)] |
| 16. | Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42-48. [PubMed] [Cited in This Article: ] |
| 17. | Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821-2827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 825] [Cited by in F6Publishing: 807] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 18. | Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, Galun E, Rachmilewitz J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214-2219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 771] [Cited by in F6Publishing: 810] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 19. | Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129:163-173. [PubMed] [Cited in This Article: ] |
| 20. | Rao MS, Mattson MP. Stem cells and aging: expanding the possibilities. Mech Ageing Dev. 2001;122:713-734. [PubMed] [Cited in This Article: ] |
| 21. | Gang EJ, Jeong JA, Hong SH, Hwang SH, Kim SW, Yang IH, Ahn C, Han H, Kim H. Skeletal myogenic differentiation of mesenchymal stem cells isolated from human umbilical cord blood. Stem Cells. 2004;22:617-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 215] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 22. | Bieback K, Klüter H. Mesenchymal stromal cells from umbilical cord blood. Curr Stem Cell Res Ther. 2007;2:310-323. [PubMed] [Cited in This Article: ] |
| 23. | Lee DH, Ahn Y, Kim SU, Wang KC, Cho BK, Phi JH, Park IH, Black PM, Carroll RS, Lee J. Targeting rat brainstem glioma using human neural stem cells and human mesenchymal stem cells. Clin Cancer Res. 2009;15:4925-4934. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 24. | Wang M, Yang Y, Yang D, Luo F, Liang W, Guo S, Xu J. The immunomodulatory activity of human umbilical cord blood-derived mesenchymal stem cells in vitro. Immunology. 2009;126:220-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 180] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 25. | Hao L, Zhang C, Chen XH, Zou ZM, Zhang X, Kong PY, Liang X, Gao L, Peng XG, Sun AH. Human umbilical cord blood-derived stromal cells suppress xenogeneic immune cell response in vitro. Croat Med J. 2009;50:351-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Rico MC, Castaneda JL, Manns JM, Uknis AB, Sainz IM, Safadi FF, Popoff SN, Dela Cadena RA. Amelioration of inflammation, angiogenesis and CTGF expression in an arthritis model by a TSP1-derived peptide treatment. J Cell Physiol. 2007;211:504-512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Park YW, Kang YM, Butterfield J, Detmar M, Goronzy JJ, Weyand CM. Thrombospondin 2 functions as an endogenous regulator of angiogenesis and inflammation in rheumatoid arthritis. Am J Pathol. 2004;165:2087-2098. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Liu Y, Mu R, Wang S, Long L, Liu X, Li R, Sun J, Guo J, Zhang X, Guo J. Therapeutic potential of human umbilical cord mesenchymal stem cells in the treatment of rheumatoid arthritis. Arthritis Res Ther. 2010;12:R210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 159] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 29. | Barsante MM, Roffê E, Yokoro CM, Tafuri WL, Souza DG, Pinho V, Castro MS, Teixeira MM. Anti-inflammatory and analgesic effects of atorvastatin in a rat model of adjuvant-induced arthritis. Eur J Pharmacol. 2005;516:282-289. [PubMed] [Cited in This Article: ] |
| 30. | Delgado M, Abad C, Martinez C, Leceta J, Gomariz RP. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat Med. 2001;7:563-568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 292] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 31. | Wagner JE. Umbilical cord blood stem cell transplantation. Cancer Treat Res. 1995;76:195-213. [PubMed] [Cited in This Article: ] |
| 32. | Miltenyi S, Müller W, Weichel W, Radbruch A. High gradient magnetic cell separation with MACS. Cytometry. 1990;11:231-238. [PubMed] [Cited in This Article: ] |
| 33. | Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol. 2001;Appendix 3:Appendix 3B. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 685] [Cited by in F6Publishing: 786] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 34. | Secco M, Zucconi E, Vieira NM, Fogaça LL, Cerqueira A, Carvalho MD, Jazedje T, Okamoto OK, Muotri AR, Zatz M. Multipotent stem cells from umbilical cord: cord is richer than blood! Stem Cells. 2008;26:146-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 254] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 35. | Pearson CM. Development of arthritis, periarthritis and periostitis in rats given adjuvants. Proc Soc Exp Biol Med. 1956;91:95-101. [PubMed] [Cited in This Article: ] |
| 36. | Uccelli A, Moretta L, Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol. 2006;36:2566-2573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 397] [Cited by in F6Publishing: 415] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 37. | Mao F, Xu WR, Qian H, Zhu W, Yan YM, Shao QX, Xu HX. Immunosuppressive effects of mesenchymal stem cells in collagen-induced mouse arthritis. Inflamm Res. 2010;59:219-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 38. | Choi JJ, Yoo SA, Park SJ, Kang YJ, Kim WU, Oh IH, Cho CS. Mesenchymal stem cells overexpressing interleukin-10 attenuate collagen-induced arthritis in mice. Clin Exp Immunol. 2008;153:269-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 39. | Zhou K, Zhang H, Jin O, Feng X, Yao G, Hou Y, Sun L. Transplantation of human bone marrow mesenchymal stem cell ameliorates the autoimmune pathogenesis in MRL/lpr mice. Cell Mol Immunol. 2008;5:417-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |